You may also like...
Embiid Benched: Sore Shin Keeps 76ers' Star Out Against Hawks!

Philadelphia 76ers' Joel Embiid is out for Thursday's game against Atlanta due to right shin soreness, following a knee ...
Ja Morant's Comeback Delayed: Grizzlies Star Out for Two More Weeks!

Memphis Grizzlies guard Ja Morant will miss at least two more weeks due to a sprained left elbow, with his re-evaluation...
Streaming Phenomenon: Sitcom Spin-Off Crushes Records with 832.7M Hours!
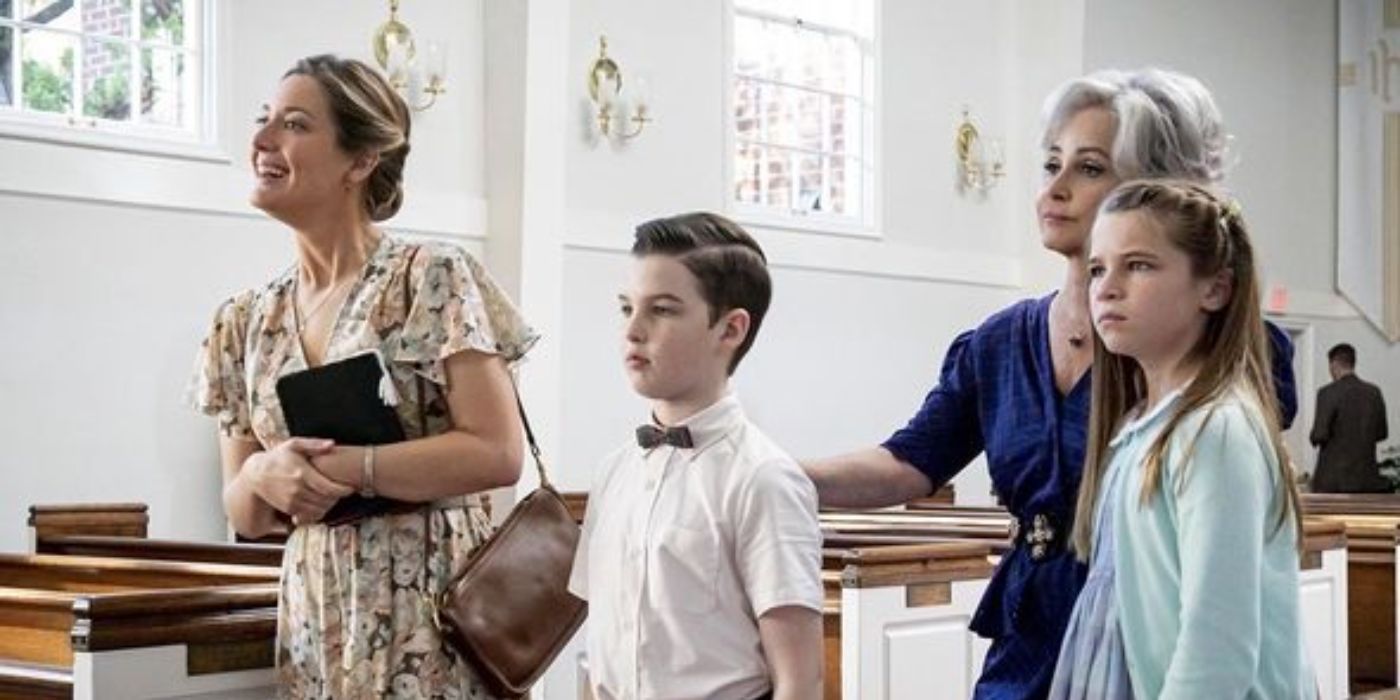
"Young Sheldon" has proven to be a highly successful sitcom spinoff, drawing immense viewership and delving into the chi...
Shocking MCU Betrayal: Downey Jr. and Evans' Returns Ruin 'Endgame' Legacy!

The potential return of Robert Downey Jr. and Chris Evans to the MCU in "Avengers: Doomsday" sparks debate, with critics...
African Royalty Reigns: Burna Boy & Tems Make Billboard Hot 100 History

Nigerian music stars Burna Boy and Tems have made history as the first African artistes with eight entries on the Billbo...
Tragedy Strikes: Florida Rapper Lil Poppa Dies at 25

Rapper Lil Poppa (Janarious Mykel Wheeler) has died at 25, confirmed on February 18, 2026, with the cause of death under...
OpenAI's India Power Play: Tata Deal for Massive AI Data Center

OpenAI has announced a landmark partnership with India's Tata Group, securing 100 megawatts of AI-ready data center capa...
OpenAI Forges Indian Fintech Frontier with Pine Labs Alliance

OpenAI and Pine Labs have partnered to integrate AI-driven reasoning into the fintech firm's payments stack, aiming to a...