BMC Musculoskeletal Disorders volume 26, Article number: 584 (2025) Cite this article
Osteoarthritis (OA) is a joint disease with a high worldwide prevalence, often leading to replacement surgery. However, despite the high success rate of replacement surgery, some patients fail to experience the intended benefits. Given the importance of patient involvement, this trial aims to assess the effect of shared decision-making (SDM) on decisional quality for patients with severe OA. Specifically, it investigates whether an in-consult patient decision aid (PtDA) enhances decisional quality, involvement, and health outcomes for patients with severe hip or knee OA.
This protocol outlines a pragmatic two-armed multicentre cluster-randomised controlled trial (C-RCT), chosen for its ability to assess the combined effect of shared decision-making (SDM) and a newly developed in-consult patient decision aid (PtDA) in routine clinical practice. The PtDA is a tool designed to help patients make informed decisions during the consultation by providing personalised treatment options, benefits, and risks. The C-RCT design is particularly well-suited to this context, as it effectively minimises contamination and bias across treatment groups. A total of 19 orthopaedic surgeons will be randomised 1:1, stratified by centre, to either continue standard consultations without the PtDA or implement SDM using the PtDA. The trial will recruit 615 patients with severe hip or knee OA. Outcomes will be measured at one week, three months, and 12 months post treatment start. The primary outcome, decisional quality, will be assessed using the Hip/Knee OA Decision Quality Instrument, focusing on patients being well-informed and receiving their preferred treatment. The secondary outcomes include patient involvement in the decision-making process and consultation durations. The tertiary outcomes include patients’ satisfaction, regret and health-related outcomes. The primary outcome will be analysed using a multilevel mixed-effects logistic regression with surgeons as random effects reporting odds ratios with 95% confidence intervals.
This study aims to contribute insights into the use of SDM facilitated by a PtDA and its impact on improving decisional quality for future patients with hip or knee OA.
www.ClinicalTrials.gov (NCT05972525), Data of registration: 06.08.2023.
Osteoarthritis (OA) is one of the most prevalent joint diseases, affecting approximately 600 million individuals worldwide [1]. The prevalence of OA is anticipated to increase due to gradually increasing obesity and age in the population with high physical activity [1,2,3,4]. OA typically affects the hip or knee and induces pain and disability, leading to reduced function and quality of life (QoL), particularly in older adults [5, 6].
Severe cases often need hip or knee replacement [7,8,9]. These surgeries are among the most frequently performed procedures worldwide [2,3,4,5,6,7,8,, 9], with proven success in terms of pain relief, increased function and enhanced health-related QoL [10, 11].
While recommendations for treating early-stage OA with non-surgical interventions (e.g., patient-education, exercise, pain medication, and, especially for knee OA, weight loss) are generally consistent [7], the decision to proceed with total joint replacement (TJR) in severe cases is influenced by patient and clinician preferences, considering factors such as symptom severity, radiological findings, age, comorbidities and QoL [4,5,6,7,8,9,10,11,, 12]. Despite its benefits, some patients undergoing TJR fail to experience the intended results [3,4,5,6,7,8,9,10,11,12,, 13]. Dissatisfaction rates after total hip replacement (THR), total knee replacement (TKR) and unicompartmental knee replacement (UKR) have been reported as 7%, 11%–18% and 10%, respectively [14,15,16,17].
Studies have identified a clear association between postoperative dissatisfaction and the misalignment of preoperative expectations with actual outcomes [16, 18, 19], while patients whose expectations are met express higher satisfaction with their surgical outcomes [16, 20]. However, it is important to acknowledge that patient preferences can sometimes be influenced by expectations that do not align with the expected outcomes from TJR surgery. A considerable number of patients choose a surgical intervention to manage joint pain thout a comprehensive understanding of what to expect from either surgery or alternative treatment options [21,22,23].
Engaging patients in shared decision-making (SDM) is recognised as a crucial strategy to enhance decisional quality, especially in preference-sensitive decisions such as to undergo TJR [24, 25]. SDM can be facilitated by patient decision aids (PtDAs), which serve to inform patients about relevant treatment options and promote the SDM process. Although over 209 randomised trials have evaluated the use of PtDAs in routine care [26], only a limited number have specifically assessed their impact on decision-making among patients with hip or knee OA [27,28,29]. Furthermore, a recent systematic review highlights the need for more robust evidence on how PtDAs influence decision quality and the overall decision-making process compared to usual care, and notes a trend towards fewer surgeries among patients receiving SDM supported by a PtDA [30]. To address this gap, the PATient Involvement (PATI) study aims to evaluate how SDM combined with an in-consult PtDA affects decisional quality, patient involvement, and health outcomes in patients with severe hip or knee OA referred for treatment.
This study adheres to the Standard Protocol Items for Randomized Trials (SPIRIT) guidelines [31] and follows the recommendations of the Standards for Universal Reporting of Decision Aids Evaluation (SUNDAE) [32]. It is reported according to the Consolidated Standards of Reporting Trials (CONSORT) extended guidelines for cluster-randomised controlled trials (C-RCTs) [33].
This study employed a two-armed, multicentre (two centres), pragmatic superiority [34] cluster-randomised controlled trial (C-RCT). Each cluster consisted of a single hip or knee surgeon, randomised (1:1 allocation) to either standard consultations or SDM using an in-consult PtDA explicitly developed for patients with hip or knee OA for this study. The PtDA supports informed decision-making during consultations by providing patients with personalised information on treatment options, including benefits and risks. The C-RCT design with randomisation of surgeons was chosen to prevent contamination by using the intervention in the control-group, as individual randomisation of patients could lead surgeons to apply the PtDA or knowledge from the intervention group to the control group, compromising the study’s integrity. Twenty clusters across two university hospitals in Denmark were randomised and stratified by centre. The scheduled consultation duration remained consistent, regardless of the intervention or control group. The primary and secondary outcomes based on patient-reported data are collected one week post-consultation (timepoint 1 [T1]), and a project nurse registered the patients’ received treatment six months post-consultation. The tertiary outcomes are assessed three months (timepoint 2 [T2]) and 12 months after treatment begins (e.g., the date of referral for non-surgical treatment or the date of surgery) (timepoint 3 [T3]) and will be reported in follow-up studies.
Specific objectives and hypotheses
Objective 1: To investigate whether using an in-consult PtDA enhances the decisional quality for patients with severe OA of the hip or knee referred for treatment.
Hypothesis 1: Patients receiving consultations using an in-consult PtDA will achieve higher decisional quality than those receiving standard consultations.
Objective 2: To investigate whether an in-consult PtDA increases patient-experienced involvement in SDM.
Hypothesis 2: Patients receiving consultations using an in-consult PtDA will report greater involvement in SDM than those who receive standard consultations.
Objective 3: To compare durations between consultations using SDM with an in-consult PtDA and standard consultations and explore the learning curve in using the in-consult PtDA, expressed as the consultation duration.
Hypothesis 3.1: The duration will not differ between consultations using the in-consult PtDA and standard consultations.
Hypothesis 3.2: The duration of consultations using the in-consult PtDA will decrease over time, indicating a learning curve associated with PtDA integration.
Objective 4: To determine whether consultations using the in-consult PtDA are superior to standard consultations regarding the level of changes in the patient-reported outcomes of pain, physical function and QoL at 3 and 12 months following surgery.
Hypothesis 4: Consultations using a PtDA are superior to standard consultations regarding the level of changes in the patient-reported outcomes of pain, physical function and QoL at 3 and 12 months after surgery.
Objective 5: To determine the association between informed patient-centred (IPC) decisions and the level of changes in the patient-reported outcomes of pain, physical function and QoL at 3 and 12 months after surgery.
Hypothesis 5: IPC decisions are positively associated with the level of changes in patient-reported outcomes related to pain, physical function and QoL at 3 and 12 months after surgery.
Objective 6: To evaluate whether consultations using a PtDA are superior to standard consultations regarding patient satisfaction at 3 and 12 months after surgery.
Hypothesis 6: Consultations using a PtDA are superior to standard consultations regarding patient satisfaction at 3 and 12 months after surgery.
Participants
Patients with hip or knee OA referred from general practice and scheduled for consultations with orthopaedic surgeons at the two study centres are screened for eligibility during the consultations, regardless of the intervention arm. Using a standardised protocol, the surgeons inform patients about the trial and obtain verbal informed consent. Patients provide their formal informed consent (Appendix) upon receiving additional trial information along with the first set of questionnaires in the week following inclusion. Although hip and knee OA differ in symptom trajectory and surgical outcomes, both groups were included in this trial to allow broader applicability of the findings. This decision aligns with the design of several comparable international studies [35]. While the study was not powered to detect joint-specific differences, stratified analyses by joint (hip vs knee) are planned to explore potential variations in outcomes.
The inclusion criteria for patients are:
Patients with the following will be excluded:
Interventions
Two new in-consult PtDAs for patients with severe OA in the hip or knee were developed and tested between October 2022 and August 2023. The development process involved a steering group consisting of two clinicians with expertise in OA in the hip or knee, one in SDM, and one with experience in orthopaedic qualitative research, along with patients, their relatives, and surgeons who were randomised to the intervention group in the c-RCT. The PtDAs were developed using a generic template, adhering to the criteria set by the International Patient Decision Aid Standards [36, 37]. Both PtDAs, titled ‘Which Treatment Is Right for Me?’ (Fig. 1), are presented in booklet form and represent the first editions of such aids for hip and knee OA in Denmark. The development process involved eight iterative rounds, followed by final testing with 15 newly recruited patients and surgeons randomised to the c-RCT. The final versions of the in-consult PtDAs are structured into five sections, following the framework outlined by Steffensen et al. [38]. These include option cards that illustrate the pros and cons of each treatment (surgical vs. non-surgical), simplified statistics (expected prosthesis survivorship, surgical risks, and expected outcomes), and patient stories.
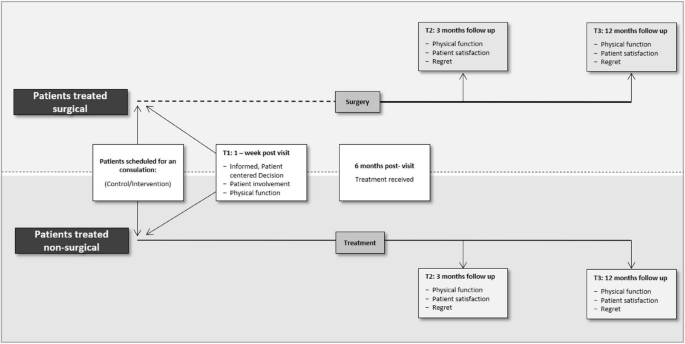
Surgeons in the intervention group received a three-hour introduction and training course on the SDM concept and using an in-consult PtDA the day before the trial commenced at each of the two centres. This introduction also provided information about screening, recruitment, intervention and survey delivery at specific time points. Surgeons in the control group participated in a mandatory introductory course covering trial design with detailed task discussions. The sequence of activities within the orthopaedic clinic flow is illustrated in Fig. 2.
Study intervention and assessment timeline for patients treated surgically and non-surgically. In the treatment pathway where patients are treated with surgery, the dotted line represents the waiting time between the inclusion date (the date patients are enrolled in the study or treatment plan) and the surgery date
Outcomes
Primary outcome:
Secondary outcomes:
Tertiary outcomes:
Patients with severe OA of the hip or knee referred from general practice and scheduled for appointments with orthopaedic surgeons at the two centres were screened for eligibility by the surgeon at the end of their consultation, irrespective of the intervention arm.
Setting and enrolment strategies
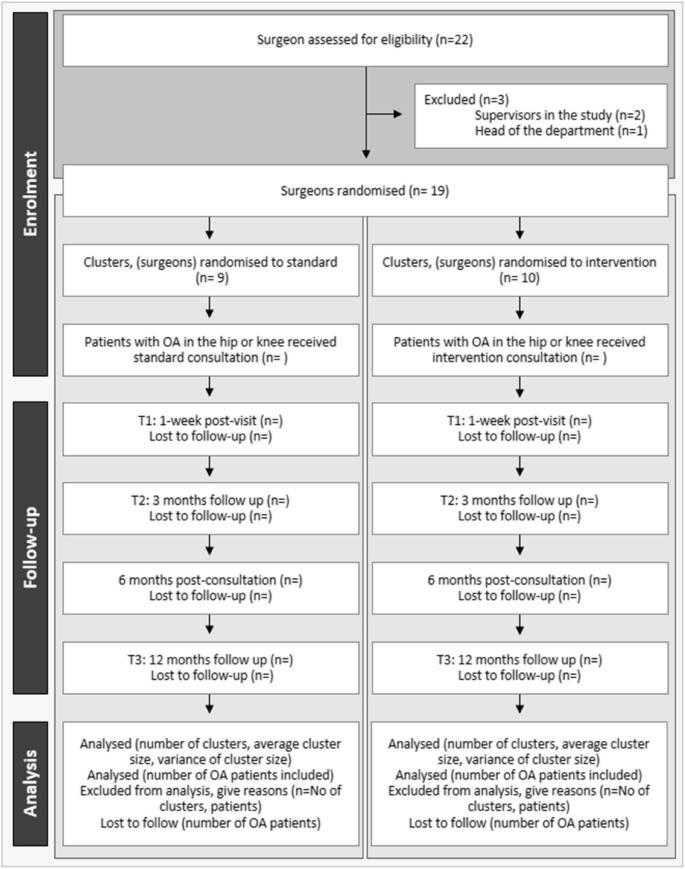
The study recruited patients and surgeons from the orthopaedic departments specialising in fast-track hip and knee replacement at two university hospitals in the Region of Southern Denmark. These departments collectively perform almost 2,700 out of the approximately 26,000 THR, TKR and UKR surgeries performed annually in Denmark [46, 47]. An inclusion period of 12 months was expected, with an inclusion rate of 23% involving weekly enrolment of 11 patients and an anticipated dropout rate of 20%. The inclusion flow is illustrated as a CONSORT flow diagram (Fig. 3) [33].
CONSORT flow diagram, extended version for C-RCTs at both cluster and individual levels. Clusters: surgeons; T1: one-week post-visit; T2: three months after treatment; T3: 12 months after treatment
Trial dates
Patient enrolment began in October 2023 at the first centre and in November 2023 at the second centre. The 12-month follow-up period commenced after patients received surgical or non-surgical treatment. Due to current waiting lists, the data collection period was estimated to last approximately two years, with an anticipated completion during the summer of 2026.
Randomisation and blinding
In October 2022, orthopaedic surgeons specialised in hip or knee replacements from both centres were randomised for the PATI study. Before the start of the trial, surgeons in the intervention group participated in the development phase of two new in-consult PtDAs (Fig. 2) that will be used in the intervention arm. A 1:1 randomisation was implemented using a computer-generated randomisation schedule, stratified by the surgeons’ employment centres and using permuted blocks of size 10. Additional blocks, each with a size of two, were introduced to accommodate potential changes, such as surgeons leaving or newly hired to the departments during the trial period.
An independent data manager created the computer-generated list of random numbers using the randomisation tool in REDCap [48]. The administrator responsible for the PATI study and the randomisation procedure will remain blinded to the sequence of random numbers within the blocks throughout the trial period. The randomisation of the clusters was disclosed to the two centres before the start of the trial. Patients will not be blinded to the interventions but will not be provided with explicit information about the intervention or control assignments. Similarly, the surgeons, project managers, and project nurses involved in the trial will not be blinded.
Data collection
Upon enrolment, patients will complete assessments via REDCap, entering their responses directly for all patient-reported outcomes. To ensure data completeness, the ‘required fields’ option will be activated to prevent missing items in the questionnaires. In the event of no response, automated reminders will be sent after three and six days. If there is still no reply, the local project nurse will contact the patient with up to two phone calls. Patients who remain unresponsive will be marked as ‘lost to follow-up’ for the relevant time point in the database.
At baseline (T1), demographic data will be collected, including participants’ age, sex, education level, income status, whether they received a PtDA handout (yes/no), and a self-reported pain score on a five-point Likert scale. At the cluster level, information will be gathered on surgeons’ age, sex, years of surgical experience, and joint specialty (hip or knee) (Table 1).
Sample size
The required sample size was estimated considering the possibility of some surgeons leaving the two centres during the trial period. Therefore, the sample size was calculated based on 15 clusters (surgeons) with an assumed intraclass correlation coefficient (ICC) of 0.02, which represents how strongly individuals within clusters are related A superiority difference of 0.15 was used, informed by data from a comparable American study that investigated the differences between sites with and without efforts to use SDM [39, 51, 52]. This resulted in a proportion of 0.40 of patients in the intervention group achieving high decisional quality compared to 0.25 in the control group [35, 39]. A total of 615 patients will be enrolled to achieve a statistical power of 80% at a two-sided significance level of 0.05, with approximately 41 patients per cluster. Of these, 328 and 287 patients will be allocated to the intervention and control groups, respectively, though the exact group assignment will be determined during the randomisation process, accounting for an expected 20% loss to follow-up.
Statistical analysis plan
The statistics will be reported according to the guidelines outlined by the Enhancing the Quality and Transparency of Health Research (EQUATOR) network [53] and CONSORT [33] statements. A detailed statistical analysis plan was developed with the study protocol to ensure transparent and reproducible statistical methods.
The primary and secondary outcomes will be statistically analysed using mixed-effect models based on linear or binomial regression according to the data type of interest [54]. Due to the hierarchical structure of the data, surgeons will be incorporated as random effects. The analysis will be performed using the intention-to-treat approach, and in case of a cross-over, a per-protocol analysis will be conducted as sensitivity analysis [54]. Given the common presence of ceiling effects in several SDM assessment scores, a sensitivity analysis will be conducted using a mixed-effect Tobit regression model to explore their potential influences.
Consultation time will be analysed using a t-test if normally distributed and a Wilcoxon rank sum test otherwise. Histograms will be visually inspected to assess the normality assumption. The learning curve will be explored through descriptive analysis.
The proportion of missing data will be explored. If less than 5% of the data is missing, a complete case analysis will be performed. If more than 5% of the data is missing, the missing data structures will be investigated, considering potential imputation methods.
Data monitoring and auditing
Given this study’s minimal risk, an external Data and Safety Monitoring Board was not established. Instead, the study manager and local project nurses are conducting meticulous internal data monitoring. The study manager and project nurses hold weekly meetings, either in person or by phone, to ensure adherence to the protocol and the smooth progression of the project.
All aspects of participant enrolment, including recruitment and survey response rates across the two centres, are being systematically tracked. No interim analyses are planned for this study.
Protocol amendments
Any substantial adjustments to the protocol will be appropriately registered at www.ClinicalTrials.gov, reported to The Regional Committees on Health Research Ethics for Southern Denmark and addressed in the primary C-RCT publication.
Study participant consent
Before the C-RCT began, a comprehensive kickoff meeting was conducted to engage all study staff from each centre. In this meeting, site team members were briefed about its objectives, their respective roles and the anticipated timeline for enrolment. This meeting was held to foster an open communication environment, encouraging study staff and surgeons to contribute with suggestions or raise any concerns related to the trial material and setup.
Patient consent
Eligible patients are presented with verbal and written information about the study, including the privacy protection concept according to ethical standards outlined in the Declaration of Helsinki [55]. Then, the patients express their voluntary agreement to participate by signing an informed consent form within the REDCap platform at T1.
This study protocol outlines the methodology for a PATI study, a pragmatic multicentre, C-RCT comparing standard consultations in orthopaedic outpatient clinics for patients with OA of the hip or knee with the SDM intervention facilitated through an in-consult PtDA. The PtDA aims to facilitate dialogues concerning complex medical information for patients and their families and to clarify values and preferences, enabling them to make informed, personalised decisions in collaboration with healthcare providers. The facilitation of SDM and using an in-consult PtDA have been shown to improve decisional quality [12].
While past studies have highlighted the value of PtDA delivery in orthopaedic surgery, the number of studies examining the impact of PtDA among patients with OA of the hip or knee remains limited [27]. This study specifically focuses on patients with OA of the hip or knee, exploring the potential benefits and implications of implementing SDM and PtDA in this context. Its cluster-randomised design enhances the robustness of the C-RCT, allowing it to evaluate the effectiveness of the intervention across two centres.
Describing the study protocol underscores the importance of methodological rigour in investigating the impact of SDM and PtDA in orthopaedic settings. The insights gained from this study can potentially inform and improve patient decision quality for those facing decisions related to OA treatment of the hip or knee.
This study had several potential limitations that should be acknowledged. Firstly, the enrolment of participants by surgeons may not be rigorous, introducing possible selection bias. Secondly, the lack of standardised criteria for defining"severe"OA and determining eligibility for JR is a known issue [56]. Current definitions are subjective, often relying on symptoms, radiographic findings, and failure of non-surgical treatments, which can vary considerably in clinical practice. This lack of standardisation may affect participant selection and limit the generalisability of the findings. Thirdly, the extent of SDM and the use of the in-consult PtDA within the intervention group depend on the surgeons. These factors may be influenced by the surgeons'reluctance, workload, or inexperience in using a PtDA. Furthermore, these potential limitations will be assessed through unannounced observations in their consultations and discussions regarding the use of the in-consult PtDA. However, surgeon-specific effects may be partially accounted for by including the surgeon as a random effect in the statistical analyses. Fourthly, conducting the C-RCT may influence surgeons in the control group to outperform their usual practice regarding information and patient involvement, even though they have not been involved in developing the PtDA. Fifthly, while the surgeons, patients, project nurses and project manager are not blinded to the intervention, the statistician will be blinded. Finally, there are generally long waiting lists for both consultations in the outpatient clinic and surgery. Consequently, patients increasingly choose to undergo surgery at private hospitals because of the extended waiting times in the public system, which could affect their commitment during the follow-up period.
The funders will not be involved in the study’s design, interventions, data collection, data management, statistical analysis, interpretation of results, manuscript writing or the decision to submit the manuscripts for publication.
No datasets were generated or analysed during the current study.
- ADL:
-
Activities of daily living
- Cat:
-
Categorical
- C-RCT:
-
Cluster-randomised controlled trial
- Con:
-
Continues
- CONSORT:
-
Consolidated Standards of Reporting Trials
- DQI:
-
Decision Quality Instrument
- EQ-5D-5L:
-
EuroQol five-dimension five-level questionnaire
- EQUATOR:
-
Enhancing the Quality and Transparency of Health Research
- FJS:
-
Forgotten Joint Score
- HK-DQI:
-
Hip/Knee Osteoarthritis Decision Quality Instrument
- IPC:
-
Informed patient–centred
- OA:
-
Osteoarthritis
- OHS:
-
Oxford Hip Score
- OKS:
-
Oxford Knee Score
- OPEN:
-
Open Patient Data Explorative Network
- PATI:
-
PATient Involvement
- PtDA:
-
Patient decision aid
- QoL:
-
Quality of life
- REDcap:
-
Research Electronic Data Capture
- SDM:
-
Shared decision-making
- SPIRIT:
-
Standard Protocol Items for Randomized Trials guidelines
- SUNDAE:
-
Standards for Universal Reporting of Decision Aids Evaluation
- THR:
-
Total hip replacement
- TKR:
-
Total knee replacement
- TJR:
-
Total joint replacement
- T1:
-
Timepoint 1
- T2:
-
Timepoint 2
- T3:
-
Timepoint 3
- UKR:
-
Unicompartmental knee replacement
We express our gratitude to the orthopaedic surgeons, local project nurses and nurses in the outpatient clinics who are involved in interventions, patient enrolment and data management at the Departments of Orthopaedic Surgery at Lillebaelt Hospital–Vejle and University Hospital of Southern Denmark as well as the Department of Orthopaedic Surgery and Traumatology at Odense University Hospital, Odense and Svendborg. We also appreciate the support and assistance of OPEN in data management and access to REDCap and OPEN storage.
Open access funding provided by University of Southern Denmark This C-RCT was provided unrestricted internal funding by the Departments of Orthopaedic Surgery at Lillebaelt Hospital–Vejle and University Hospital of Southern Denmark as well as the Department of Orthopaedic Surgery and Traumatology at Odense University Hospital. External funding was obtained from the Region of Southern Denmark (22/26219) and the Research Fund at Lillebaelt Hospital.
The potential risk of harm is considered minimal, as the intervention involves a new approach to communicate and involve the patients during the consultation. Participants will receive information orally during the consultation from the surgeon and written details via e-Boks, including a link to the electronic questionnaire. After providing oral consent, participants have a minimum of 24 h to consider before signing or refusing to sign the informed consent form at T1. Data collection and processing were approved by the Region of Southern Denmark (Journal No. 22/9955). Approval from the Danish Health Research Ethics Committee is not required for studies involving questionnaires or observations, as they are not classified as interventions under the Committee Act (decision on non-approval project ID: S-20200137). The C-RCT are registered on www.ClinicalTrials.gov (NCT05972525), with registration date: 06.08.2023. Data will be stored in the Open Patient Explorative Patient Network (OPEN), in accordance with the European General Data Protection Regulation. Authorship eligibility will follow guidelines outlined [57].
Not required.
CV was reimbursed for travel expenses by Stryker, paid to the institution, which are unrelated to this study. All other authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Pedersen, T.A., Lindberg-Larsen, M., Jensen, C.M. et al. Impact of an in-consult patient decision aid on decisional quality, involvement, and health outcome for patients with severe hip or knee osteoarthritis – a study protocol for a multicentre, cluster randomised controlled trial (PATI-study). BMC Musculoskelet Disord 26, 584 (2025). https://doi.org/10.1186/s12891-025-08856-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12891-025-08856-w