BMC Veterinary Research volume 21, Article number: 430 (2025) Cite this article
AbstractSection Background
Dental calculus (DC) in dogs is a common oral health issue that can result in discomfort and may lead to systemic complications, if not managed effectively. While dental care practices are often overlooked by many owners, it remains crucial to identify and address possible DC risk and protective factors. The present retrospective cross-sectional study sought to examine the early life factors contributing to the emergence of owner-reported DC in dogs later in life, emphasizing the impact of modifiable variables. The study examined exposure data across five pivotal early life stages, starting prenatally and extending into adolescence. Multivariate logistic regression analyses using backward stepwise deletion were performed using data from 4771 dogs, including 2360 cases and 2411 controls, to examine the potential associations between DC in dogs and 29 distinct early life variables across five statistical models. The study incorporated a range of independent variables, including dietary, environmental, demographic, domestic, and immune-related factors. Additionally, the study aimed to compare between energy value and macronutrient composition of non-processed meat-based diets and ultra-processed carbohydrate-based diets available on the Finnish market.
AbstractSection Results
Our research indicated that dogs fed a non-processed meat-based diet during the weaning period, puppyhood, and adolescence, which corresponds to the age range of 1 month to 1 or 1.5 years, were associated with a significantly lower risk of developing DC later in life. Conversely, dogs consumed an ultra-processed carbohydrate-based diet during the same periods was associated with a significantly higher risk (p = 0.01, p = 0.001, and p = 0.02 for weaning, puppyhood, and adolescence, respectively). Additionally, residing with other dogs was significantly associated with a decreased likelihood of DC development later in life (p = 0.015). Moreover, from the non-modifiable factors model, a maternal history of DC and small size of the dog were strongly associated with an increased risk of DC development later in life.
AbstractSection Conclusions
In addition to the well-known non-modifiable risk factors, such as age and size of the dog, the current study revealed potential associations between modifiable early life exposures and development of DC later in life, including early diets and living with other dogs. However, controlled diet-intervention studies remain necessary to substantiate these connections.
For several decades dental calculus (DC), which is also known by the name of tartar in dogs, has been recognized as a highly prevalent and clinically significant oral health issue in dogs, originating from plaque accumulation [1]. An acquired enamel pellicle is formed on the tooth surface, providing a base for the accumulation of a sticky microbial biofilm, known as dental plaque, which begins to develop within a few hours after a meal [1]. This process involves interactions between anaerobic bacteria, calcium and phosphate salts from saliva [2, 3]. Plaque starts to harden within 24 hours, eventually transforming into calcified deposits on tooth surfaces, resulting in the formation of calculus. In case of the presence of pathogenic bacteria within the biofilm, particularly in the subgingival DC, it may further progress into periodontal disease [2,3,4,5]. Periodontal disease is characterized by inflammation and damage to the gums, teeth, and other oral structures in the mouth [3]. Periodontal disease in dogs may be accompanied by pain, halitosis, and edentulism [3]. In some cases, supragingival DC can remain sterile and non-pathogenic, acting as a stable deposit that does not progress to periodontal disease [6]. Likewise, acute PD can occasionally present in the absence of DC [7]. However, DC and periodontal disease are most seen concurrently in dogs. Recently, DC has been included in the explanatory texts accompanying images of grades II-IV or III-IV, in two visual periodontal disease assessment tools using 0-IV scales [8, 9]. Therefore, DC constitutes a major challenge to oral health in both canine and human populations [3, 11, 128], and it can also contribute to other major health issues and systemic diseases such as cardiovascular-related diseases, if not adequately managed [12,13,14,15,16]. An epidemiological study conducted in the Czech Republic estimated that over 60% of dogs exhibit DC [17], while a study from Belgium found that 31% of dogs had DC [18]. Similarly, a Swedish study reported a notably higher prevalence of 83.3% [19]. Collectively, the previous research underscores the widespread and pervasive nature of the disease within dog populations [8, 128].
The aetiology of DC is multifactorial and includes both non-modifiable and modifiable predisposing factors. Increased age has long been known as a risk factor [19, 20]. Small size dogs were found to be highly predisposed to the disease compared to large or medium sized dogs [6, 21]. Furthermore, different types of diets [1, 22,23,24,25,26,27,28,29], microbiological influences [30,31,32,33], and other environmental factors such as stress, oral care and chewing habits [34, 35], are implicated [21, 36]. Notably, dental plaque can be removed through regular oral hygiene such as brushing the dog´s teeth or by mechanical abrasion provided by an appropriate diet [23]. However, a recent study showed that less than 4% of dog owners brush their dog’s teeth on a daily basis, and 46% have never brushed their dog’s teeth [37]. As dental care practices are overlooked by many owners, it remains crucial to identify and address possible modifiable DC risk and protective factors.
Nutrition plays a significant role in promoting overall health, especially oral health, for both humans and dogs throughout all stages of life [23, 38, 39]. Despite the extensive literature available on the impact of diet and other factors on periodontal health in both canine and human subjects [25, 39,40,41,42,43,44], there have been no studies examining the role of early life exposures, such as dietary and environmental factors, on DC development in dogs later in life. In the current study we hypothesize that early life exposures, including both modifiable (dietary and environmental factors) and non-modifiable (including age, breed, size of dogs, maternal history of the disease) factors, are associated with the risk of developing dental calculus in dogs later in life. Therefore, in order to test our hypothesis and address this gap in knowledge, a retrospective cross-sectional study was conducted to identify the early life risk factors associated with the development of owner-reported DC in dogs later in life, with a focus on modifiable risk factors. If these could be identified, they could potentially be addressed as a preventive strategy in veterinary oral health practices and by pet owners. Additionally, the study aimed to compare between the energy values and macronutrient composition of non-processed meat-based diets and ultra-processed carbohydrate-based diets available on the Finnish market.
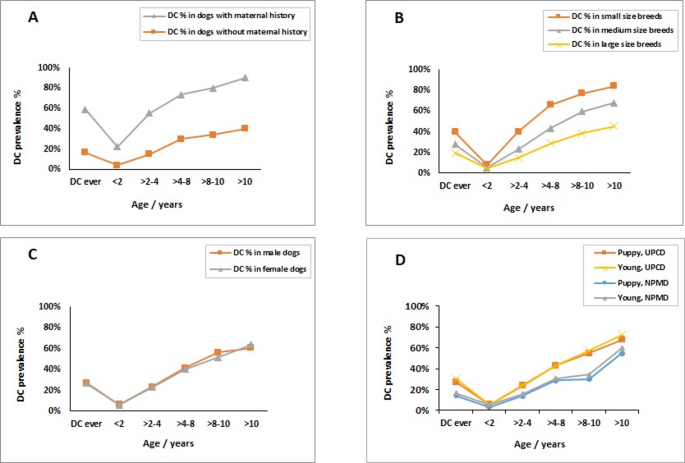
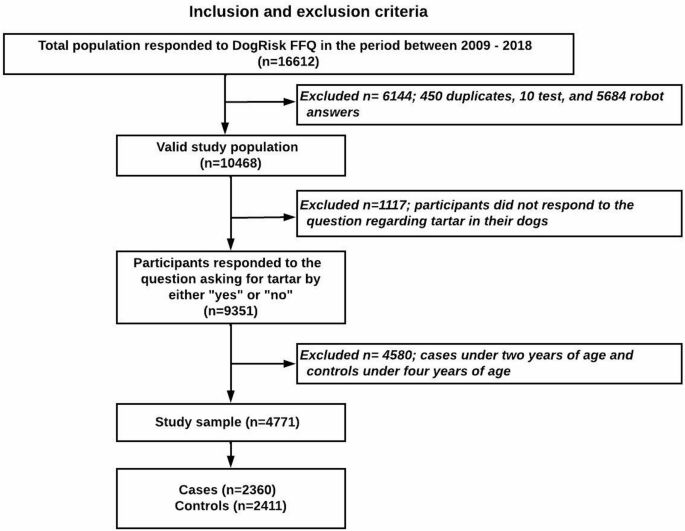
The study examined a total of 4771 dogs, of which 2360 dogs were owner-reported to have DC and 2411 dogs were reported to be without DC. The mean age ± SD of all the dogs that participated in the study, as well as the age of the dogs with DC and the control group, was measured to be 5.1 ± 3.13, 6.37 ± 3.41, and 4.42 ± 2.75 years old, respectively. Moreover, the mean age of DC onset ± SD was measured for the population with DC data (n = 9351) to be 3.45 ± 2.54. The prevalence of owner-reported DC in Finnish dogs, as estimated from the DogRisk FFQ answered by the overall population (n = 10468) between 2009 and 2018, was found to be 24%. In addition, the prevalence of DC in different age groups after stratification of the study population according to maternal history of DC, the size of the dog, gender, and puppyhood and adolescence diets was calculated, as shown in Fig. 1. Table 1 displays the descriptive statistics of the study population and covariates, as well as the distribution of the categorical variables within the study cases and controls.
Prevalence of dental calculus within different age groups in the study population (n = 10468), stratified by maternal history of DC (A), the size of the dog’s breed (B), gender (C), and diet during puppyhood and adolescence (D)
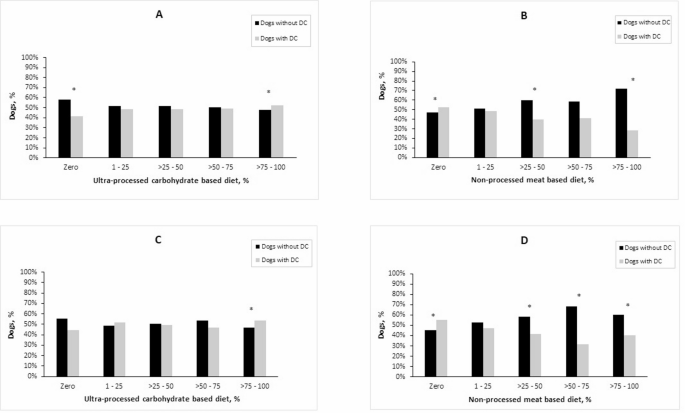
Furthermore, the study analysed the differences between dogs with DC and dogs without DC, after dividing the study sample into five groups, according to their proportional consumption of UPCD and NPMD during puppyhood and adolescence, respectively (Fig. 2). During the puppyhood period, dogs that consumed no UPCD, and/or consumed 25–50% or 75–100% of their diet as NPMD, showed a significant reduction in DC prevalence. On the contrary, the consumption of a diet composed of more than 75% UPCD, and/or a total absence of NPMD, was significantly associated with a higher ratio of dogs with DC, compared to dogs without DC (Fig. 2A and B).
Ratios of dogs with and without dental calculus across five different groups divided according to their consumption of UPCD (A) and NPMD (B) during puppyhood, and according to their consumption of UPCD (C) and NPMD (D) during adolescence (n = 4771)
Similar results were observed for dietary intake during adolescence: groups that consumed varying proportions between 25 and 100% of their diets as NPMD showed a significantly higher proportion of dogs without DC compared to dogs with DC. Conversely, dogs whose diets were not including NPMD and/or consisted of 75–100% UPCD were significantly presented with higher DC (Fig. 2C and D).
The results of the chi square tests (X2) revealed significant associations (p < 0.05) between 16 out of the 29 exposure variables and the occurrence of owner-reported DC in dogs later in life. However, 20 independent variables in total showed associations with a p-value of < 0.2, allowing for further regression analysis. Results of the X2 tests are shown in Table 1.
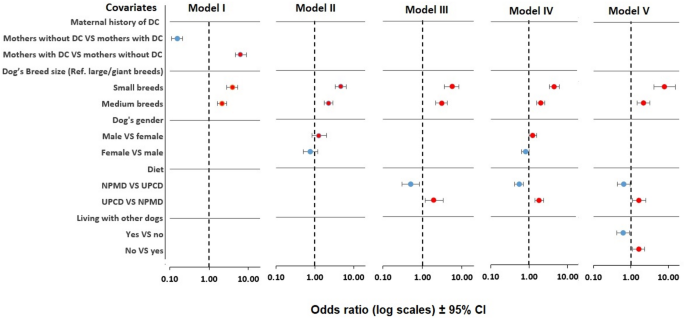
Figure 3 presents odds ratios (OR) and p-values from five multivariate regression models analysing associations between owner-reported DC in adult dogs ≥ 2 years and various factors, with adjustments for the size of the dog and gender in all models. The analyses showed seven variables that remained significantly associated with DC (p < 0.05). The significant variables associated with an increased risk of developing DC in adulthood included maternal history of DC, smaller dog size, male gender, consumption of an UPCD diet during weaning period, puppyhood, and adolescence, and being the only dog in the household. Significant variables associated with a decreased risk of developing DC included absence of maternal history of DC, larger dog size, female gender, consumption of a NPMD diet during weaning period, puppyhood, and adolescence, and living with other dogs in the same household. In model II, examining the modifiable pre- and postnatal maternal and puppy exposures, no associations remained significant, as shown in Fig. 3 and supplementary Table 1 (S1 Table).
Forest plot of early life exposures and their association with dental calculus risk in 4771 adult dogs. Model I – V were adjusted for the size of the dog and gender. Model I, non-modifiable genetic and demographic variables; Model II, modifiable perinatal maternal and puppy exposures (from pregnancy to the first four weeks of the puppy’s life); Model III, puppy’s early postnatal exposures (from one to two months old); Model IV, puppy’s late postnatal exposures (from two to six months old); and Model V, adolescent exposures (from six to eighteen months old). OR values over 1 mean that the variable was a potential risk (= positive correlation), whereas OR values under 1 may be potentially protective (= negative correlation). DC: dental calculus, CI: confidence interval, NPMD: non-processed meat-based diet, UPCD: ultra-processed carbohydrate-based diet, VS: versus
In a supplementary sub-study, we calculated the macronutrient composition of 54 commercially available dog foods on the Finnish pet food market at the time of the questionnaire. The foods were randomly selected and included 13 NPMD products and 41 UPCD products. The macronutrient composition was calculated per 1000 kcal metabolizable energy (g/1000 kcal, ME). The results were presented as mean ± standard deviation (SD), as well as the relative energy contribution of protein, fat, and carbohydrate. The protein/fat/carbohydrate (P/F/C) energy ratios were calculated per 100 g of diet on a fresh matter basis as presented in Table 2.
We examined the relationship between a range of early life exposures, from prenatal to adolescent life, and the occurrence of DC in dogs aged two years or older. This retrospective cross-sectional study provides new insights into the influence of modifiable and non-modifiable early life factors on the development of DC in adult dogs. The current study reports significant associations between weaning, puppyhood, and adolescence diets and the incidence of DC in dogs later in life (Figs. 3 and S1 Table). Moreover, among the non-modifiable factors, we found that the size of the dog, maternal history of DC, and gender were significantly associated with the incidence of DC in adult dogs (Figs. 1 and 3). The consistent results of a significantly decreased risk of adult DC with NPMD consumption across three postnatal phases, along with the narrow 95% confidence intervals (CI) of the odds ratios (ORs), indicate that the results are robust (Fig. 3 and S1 Table).
A broad range of literature has previously verified a diet and nutrition - oral health relationship in humans and dogs [22, 25, 40,41,42,43,44,45,46]. However, to the best of our knowledge, this is the first study to analyse early life, perinatal, modifiable risk factors and their potential association with the development of DC in dogs later in life.
In this sub-study, the average protein: fat: carbohydrate (PFC) energy ratio of the calculated NPMDs were 30%:69%:2%, while the average PFC energy ratio of UPCDs was 26%:33%:41%, respectively. These results indicate that UPCDs contain a substantial amount of carbohydrates, whereas NPMDs contain a significant amount of animal derived fats (Table 2). Previous studies have shown that animals tend to select foods that meet their specific macro- or micronutrient requirements [47, 48]. A study reported that dogs self-select diets with a PFC energy ratio between 38%:59%:3% and 34%:62%:4% [47], whereas wolves, prefer diets with PFC energy ratios ranging from 54%:45%:1% [49] to 30%:63%:7% [48]. These preferred macronutrient distributions are broadly consistent with the ratios identified in the NPMD in the current study. Given that dogs have no established minimum dietary requirements for soluble carbohydrates or starches [50,51,52], this supports the fact that dietary preferences are likely driven by physiological needs. In addition to macronutrient composition, the preference of the NPMDs by the canines may also be influenced by other factors, such as diet palatability, aroma, and the psychological satisfaction compared to UPCDs [53, 54].
In children it has been reported that postnatal nutrition, during the pre- and post-eruptive stages of deciduous and permanent teeth, can have a significant impact on the evolution and integrity of the oral tissues which can influence the likelihood of oral diseases later in life [39]. The diets examined in the current study, comprising the weaning diet, the puppy diet, and the adolescent diet, are typically provided to puppies beginning at the age of three weeks, when their deciduous teeth emerge, and continuing until they reach the age of 1-1.5 years, when all of their permanent teeth have already fully erupted, which happens around the age of 7 months [55,56,57,58]. A nutritionally balanced diet is essential for puppies to maintain normal development and formation of the teeth, tooth mineralization, and strength of the enamel, resulting in a long-lasting effect on oral health [40]. The nutritional intake, particularly during puppyhood and adolescence, prior to or following the eruption of permanent teeth, has a significant long-term impact on the teeth, maxillary bones, the integrity of the oral cavity mucosa, the infection resistance, and the longevity of the tooth [40].
The oral microbiome is another major determinant of oral health [59,60,61]; its dysbiosis predisposes both humans [62, 63] and dogs [64,65,66] to inflammation and disease. In humans’ early exposures, particularly diet, have long-lasting effects on oral and systemic health [67, 68,69,70], where the early-life diet has been shown to be the most influential factor in microbiome development [71,72,73,74]. Diet provides essential nutrients for oral microbes [75], while high-glycaemic diets promote pathogenic bacteria and suppress beneficial ones [76]. Moreover, diet composition and consistency influence the oral environment by altering plaque bacteria, stimulating salivary flow, and increasing food contact with oral surfaces [40]. This is consistent with our findings of the dog’s diet, reflecting the differential impacts of the diet composition on the oral microbiome and its crucial role in modulating the canine oral health.
The influence of diet composition and consistency on DC has been previously reported [40]. Notably, at least one study has reported that dogs consuming raw diets exhibit reduced level of DC and improved breath quality compared to those fed commercial diets [77]. Furthermore, anecdotal evidences suggest that DC in dogs could be reversed or controlled by diet [31, 78]. The effect of high carbohydrate diets has often been identified as a prominent factor in plaque accumulation in humans [79, 80]. It is relevant to note that not only the dogs’ oral diseases resemble those of humans, also the dietary patterns of dog feeding resemble those of human nutrition [81, 82]. The human western diet, and the so called Standard American Diet, resembles the UPCDs dry dog feeds, typically fed to dogs [82,83,84]. According to research on humans, excessive carbohydrate intake was found to contribute to gut and oral microbial dysbiosis which associated with chronic inflammation [85], and formation of large amounts of dental plaque [79, 80]. This is in tune with our finding of the UPCDs, which typically are rich in soluble carbohydrates and starch [40] (see Table 2), associating with increased risk of DC development (Fig. 3). Oba et al. [86] analysed the oral microbiota associated with detrimental effects of the oral tissue after eating dry dog food for few months and found that the following bacterial phyla predominated with development of dental plaque of the teeth surfaces: Actinomyces, Corynebacterium, Capnocytophaga, Leptotrichia, and Neisseria. Several studies on dogs have shown that consuming raw meat-based diets isassociated with greater diversity in gut microbiota compared to consuming dry dog foods [87,88,89]. A human study demonstrated that a low carbohydrate to fibre ratio was associated with a reduced risk of periodontitis [90]. Another human study found that the consumption of a low-carbohydrate high-fat/ketogenic style diet induced changes in the oral microbiome, where the relative abundances of Haemophilus, Neisseria, and Prevotella spp. were reduced, and the relative abundance of Streptococcus spp. was increased [91]. Interestingly, the Haemophilus, Neisseria, and Prevotella spp were found to be prevalent in dental plaque of both canines and healthy adults [92,93,94]. Therefore, we propose that the consumption of NPMD by dogs, resembling a low-carbohydrate high- fat/ketogenic diet mentioned in the previous research, would be associated with a reduction in the dental plaque prevalent bacteria mentioned above, and subsequently a reduction in the DC development in dogs. Although, direct studies on the oral microbiome of dogs fed raw diets are limited, several studies have investigated the effects of raw feeding on the gut microbiome, which may offer insights into potential impacts on dog’s oral health [95]. However, other conflicting studies has reported that pathogens are commonly found in both NPMD [96,97,98] and UPCD [97,98,99,100], although clinical illness in dogs eating NPMD and disease transmission to humans are rare [101].
Domestic dogs on species-appropriate diets (NPMD) typically consume low-carbohydrate, high-protein, and fiber-rich foods such as cartilage, bones, raw vegetables, and fruits [102]. The NPMD usually includes whole animal parts, like chicken wings or drumsticks, large bones with the cartilage end-pieces, whole animal legs, or marrow-bones. These components require shredding and tearing of flesh and attached connective tissue which is accompanied with prolonged mastication. This activity takes more time, and the dietary dental flossing cleans the teeth naturally by removing the bacterial biofilm from the tooth surfaces, while dry kibble is usually consumed quickly with minimal chewing, allowing for plaque accumulation [53]. Moreover, these components act as “nature’s toothbrushes” and help to clean the teeth [40] and reduce dental plaque and calculus formation [22 30]. This can also be seen in wild canines and domestic dogs on NPMD, both typically consume low-carbohydrate, high-protein, and often animal-fiber-rich foods including cartilage, tendons, sinews, raw vegetables, and fruits, as well as hard foods, like bones [30]. This is in accordance with a study on captive cheetahs compared a natural diet including raw meaty bones to a commercial dry kibble diet and reported a significantly reduced DC score in the group eating meaty bones [53]. Likewise, an Australian study comparing scores of dental calculi between domestic cats eating canned moist food and feral cats eating wild carcasses, reported significantly higher DC scores in the domestic cats, compared to the feral cats [46].
Moreover, three studies conducted on beagle dogs have found that the consumption of raw bones significantly reduced the DC accumulation on the tooth surfaces by 81–90% within a 2–3 week period [30,31,32]. Two of these studies reported no adverse health outcomes such as tooth fractures, gastrointestinal obstructions, or oral lesions [30, 32]. However, Pinto et al. (2021) observed that some gingivitis had transitioned into minor gingival trauma [31]. Additionally, two dogs in the same study were found to have small bone fragments lodged between their teeth [31]. Similarly, an analysis of 29 African wild dog skulls from the early 19th century, that most likely had been consuming a natural diet primarily consisting of wild antelope, found evidence of tooth wear in 83% of the individual skulls, and tooth fractures in 48%, and 7% reported DC [103].
In agreement with our findings, a broad range of literature has reported that dry regular dog food is involved in the development of DC in dogs and does not help in reducing the risk of periodontitis [24, 27, 86, 104, 105]. Similarly, Oba et al. (2021) found that the oral health score for dental plaque and DC ranged from severe to moderate (9/12 score of plaque and 6/12 score for calculus) in healthy dogs fed dry kibble [105]. In another study, the same authors compared oral health scores between two groups of healthy dogs eating dry dog food and wet dog food, both ultra-processed and carbohydrate-dense, and they found that both diets were associated with the development of plaque, calculus, and gingivitis with no significant difference between the two groups, however plaque accumulation tended to be less in the dogs consuming the dry food [86]. Based on our findings, NPMD appears to exert a protective effect. This effect might be attributable to many diet-related factors including the physical composition, anti-inflammatory properties, enhanced immune function and/or reduced oxidative stress [101, 106]. Further research on the topic is required to elucidate the relationship between diet and DC accumulation in dogs.
In the current study we found that male dogs were significantly associated with increased risk of DC development, while female dogs were associated with a lower risk. This has been seen in previous research [8, 107, 108]. However, another study reported that DC was prevalent in 50% of female dogs and 44.3% of male dogs [109]. It is unclear whether there could be a behavioral, hormonal or other related reason behind this and more research is needed.
Furthermore, we found that living with other dogs, significantly decreased the risk of adult DC (OR = 0.6) compared to subjects reared alone, while being reared without other dogs in the same household significantly increased the risk (OR = 1.6) (Fig. 3). This result agrees with our previous reports showing reduced risks of canine atopic dermatitis and otitis in dogs raised with their dams in the same household [110, 111]. Studies have shown that regular interactions within a household environment, such as dogs interacting with each other and with their owners through oral and skin contact, influence the adaptation of the immune system, which occurs via sharing the same microbiome and exposure to similar environmental stimuli [112,113,114,115]. Moreover, dogs tend to derive greater enjoyment from engaging in play-biting and chewing each other’s toys and bones as opposed to doing so independently, which may result in an increase in gnawing behaviour. Further research on this topic is needed.
Based on the multivariate logistic regression analysis of the non-modifiable risk factors, maternal history of DC was strongly associated with a significantly increased risk of DC development in their offspring later in life, with an odds ratio of 7.6 compared to dogs without maternal history of DC (Fig. 3). This supports previous findings showing that maternal history of diseases like atopic dermatitis, inflammatory bowel disease, and otitis in dogs strongly impacted the offspring’s likelihood of developing the same diseases [110, 111, 116]. Similarly, a human study indicated that there was an association between maternal periodontitis and the risk for childhood and adolescent periodontitis in their children [117]. Although there are no previous studies stating that a maternal history of DC is a contributing factor in the disease development in the following generation of dogs, nor addressed the heritability of DC, a genetic component of periodontal disease has been recognized [118]. Researchers have reported a potential association between the TLR9 gene variant, as well as the IL1A and IL1B gene variants, and the predisposition to develop periodontal disease in dogs [118,119,120]. Michalowicz et al. (2000) estimated that chronic periodontal disease has a 50% heritability in humans [121]. Even so, it is worth noting that genetic variability might be one of several factors that adjusts an individual’s susceptibility to a disease. Several studies on humans, dogs, and murine models reported that there was a correlation between the maternal oral microbiome and the neonatal oral microbiome through an intrauterine blood-borne dissemination route [122, 123, 124, 125, 126, 127]. This suggests that the early colonization of the neonatal oral microflora starts even before birth, which is crucial for the normal development of oral and overall health later in life [122]. Furthermore, neonates and young puppies can also acquire additional oral microbiota from their surroundings even after birth. According to Pérez-Serrano et al. [128] dental plaque bacteria in dogs can be transmitted among co-inhabitants, such as owners and other dogs, within the same household. We assume that maternal oral bacteria from dams with DC are transferred to their offspring after birth, through interactions between mother and puppies within a shared environment [129]. Therefore, it is crucial to implement strategies that improve maternal oral health before and during pregnancy. This proactive approach is essential for reducing the likelihood of dental diseases manifesting in the offspring during adulthood. Moreover, it is important to note that the presence of the disease in the dogs’ mothers does not necessarily indicate that it is genetically inherited. It is possible that the disease was acquired directly from the mother through the oral microbiota or from a shared environment, such as a common diet, as discussed earlier.
From the same non-modifiable model, we observed that the dog breed, or purely the dog size, is a significant predisposing factor for the development of DC in dogs. Our findings indicate that dogs belonging to small and medium-sized breeds have a significantly higher likelihood of developing DC, with an odds ratio of 3.7 and 2.14, respectively, compared to dogs belonging to large or giant breeds (Fig. 3). These findings align with the literature, which posits that small-sized dogs are at a greater risk of developing DC later in life [6, 14, 21, 130, 131]. Smaller dog breeds are more prone to periodontal disease than larger breeds for several key reasons. Compared to large dogs, small dogs often have relatively large teeth that are proportionally too large for their jaws [132], which leads to overcrowded teeth promoting plaque buildup. Furthermore, the larger contact area between the teeth and gums in small dogs can result in a more significant inflammatory response. Additionally, small dogs possess less alveolar bone relative to their large teeth, making the pathological effects of periodontal disease considerably more severe than in larger breeds [133].
The major strength of the current study is the use of a validated questionnaire data [134] with a high response rate, allowing for standardized and consistent data collection. Moreover, the questionnaire was distributed widely across Finland, thus enabling us to reach diverse populations and collect data from different demographic groups for a population level-analysis. The exploited data in the current study features a retrospective cross-sectional design, specifically diet related questions, environmental exposures, and other covariates were asked over five perinatal life periods, including the pre-natal, neo-natal, weaning, puppyhood, and adolescence stages. The questionnaire setup allows for tracking the changes in the exposome and the evaluation of its impact on the studied outcome over time, retrospectively. Furthermore, the DogRisk FFQ is tailored to capture a comprehensive array of confounding variables, including demographic characteristics, lifestyle factors, environmental factors, maternal medical history, and other relevant covariates, which enhance the validity and reliability of the study findings. Another important strength in the current study is that we considered the possibility of reverse causality and its potentially biased results. To avoid that, we restricted our analysis to dogs over the age of two in the case group and dogs over the age of four in the control group. These age cut-offs were based on the average age of DC development in dogs as reported in our study to be 3.45 years, thereby we can suggest that the exposures during the first two years of life can cause the outcome, but not the opposite.
It is also essential to acknowledge the potential limitations of the current study, including the reliance on owner-reported data and the possibility of recall bias. However, the ability of owners to assess DC in their dogs was verified in previous research [37]. Moreover, owners’ responses in the current study were validated by asking the owners to answer a set of extra questions about DC. In addition to the main question asking whether the dog has or have had DC, we asked extra questions such as the age at which DC started to develop for the first time and whether it had been recurrent, etc. These correlated questions were used to validate the owners’ responses regarding the DC main question. We also acknowledge that the mean age in the control group was lower than the mean age in the cases, even though we set the age limits to be more than 4 years for the controls and more than 2 years for the cases based on the mean age of DC onset from the current study and literature. Furthermore, the retrospective cross-sectional study design limits our ability to establish causation, therefore, further diet intervention studies are imperatively required to establish the causation.
In summary, the present study underscores the significance of early life exposures in modulating the risk of DC development in dogs later in life. We found that dogs fed a NPMD diet during weaning, late puppyhood and adolescence showed a significantly lower risk of developing DC later in life, whereas those consuming UPCD had a significantly higher risk. Moreover, living with other dogs was also significantly associated with a lower risk of DC development later in life. Additionally, the maternal history of DC was strongly associated with a high risk of DC development in offspring during adulthood, compared to dogs without a maternal history of DC, however it remains unclear whether shared genetic or environmental factors are primarily responsible. Furthermore, dogs belonging to small breeds and older dogs were significantly more susceptible to develop DC later in life, than dogs belonging to medium or large breeds and younger dogs. Although the current study suggests an association between early life exposures and later onset of DC in dogs, further longitudinal dietary interventions are required to verify our observations.
This retrospective, cross-sectional epidemiological study aimed to analyse the potential early life risk factors associated with the subsequent development of owner-reported DC in dogs. The study utilized the validated [134] Helsinki University DogRisk food frequency questionnaire (FFQ) for owner-reported data collection with a retrospective cross-sectional design (a copy is available in Finnish at: http://bit.ly/427aGBa). The DogRisk FFQ was available to dog owners between 2009 and 2021. The FFQ was available only in Finnish and aimed to capture information on the different types of diets regularly consumed by Finnish dogs, throughout their lives, and their dams’ dietary patterns during pregnancy and lactation. The FFQ also included sections on demographic details, dog characteristics, background history, environmental exposures, and the presence or absence of different diseases in the dogs and their dams. Additional information about the DogRisk FFQ has been published in prior research [110, 111, 116]. The DogRisk FFQ was announced on popular social media platforms, at dog fairs, dog clubs, dog magazines, pet food stores, veterinary clinic websites and in veterinary clinic waiting rooms. The questionnaire was directed to dog-owners, but no human data was collected. Therefore, a waiver for informed consent was obtained from the Research Ethics Committee on Animal Research at the University of Helsinki, Finland. However, the FFQ has an ethical approval (29.4.2016) from the University of Helsinki Viikki Campus Ethical Board for the purpose of publication.
The study included dogs of various breeds, both genders and of both castrated and intact status. At the time of data retrieval, a total of 10,468 participants had responded to the DogRisk FFQ. Of these, 9,351 had answered the study’s main outcome (dependent) variable: “Has your dog suffered from tartar/dental calculus?” with one of two available answers: “Yes” or “No”. According to the literature, DC often manifests before the age of three years [20, 37], while the mean age of DC onset ± SD in the current study was found to be 3.45 years ± 2.54. Therefore, the control dogs had to be more than four years old, whereas the cases were chosen from dogs that were over two years of age, in order to avoid reverse causality. Accordingly, 4,580 subjects were excluded from the analysis, as shown in the study flow chart (Fig. 4).
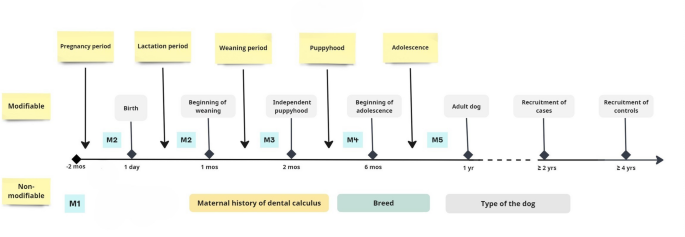
The study examined one dependent variable for its possible association with 29 explanatory discrete variables (Table 1). All participated dogs (including mixed breed dogs) were categorized into three groups according to the size of the dog; small size breeds < 10 kg, medium size breeds from 10 to < 25 kg, and large size breeds ≥ 25 kg. The study covariates were tested and assorted in five models depending on whether the exposure was modifiable or non-modifiable, and on the timing of the exposure, as outlined in the study timeline in Fig. 5 and shown in Table 1.
Therefore, the multivariate regression models were labelled accordingly, Model I, which pertained to non-modifiable genetic and demographic variables; Model II, which referred to modifiable perinatal maternal and puppy exposures during pregnancy through the first four weeks of the puppy’s life; Model III, which focused on the puppy’s early postnatal exposures during the age from one to two months old; Model IV, which addressed the puppy’s late postnatal exposures extending from two to six months old; and Model V, which examined adolescent exposures during the period from six to eighteen months old. The study exposure period extended from conception till the age of adolescence, where transition from one stage to another varied according to the size of the dog. The questionnaire provided putative age ranges to define adolescent dogs. Based on our dataset, we had suggested that adolescence typically occurs between 6 and 12 months in small breeds, and between 8 and 18 months in large breeds. For medium sized dogs, owners were encouraged to estimate the adolescent period based on their dog’s breed and size, which likely falls between these two ages. The tested early life variables included type of diet consumed during each life period, environmental exposures, lifestyle factors, domestic-related factors, as well as demographic and background related factors.
The five dichotomous dietary variables tested in the current study (Table 1) included two categories: non-processed meat-based diet (NPMD) and ultra-processed carbohydrate-based diet (UPCD). The NPMD can include raw meat (muscle meat from various animals), raw meaty bones (like chicken necks or wings), organ meats (liver, kidney, heart, tripe, etc.), vegetables, fruits (like berries, etc.), and supplements (depending on the dog’s needs). The UPCD refers to regular dry dog food (kibble). Diet-related variables were curated from the questions asked to dogs’ owners in the DogRisk FFQ [111]. Three open-ended questions (questions 26, 27, and 32) were used to gather information about the maternal diet during both pregnancy and lactation, as well as the puppies’ first solid diet. Based on the responses, pregnant dams, lactating dams, and their 0–2 months old puppies were categorized into two dietary groups: those consuming UPCD and those consuming NPMD, represented by three distinct dichotomous variables respectively. Dogs fed other alternative diets were excluded from the analysis. For puppies aged 2 to 6 months and young dogs aged 6 to 12 or 18 months, dietary information was obtained through owners’ responses to questions 38 and 40. in which owners reported the percentage of the puppy’s and youngster’s intake from four diet types: UPCD, NPMD, processed wet food, and home-made diets. This study focused only on two extreme groups: puppies and youngsters whose diets consisted of over 80% UPCD or more than 20% NPMD. These thresholds were selected in alignment with prior studies [110, 111, 116].
In order to highlight the major differences between the macronutrient composition and their energy contribution of the two diets tested in the current study, a sub-study analysis was carried out. A total of 54 different commercial dog foods were randomly selected form the Finnish dog food market; 13 NPMDs and 44 UPCDs, to calculate and compare their energy and macronutrient content. The NPMDs included in the analysis were either frozen or freeze-dried; with one including fermented vegetables, however, all of the diets were not heat treated. The UPCDs used in the sub-study were all extruded commercial dry foods. The included diets (54 foods) were complete and balanced according to AAFCO or FEDIAF guidelines [87, 88].
In addition, two ratio scales were employed to assess the occurrence of DC in dogs in relation to different proportions of NPMD and UPCD in their diets as follow: 0%, 1–25%, > 25–50%, > 50–75%, and > 75–100%.
It is worth noting that because the NPMDs were not heat-treated, they lacked microbial killing steps, and therefore likely contain more microbes, both beneficial and potentially pathogenic, although microbial content was not analysed in this study.
Statistical analysis was done using the IBM SPSS statistical package v. 29. Descriptive statistics, including frequencies, were used to characterize the study sample. The sample size was not pre-determined through a formal sample size calculation due to the exploratory nature of the study. The study aimed to include as many participants as possible within the data collection period to enhance statistical power. The presence or absence of a relationship between each of the tested covariates, as determined by the DogRisk FFQ, and the dependent variable were estimated using Pearson’s Chi-square test. Variables that showed a liberal association with the main outcome variable (p < 0.2) in the Pearson’s Chi-square test were included in the multivariate logistic regression models. Bonferroni correction was applied for each individual test to adjust p-values and reduce false positives. Binary logistic regression analysis was employed to assess the association between potential risk or protecting factors and the presence or absence of owner-reported DC in dogs. The five regression models were constructed in a stepwise backward elimination manner, allowing for the assessment of the independent effect of each variable while adjusting for potential confounders, considering variables with a significance level of p < 0.05 for inclusion. The entry and removal probability were set at (0.1) and (0.4), correspondingly. The adjusted odds ratios and 95% confidence intervals were calculated both for risk and protection for the dichotomous covariates using each category as a reference to quantify the strength and direction of the associations. The models were adjusted for size of the dog and gender at each of the five models. Missing values were treated by listwise deletion rather than imputation. Model fitness was weighed using the Omnibus test (p-value < 0.05), Hosmer and Lemeshow test (p-value > 0.05), and Nagelkerke’s R (for the largest value) to ensure their adequacy. The results were reported as odds ratios (OR) with a p < 0.05 and an OR below one indicating a significant negative association (potentially protective against disease) and an OR above one indicating a significant positive association (potentially risk of disease).
The data sets used in the current study available from Dr. Anna Hielm-Björkman on a reasonable request.
- DC:
-
dental calculus
- NPMD:
-
non-processed meat-based diet
- UPCD:
-
ultra processed carbohydrate-based diet
- FFQ:
-
food frequency questionnaire
- PFC:
-
protein/fat/carbohydrate ratio by energy
- DM:
-
dry matter
We thank the dog owners who participated in the study by answering the DogRisk food frequency questionnaire (DogRisk FFQ: http://bit.ly/427aGBa). We also would like to thank senior researcher Shea Beasley for letting us use our common data (the DogRisk data bank ownership is divided between AH-B (60%) and PhD Shea Beasley (40%)).
Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). Open Access funding provided by University of Helsinki (including Helsinki University Central Hospital). Instinct Pet Food (www.instinctpetfood.com), Vetcare Oy Ltd. (www.vetcare.fi), the Swedish Cultural Foundation (www.kulturfonden.fi/in-english; Grant number 13/3307 − 1304), Victoriastiftelsen grant, MUSH Ltd. (www.mushbarf.com), Moomin characters Ltd. (www.moomin.com/en/), and Muurla Ltd have all funded the data collection and analysis for this study. All authors are on university salary or student grants. There has been no additional external funding received for this study. The funders had no role in study design, data collection, data analysis, reporting the results, decision to publish, or preparation of the manuscript.
The questionnaire was directed to dog-owners, but no human data was collected. Therefore, a waiver for informed consent was obtained from the Research Ethics Committee on Animal Research at the University of Helsinki, Finland. However, it has an ethical approval (29.4.2016) from the University of Helsinki Viikki Campus Ethical Board for the purpose of publication.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Hemida, M.B.M., Holm, S., Eklundh, M. et al. Modifiable early life risk factors for dental calculus in dogs: a retrospective cross-sectional study in Finland. BMC Vet Res 21, 430 (2025). https://doi.org/10.1186/s12917-025-04885-8









:max_bytes(150000):strip_icc()/sean-diddy-combs-Pre-GRAMMY-Gala-2020-50-cent-atlanta-may-2024-070225-b887b8e9d2c14ee0b7878443ba8453f3.jpg)


