Nutrition Journal volume 24, Article number: 101 (2025) Cite this article
Potassium plays an important role in glucose metabolism and blood vessel. However, there is a lack of systematic research on the intake of potassium and diabetic microvascular complications. The aim of this study was to explore whether inadequate potassium intake increases the risk of developing diabetic microvascular complications, diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy using the UK Biobank database.
This study included 22,395 subjects with type 2 diabetes mellitus at baseline. Urinary potassium and creatinine were measured by potentiometry and photometric assay respectively. Dietary potassium intake was measured using the 24-hour dietary recall method. The occurrence of microvascular complications was determined using ICD-10 codes from cumulative hospitalization records and death records in the national death registry. Cox proportional hazards models were used to explore the relationship between urinary potassium-to-creatinine ratio, dietary potassium, and overall and individual microvascular complications, generating hazard ratios (HRs) and 95% confidence intervals (CIs).
Compared with the minimum potassium-to-creatinine ratio group, the highest potassium-to-creatinine ratio group had a significantly lower risk of diabetic microvascular complications (HR, 0.70 [95% CI 0.63–0.78]; P for trend < 0.001) and diabetic nephropathy (HR, 0.54 [95% CI 0.47–0.61]; P for trend < 0.001). The group with the highest dietary potassium had a significantly lower risk of diabetic nephropathy (HR, 0.48 [95% CI 0.29–0.80], P for trend = 0.005) than the minimum dietary potassium group. The restricted cubic spline results showed a non-linear relationship between urinary potassium-to-creatinine ratio and overall microvascular complications and diabetic nephropathy, with nonlinear P values of 0.009 and < 0.001, respectively, and a generally declining trend.
The urinary potassium-to-creatinine ratio was significantly negatively associated with overall diabetic microvascular complications and diabetic nephropathy.
Diabetes mellitus is a global health problem: the 10th edition of The International Diabetes Federation Diabetes Atlas indicates that there were 536.6 million individuals affected by diabetes globally in 2021 [1]. Long-term hyperglycemia can induce microvascular damage. Diabetic microvascular complications are a group of chronic diseases including diabetic nephropathy, retinopathy, and neuropathy. Epidemiological data show that among people with diabetes, the prevalence of diabetic nephropathy, retinopathy, and neuropathy is 10.8–30% [2,3,4], 27.9% [5], and 50.8% [6], respectively. By affecting kidney function, vision, and limb function, these complications significantly diminish patients’ quality of life and place a heavy strain on both families and society [7].
Potassium (K) is a vital macronutrient for human health, predominantly located within the intracellular fluid, with only approximately 2% present in the extracellular fluid [8]. According to the United States Department of Agriculture’s (USDA) food composition data, potassium is abundantly found in legumes, vegetables, fruits, potatoes, and beef. This nutrient is crucial for maintaining body homeostasis and is involved in several physiological processes, including the regulation of electrolytes, acid-base balance, and neuromuscular conduction [9]. The World Health Organization (WHO) recommends that adults consume a minimum daily potassium intake of 3510 mg to reduce the risk of hypertension and cardiovascular morbidity [10]. The Institute of Medicine (IOM) suggests an Adequate Intake (AI) of 4700 mg per day for adults [11]. In contrast, the 2023 edition of the Chinese Dietary Guidelines recommends a daily potassium intake of 2000 mg for adults [12]. The recommended intake cut-point method is utilized to estimate the prevalence of inadequate potassium intake. Potassium deficiency refers to a chronic condition characterized by consistently insufficient daily dietary potassium intake. In contrast, potassium deficiency denotes a chronic condition wherein an individual’s daily dietary potassium intake persistently remains below the minimum levels recommended by authoritative health organizations, thereby failing to satisfy the body’s essential physiological needs. Inadequate potassium intake remains a global public health concern, contributing to increased cardiovascular disease burden [13, 14]. Potassium deficiency is more prevalent in individuals with diabetes mellitus, primarily due to osmotic diuresis, medication-induced potassium loss, and suboptimal dietary management [15, 16]. Studies show that the attainment rate of potassium intake is as low as 5% in people with diabetes [17]. Inadequate potassium intake can damage pancreatic islet cells, impair insulin secretion, and increase the risk of developing diabetes [8]. Long-term potassium deficiency can also damage kidney function and decrease urine concentration [18]. Therefore, inadequate potassium intake may increase the risk of developing diabetic microvascular complications.
However, there is a lack of systematic research on the intake of potassium and diabetic microvascular complications: to date, only two studies have analyzed the association between dietary potassium and diabetic retinopathy, and their findings yielded inconsistent results. One study concluded that dietary potassium was negatively correlated with diabetic retinopathy, whereas the other found no significant association between these variables [19, 20]. Therefore, the aim of this study was to explore whether inadequate potassium intake increases the risk of developing diabetic microvascular complications, diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy using the UK Biobank database.
The subjects and data for this study were derived from the UK Biobank database. The UK Biobank database is the largest biomedical public resource that comprehensively integrates a variety of data including genetics, lifestyle, and health. The database began recruiting volunteers aged 40–69 years in 2006, and by 2010 had accumulated approximately half a million participants, whose primary care data from the medical systems of Scotland, Wales, and England were then followed up continuously [21]. The present study was performed under application number 92014.
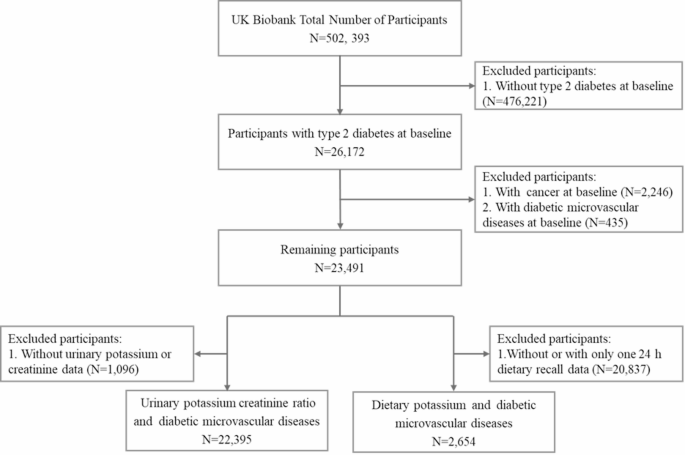
This study included 26,172 subjects who were diagnosed with type 2 diabetes at baseline, with type 2 diabetes determined based on International Classification of Diseases (ICD)-10 coding, fasting blood glucose, glycated hemoglobin, and self-reported information at baseline [22]. After excluding individuals who self-reported having cancer at baseline (N = 2,246), those who had already developed microvascular complications at baseline (N = 435), and those with missing urinary potassium or urinary creatinine at baseline (N = 1,096), a total of 22,395 subjects were included in the analysis. When exploring the association between dietary potassium and diabetic microvascular complications, we only included subjects with at least two 24-hour dietary recalls (N = 2,654) (Fig. 1). The UK Biobank database was approved by the North West Multi-center Research Ethics Committee, and all participants signed written informed consent forms [23].
Because urinary potassium accounts for 77% of total potassium intake, it is considered a good surrogate for dietary potassium intake [24]. Therefore, the main exposure factor in this study was urinary potassium. Each participant provided a 9 mL urine sample during the baseline survey, which was then stored at 4 °C and transported to the laboratory for testing. When testing urinary potassium, the specimen was prediluted before being measured by potentiometry, with a range of 2–200 mmol/L. Urinary creatinine was measured by photometric assay, with a range of 88–44,200 µmol/L; if the result exceeded the upper limit, the sample was diluted and re-analyzed. All tests were conducted using the same clinical chemistry analyzer in a laboratory accredited by the international standard ISO 17,025 [25]. Because creatinine is excreted at a nearly constant rate in the human body, urinary potassium was expressed as the urinary potassium-to-creatinine ratio, to account for variation in participants’ daily urine volume [26].
Additionally, dietary potassium intake was measured using the 24-hour dietary recall method. The UK Biobank issues an online dietary questionnaire four times after an initial in-person assessment at baseline, and participants can repeatedly fill in dietary information by receiving emails or logging onto the website. Although a single 24-hour diet recall questionnaire has poor representativeness, multiple 24-hour diet recall questionnaires are representative of a person’s long-term dietary habits and nutritional intake [27]. Therefore, we included subjects who completed at least two 24-hour dietary recall surveys and calculated the average of multiple measurements as the dietary potassium intake.
Primary care data were obtained from the medical systems of Scotland, Wales, and England. The occurrence of microvascular complications during the follow-up process was determined using ICD-10 codes from cumulative hospitalization records and death records in the national death registry. The diagnostic codes for diabetic nephropathy are ICD-10: E11.2, E14.2, and N18.0–N18.9; the diagnostic codes for diabetic retinopathy are ICD-10: H28.0, H36.0, E11.3, and E14.3; and the diagnostic codes for diabetic neuropathy are ICD-10: E11.4, E14.4, G59.0, G62.9, G63.2, and G99.0 [28]. Participants were followed prospectively until the occurrence of an outcome, death, or the date of loss to follow-up (September 30, 2021), whichever came first.
The sociodemographic characteristics of the study subjects were obtained through a touch-screen questionnaire at the baseline survey and included age, sex, ethnicity, professional qualifications, smoking status, alcohol consumption, and physical activity. Professional qualifications was divided into five categories: College or University, A levels/AS levels or equivalent, O levels/GCSEs or CSEs or equivalent, NVQ or HND or HNC or equivalent, and None of the above. A levels/AS levels or equivalent refers to the General Certificate of Education Advanced Level (A-Level) and equivalent international secondary qualification. O levels/GCSEs or CSEs or equivalent refers to the General Certificate of Secondary Education (GCSE) or an internationally recognized equivalent qualification (e.g., International General Certificate of Secondary Education, IGCSE). NVQ or HND or HNC or equivalent encompasses key qualifications within the UK vocational and higher education framework, respectively corresponding to skill-based certifications (e.g., NVQs), applied diplomas (e.g., HNDs), and short-cycle credentials (e.g., HNCs). Smoking status was divided into three categories: never smokers, former smokers, and current smokers. Alcohol consumption was divided into six categories: never drinkers, former drinkers, occasional drinkers, weekly drinkers, and daily drinkers. Self-reported physical activity was collected through the International Physical Activity Questionnaire short form [22], and total physical activity was calculated and expressed in metabolic equivalents. The Townsend Deprivation Index is a socioeconomic indicator proposed by sociologist Peter Townsend in 1987, which is calculated by collecting census data and assigning scores to different areas according to postal codes, with higher scores indicating a lower socioeconomic status [29]. The calculation method for the duration of diabetes was the time of the baseline survey minus the time of the first diagnosis of diabetes, in years.
During the baseline survey, nurses conducted physical measurements and examinations on participants, including height, weight, and blood pressure. BMI was calculated by dividing weight in kilograms by the square of height in meters, and the results were categorized into four groups: less than 18.5 kg/m2, 18.5–24.9 kg/m2, 25–29.9 kg/m2, and 30 kg/m2 or above [30]. Blood pressure was measured using the average of two automatic readings from an Omron 705 IT electronic blood pressure monitor (OMRON Healthcare, Hoofddorp, the Netherlands). Biochemical indicators were measured using blood samples collected at the time of recruitment. The Beckman Coulter AU5800 biochemical analyzer (Brea, CA, United States) was used to determine levels of fasting glucose and triglycerides using the hexokinase and glycerol-3-phosphate–peroxidase methods, respectively. Estimated glomerular filtration rate (eGFR) was calculated using the 2021 Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation [31].
Analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC, USA) software and R (version 4.2.1; Vienna, Austria), and a P value < 0.05 was deemed statistically significant. Continuous variables were expressed as mean ± standard deviation and categorical variables were represented by frequency and percentage. Analysis of variance or chi-square tests were used to compare the baseline characteristics of populations divided into quartiles based on urinary potassium-to-creatinine ratio or dietary potassium intake. The intraclass correlation coefficient (ICC) was calculated using a one-way random effects model via the PROC MIXED procedure in SAS for repeated dietary measurements. The Pearson correlation coefficient between the urinary potassium-to-creatinine ratio and dietary potassium intake was calculated through correlation analysis.
Cox proportional hazards models were used to explore the relationship between urinary potassium-to-creatinine ratio, dietary potassium, and overall and individual microvascular complications, generating hazard ratios (HRs) and 95% confidence intervals (CIs). Initially, urinary potassium-to-creatinine ratio and dietary potassium were treated as continuous variables to investigate the risk changes in microvascular complications for each standard deviation increase. Subsequently, urinary potassium-to-creatinine ratio and dietary potassium were categorized into quartiles, with the lowest quartile serving as the reference group, to explore the risk changes in the other quartiles and to conduct a test for trend. In the multivariable model, adjustments were made for age, sex, ethnicity, professional qualifications, Townsend deprivation index, smoking status, alcohol consumption, BMI (as a categorical variable), physical activity, baseline insulin use and duration of type 2 diabetes. When analyzing dietary potassium, further adjustments were made for total energy intake. To explore the non-linear relationship between urinary potassium-to-creatinine ratio, dietary potassium, and microvascular complications, multivariable-restricted cubic regression splines with 4 knots were conducted.
To assess the stability of the study results, we also conducted sensitivity analyses. Considering the impact of death on the outcomes, we applied competing risk models (cause-specific models and sub distribution hazard models), where death that occurred before reaching the microvascular complications outcome was considered a competing risk and was thus censored. In addition, multiple imputation was used to impute missing values for covariates and further adjustments were made in the multifactorial models for blood glucose, blood pressure, and triglycerides. We stratified the study subjects into different subgroups based on sex, BMI, and duration of diabetes to explore the relationship between potassium and microvascular complications in populations with different characteristics.
The baseline characteristics of the study subjects are presented according to urinary potassium-to-creatinine ratio quartiles in Table 1. In this population of 22,395 individuals, the mean age of the subjects was 59.31 ± 7.23 years, with 62% being male. Participants with a higher urinary potassium-to-creatinine ratio tended to be older, more often female, and predominantly Caucasian, with higher professional qualifications, a lower Townsend deprivation index, a lower smoking rate, lower BMI, higher levels of physical activity, and lower fasting blood glucose and triglycerides. The baseline characteristics of the subjects according to dietary potassium quartiles are detailed in Supplementary Table 1. The median dietary potassium intake of the total population was 3039.14 mg/day, which was less than the recommended dietary intake suggested by WHO. The inter-rater reliability analysis using a two-way random-effects intraclass correlation coefficient (ICC) for absolute agreement among repeated dietary measurements indicated poor consistencies: ICC = 0.38, 95% CI [0.35, 0.41].
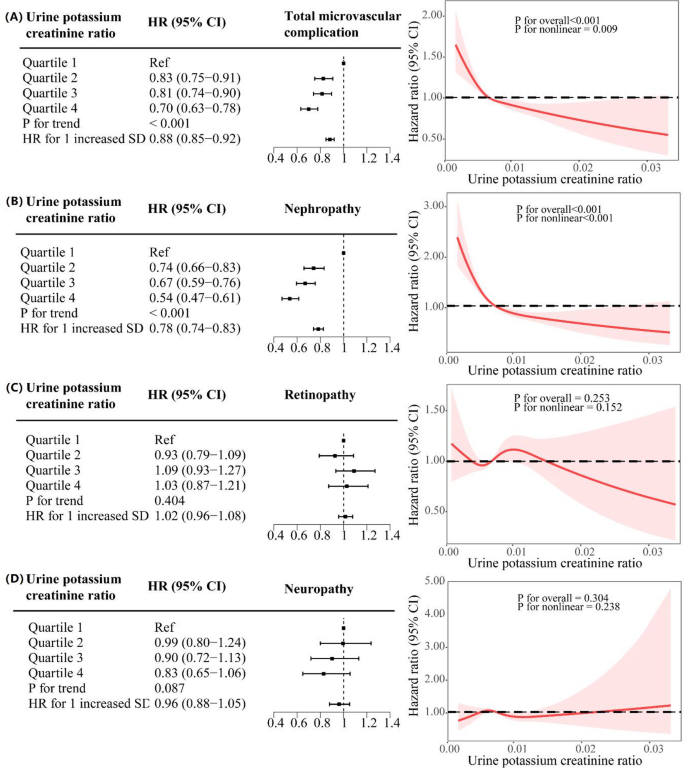
The Pearson correlation coefficient between the urinary potassium-to-creatinine ratio and dietary potassium intake was calculated through correlation analysis, yielding a statistically significant but weak positive association (r = 0.12, P < 0.001). The relationship between urinary potassium-to-creatinine ratio and diabetic microvascular complications is detailed in Fig. 2. The relationship between dietary potassium and diabetic microvascular complications is detailed in Supplementary Fig. 1. The incidence rates of diabetic microvascular complications in Q1, Q2, Q3, and Q4 of urinary potassium-to-creatinine ratio were 22.31%, 19.57%, 19.70%, and 16.88%, respectively. The multifactorial Cox regression model showed that compared with the Q1 group, the Q4 group had a significantly lower risk of diabetic microvascular complications (HR, 0.70 [95% CI 0.63–0.78]; P for trend < 0.001) and diabetic nephropathy (HR, 0.54 [95% CI 0.47–0.61]; P for trend < 0.001). However, there was no significant difference in the risk of diabetic retinopathy or diabetic neuropathy (HR, 1.03 [95% CI 0.87–1.21], P for trend = 0.404; HR, 0.83 [95% CI 0.65–1.06], P for trend = 0.087, respectively). When the urinary potassium-to-creatinine ratio was treated as a continuous variable, the results showed that for each standard deviation increase in urinary potassium-to-creatinine ratio, the overall HRs of microvascular complications and diabetic nephropathy were as follows: 0.88 (95% CI 0.85–0.92) and 0.78 (95% CI 0.74–0.83), respectively (P for trend < 0.001 for both).
The relationship between urinary potassium-to-creatinine ratio (N = 22,395) and diabetic microvascular complications. (A) Total diabetic microvascular complications; (B) Diabetic nephropathy; (C) Diabetic retinopathy; (D) Diabetic neuropathy. Adjusted for age, sex, ethnicity, professional qualifications, smoking status, alcohol consumption, BMI, Townsend deprivation index, baseline insulin use and duration of diabetes. HR, hazard ratio; CI, confidence interval
The analysis of the association between dietary potassium with diabetic microvascular complications yielded similar results. After further adjustment for energy intake, the group with the highest dietary potassium (Q4) had a significantly lower risk of diabetic nephropathy (HR, 0.48 [95% CI 0.29–0.80], P for trend = 0.005) than the Q1 group.
The restricted cubic spline results showed a non-linear relationship between urinary potassium-to-creatinine ratio and overall microvascular complications and diabetic nephropathy, with nonlinear P values of 0.009 and < 0.001, respectively, and a generally declining trend. No association was found between urinary potassium-to-creatinine ratio and diabetic retinopathy or neuropathy(Fig. 2). Unlike the urinary potassium-to-creatinine ratio, no non-linear relationship was found between dietary potassium and the risk of microvascular complications, which showed an overall linear declining trend(Supplementary Fig. 1).
All sensitivity analyses, including the use of sub distribution hazard models and cause-specific hazard models for competing risk model analysis, multiple imputations for missing covariates, and further adjustment for blood glucose, blood pressure, and blood lipids, showed no significant differences from the results of the aforementioned Cox regression risk model(Supplementary Table 2, Supplementary Table 3). Moreover, sensitivity analyses excluding participants with estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m² were conducted, with results detailed in Supplementary Table 4. Results showed no significant differences from the results of the aforementioned Cox regression risk model after excluding chronic kidney disease. Subgroup analysis results showed that the relationship between urinary potassium-to-creatinine ratio and dietary potassium with the risk of microvascular complications was essentially consistent across patients categorized by sex, BMI, and duration of diabetes(Table 2, Supplementary Table 5).
This is the first large-scale prospective cohort study to systematically analyze the association of urinary potassium-to-creatinine ratio and dietary potassium with diabetic microvascular complications using the UK Biobank database. The median dietary potassium intake was 2342.71 mg/day, 3170.95 mg/day, 3696.75 mg/day, and 4656.53 mg/day in Q1, Q2, Q3, and Q4, respectively. And only Q3 and Q4 reached recommended intake suggested by WHO. Our study found that the urinary potassium-to-creatinine ratio was significantly negatively associated with overall diabetic microvascular complications (HR, 0.70 [95% CI 0.63–0.78], P for trend < 0.001), but when analyzed by complication, this association only remained in diabetic nephropathy (HR, 0.54 [95% CI 0.47–0.61], P for trend < 0.001); no association was found between diabetic retinopathy and diabetic neuropathy. We also conducted sensitivity analyses and subgroup analysis, with the results remaining stable.
The negative association we observed between urinary potassium-to-creatinine ratio and both overall diabetic microvascular complications and diabetic nephropathy is the first of its kind. However, despite a lack of directly supportive literature, there are plausible mechanisms that may underlie these associations. Potassium has beneficial effects on oxidative stress [32], blood pressure [33], and vascular function [34, 35], all of which could prevent microvascular complications. In addition, the anatomical structure of the kidney is rich in blood vessels, and kidney function is closely related to vascular conditions [36]. Animal studies show that under low potassium conditions, macrophage infiltration increases, and the expression of monocyte chemoattractant protein-1 and tumor necrosis factor-α increases. Meanwhile, vascular endothelial growth factor and endothelial nitric oxide synthase levels decrease, which leads to perivascular capillary injury and kidney disease [37]. Low potassium conditions can also directly lead to a variety of histological or functional changes in renal units [38].
Our study indicated no significant association between potassium intake and diabetic retinopathy, with only a few previous studies exploring this relationship. A prospective cohort study by Tanaka et al., which included 978 cases of type 2 diabetes, obtained the average dietary potassium intake of the subjects through both food frequency questionnaires and 24-hour dietary surveys at baseline; they found no association between dietary potassium and diabetic retinopathy, consistent with our findings [20]. However, a cross-sectional study by Chen et al., using the NHANES database and including 5,321 cases of type 2 diabetes, concluded that dietary potassium was negatively correlated with diabetic retinopathy [19]. However, it should be noted that as a cross-sectional study, the findings of Chen et al. only indicate correlation, rather than causality; in addition, the study used a single 24-hour dietary survey method, which could lead to inaccurate calculation of dietary potassium. Therefore, there is limited evidence to suggest an association between potassium intake and diabetic retinopathy, possibly because the inflammatory response stimulated by a high-glucose environment is the main risk factor for retinopathy [39].
Diabetic neuropathy mainly includes peripheral neuropathy, autonomic neuropathy, and central neuropathy, with the first being the most common [40]. Currently, there are no reports on the relationship between either dietary potassium or urinary potassium and diabetic neuropathy. Our results suggest that potassium intake is not related to diabetic neuropathy, possibly because the pathogenesis of diabetic neuropathy is complex, and related to both vascular damage and neuronal cell damage, with the latter being more significant. Common mechanisms for the development of diabetic neuropathy are as follows: first, abnormalities in multiple signaling pathways and dysfunction of neuronal organelles caused by hyperglycemia and hyperlipidemia [41, 42]; second, the loss of neurotrophic signals due to abnormalities in the insulin signaling pathway, which inhibit the growth of neuronal cells and promote their apoptosis [43]; and third, peripheral vascular damage, which leads to insufficient oxygen supply in the microcirculation, indirectly causing neuronal cell damage [44].
There are some limitations to our study. First, the study subjects are not globally representative: with 88.26% of the UK Biobank database’s recruited population being British and white, the results of this study cannot be extrapolated to other populations. Second, the urine specimens collected were single random urine samples, which may affect accuracy; however, this is a commonly used method in epidemiological studies, and previous studies have shown that this method is reliable [25, 45]. Third, because disease diagnosis is linked to the healthcare system, there may be a misclassification of subjects who have diabetic microvascular complications but have not sought medical attention to the negative diagnosis group, thus reducing the study’s ability to detect associations. Fourth, although we adjusted for as many potential confounding factors as possible, we cannot completely rule out residual confounding or unmeasured factors.
Our study suggests that inadequate potassium intake may increase the risk of diabetic microvascular complications, particularly diabetic nephropathy. Therefore, clinical practitioners should monitor the potassium intake of patients with diabetes, and if possible, include the urinary potassium-to-creatinine ratio in routine examinations for people with diabetes to identify high-risk groups for diabetic nephropathy as early as possible. Finally, whether supplementing potassium in patients with diabetes with inadequate potassium intake would reduce the risk of diabetic nephropathy is unknown, and more research is needed in the future to explore this.
Availability of data and materials: The data that support the findings of this study are available on the UK Biobank website (https://www.ukbiobank.ac.uk/).
- AI:
-
Adequate intake
- A/AS:
-
Advanced
- BMI:
-
Body mass index
- CI:
-
Confidence interval
- CKD-EPI:
-
Chronic kidney disease epidemiology collaboration
- CSE:
-
Certificate of secondary education
- eGFR:
-
Estimated glomerular filtration rate
- GCSE:
-
General certificate of secondary education
- HNC:
-
Higher national certificate
- HND:
-
Higher national diploma
- HR:
-
Hazard ratio
- ICC:
-
Intraclass correlation coefficient
- ICD-10:
-
The international classification of diseases version 10
- IOM:
-
Institute of medicine
- IQR:
-
Median and interquartile range
- NK:
-
Not known
- NVQ:
-
National vocational qualification
- O:
-
Ordinary
- USDA:
-
United States department of agriculture
- WHO:
-
World health organizatio
Our grateful appreciation is extended to all the people and research staff who participated in the UK Biobank study. We thank Philippa Gunn, D.Phil., from Liwen Bianji (Edanz) (www.liwenbianji.cn) for editing the English text of a draft of this manuscript.
This work was partly supported by the Key project of Hubei Natural Science Foundation Innovation and Development Joint Fund (Grant number 2023AFD031); the Science and Technology Research Key Project of Education Department of Hubei Province China (Grant number D20212602); and the Hubei Natural Science Foundation Program (Youth Project) (Grant number 2023AFB211).
The UK Biobank database was approved by the North West Multi-center Research Ethics Committee, and all participants signed written informed consent forms.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Li, C., Zeng, J., Huang, X. et al. The association between urinary and dietary potassium and diabetic microvascular complications: insights from UK Biobank data. Nutr J 24, 101 (2025). https://doi.org/10.1186/s12937-025-01161-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12937-025-01161-1