BMC Veterinary Research volume 21, Article number: 354 (2025) Cite this article
Optimizing glucose control is one of the primary goals of diabetes management. This study assessed the feasibility and accuracy of a remote real-time continuous glucose monitoring system (RT-CGMS) integrated with intelligent tracking in diabetic dogs. Seven Beagle dogs were monitored using interstitial sensors across different configurations: adhesive only, adhesive with protective garments, and garments combined with an innovative glucose monitoring approach for remote transmission. Sensor wear time was slightly longer with garments (8.2 ± 6.7 vs. 5.8 ± 3.1 days; P > 0.05). Valid data acquisition was significantly higher in the remote-monitoring group [95 (84, 96)] compared to Group 1 [67 (47, 78)] and Group 2 [76 (64, 80), P < 0.001 for both]. A strong correlation was found between RT-CGMS and PBGM measurements (r = 0.904). Calibration improved accuracy at glucose levels ≥ 5.5 mmol/L, reducing MARD from 28.5 to 14.5% and increasing Bland-Altman agreement from 48 to 67%. However, MAD slightly increased in the < 5.5 mmol/L range (2.2 to 2.7 mmol/L). Frequent hyperglycemia, high variability, and glucose excursions were observed. In conclusion, RT-CGMS with intelligent tracking improved data continuity and accuracy in diabetic dogs. Future research should focus on improving the system’s sensitivity under hypoglycemic conditions and exploring its broader applications, including its role in enhancing in-hospital glucose management, utilizing big data to facilitate online diagnostics and offline follow-up care, providing guidance for daily glucose stabilization, enabling personalized veterinary services, and offering subscription-based health reports for pet owners.
The diabetic dog model, induced by relatively high doses of streptozotocin (STZ), exhibits insulin-dependent hyperglycemia with significant variability and an increased risk of hypoglycemia and ketosis. As a result, continuous glucose monitoring (CGM) is essential for the effective management of these potentially life-threatening conditions. Traditional blood glucose monitoring methods, such as capillary measurements using portable blood glucose meters (PBGM), present substantial limitations in diabetic dogs. These include a limited number of time points, the absence of real-time or remote data transmission, and low animal compliance due to the stress caused by repeated sampling. Moreover, this method often requires venipuncture, which can cause discomfort and may miss glycemic peaks or nadirs occurring between sampling intervals [1]. This concern remains highly relevant today. For example, PBGM devices have been shown to detect fewer than 10% of hypoglycemic events compared to CGM systems, underscoring their inadequacy for dynamic glucose tracking in diabetic dogs [2].
Electrochemical sensors have emerged as the preferred technology for measuring interstitial glucose due to their high sensitivity, selectivity, long-term stability, broad detection range, and rapid response time [3, 4]. In veterinary settings, most studies to date have employed intermittently scanned continuous glucose monitoring (isCGM) systems-such as the FreeStyle Libre sensor- to track interstitial glucose levels in diabetic dogs [2, 5, 6, 7]. However, these devices require manual scanning every eight hours to reconstruct a 24-hour glucose profile, lacking both automated, real-time data transmission and remote access. Additionally, they do not support calibration with blood glucose reference values, which may limit the accuracy and responsiveness of glucose monitoring, especially in emergency situations. Moreover, achieving successful CGM in dogs presents unique challenges. Previous studies have identified dense fur and the tendency of dogs to dislodge sensors as major barriers to effective sensor adhesion and long-term function [8]. These factors compromise sensor performance and highlight the need for innovative solutions to enhance wearability. In response, our study introduces the use of a protective garment– an approach not previously explored in veterinary CGM– to improve sensor stability and functional duration.
To further advance glucose monitoring in animals, we integrated an innovative glucose monitoring approach: the Osense™ Air (AccuraLink Technologies Ltd., Beijing, China). This device is part of the intelligent Vitals Tracking-Glucose Management System (iVT-GMS), which has been applied in hospital settings for diabetic patients but has not been evaluated in animals. The system enables automated, continuous glucose monitoring capabilities, reducing the risk of hypoglycemia, and minimizes animal stress, thereby improving both experimental accuracy and animal welfare.
Therefore, the objective of this study was to evaluate the feasibility and accuracy of a real-time continuous glucose monitoring system (RT-CGMS) enhanced with intelligent tracking technology in a diabetic dog model.
Seven healthy female Beagle dogs were purchased from the Beijing Keyu Animal Breeding Center (Beijing, China). Diabetes was induced via intravenous injection of streptozotocin (STZ) at a dosage of 30 mg/kg following an overnight fast. Dogs that maintained normoglycemia (defined as fasting blood glucose levels < 5.5 mmol/L) for 1–4 months after the initial injection received a second dose of STZ. Persistent fasting blood glucose levels consistently above 11.1 mmol/L were used to confirm the diabetic status [9]. Table 1 summarizes the baseline characteristics of the diabetic dogs. Each dog underwent between one and seven CGM monitoring sessions during the study period.
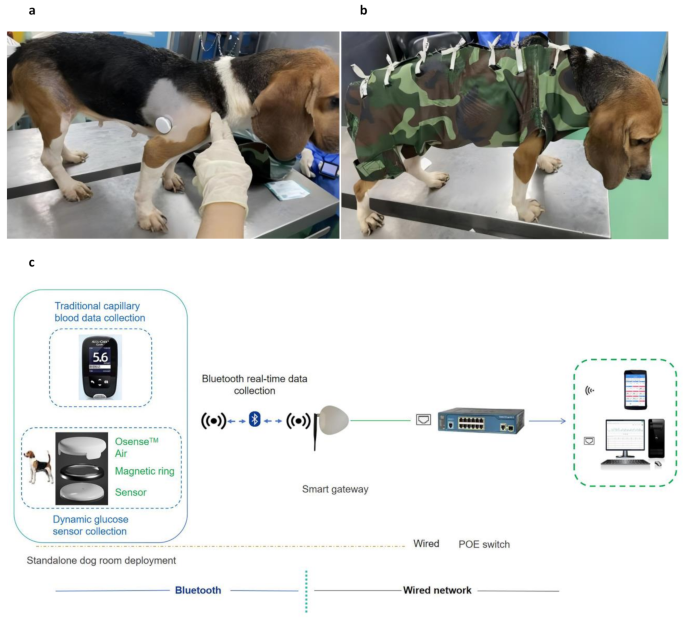
Glucose sensors were affixed to the dorsal mid-shoulder region of each dog after clipping a 10 × 10 cm area of fur and thoroughly cleaning the site with 70% alcohol (Fig. 1a). Monitoring sessions were divided into three groups based on the use of protective garments and integration of the Osense™ Air device. Please note that the group sample size (n) refers to the number of sensors used, not the number of individual dogs, as some dogs participated in multiple sessions.
Sensors were applied using only 3 M vetbond tissue adhesive (Minnesota Mining and Manufacturing Company, Minnesota, USA), without any additional fixation or transmission devices.
Sensors were secured using both adhesive and a custom-designed protective garment made of tear- and scratch- material to improve wearability (Fig. 1b).
This configuration combined the use of protective garments with the Osense™ Air, a near-field communication (NFC)-to-Bluetooth transceiver that enable real-time remote data transmission from the FreeStyle Libre sensor.
Automatic Glucose Management System for Diabetic Dogs. Composition and deployment of the continuous glucose monitoring system in a diabetic canine model. (a) Placement of the FreeStyle Libre sensor and Osense™ Air device on the dorsal mid-shoulder region following site preparation. (b) Sensor fixation enhanced with custom-designed protective garments made from durable, tear-resistant materials to improve wearability. (c) Schematic of the iVT-GMS, illustrating real-time data transmission from the NFC-enabled Osense™ Air device via Bluetooth to a smart gateway, with optional PoE-based wired network integration for multi-platform glucose data visualization. iVT-GMS, Intelligent Vitals Tracking–Glucose Management System; NFC, near-field communication; PoE, power over Ethernet
The groups were implemented in a stepwise fashion: starting with the baseline configuration (Group 1), followed by the introduction of protective garments (Group 2), and finally the integration of the Osense™ Air device (Group 3). This staged approach allowed us to assess the incremental effects of each component on sensor retention, data stability, and transmission efficiency.
After a one-hour of initialization period post-attachment, sensors began measuring interstitial glucose. Each sensor was programmed to record interstitial glucose every 15 min, yielding a theoretical maximum of 96 data points per day under ideal conditions. In Groups 1 and 2, glucose data obtained manually using the Libre scanner at least three times per day. In Group 3, glucose values were collected and transmitted continuously in real-time using the Osense™ Air device. Both the sensor and transceiver were secured using a combination of tissue adhesive and customized garments to ensure optimal stability and data acquisition.
Capillary blood glucose measurements were obtained using a PBGM device (Roche Accu-Check, Basel, Switzerland) when necessary. In Group 3, calibration of the remote RT-CGMS was conducted intermittently using capillary blood glucose values obtained via PBGM. These values were wirelessly transmitted to the Osense™ mobile application, where the operator could label them as preprandial or postprandial. Calibration was usually conducted during relatively stable glycemic periods (e.g., prior to meals or insulin injection) to reduce physiological variability. Each PBGM reading was then paired with a temporally matched interstitial glucose value recorded by the RT-CGMS, allowing for precise calibration of the system’s glucose trend outputs.
To enhance continuous glucose monitoring in diabetic dogs, this study integrated the Osense™ Air device-an advanced NFC-to-Bluetooth transceiver into an intelligent RT-CGMS platform. A custom-designed magnetic ring enabled secure and precise attachment to the FreeStyle Libre sensor, ensuring stable data transfer and minimizing disconnection risk. This innovative interface significantly improved upon traditional glucose monitoring methods by enabling automated, real-time data acquisition and transmission.
Step 1: NFC-Based Data Collection.
Step 2: Real-Time Wireless Transmission.
Step 3: Smart Gateway as the Data Hub.
Step 4: Wired Network Integration via PoE.
The resulting iVT-GMS represents a next-generation platform for automated glucose monitoring in diabetic dogs. The system facilitates real-time data acquisition, immediate glucose trend analysis, and supports rapid adjustments in glycemic management protocols. By replicating in-hospital monitoring conditions within a controlled research environment, the iVT-CGMS offers a more dynamic, data-driven, and animal-friendly approach to managing diabetes in canine models (Fig. 1c).
All data were collected between July and November 2022. Statistical analyses were conducted on a per-sensor basis, with each sensor treated as an independent analytical unit. Sensor wear time, data validity, and glucose measurement accuracy were all evaluated at the sensor level. To ensure data accuracy, the following criteria were applied to define valid data:
Statistical analysis was performed using SPSS 25.0 (IBM Corp., Armonk, NY, USA). Data normality was assessed using the Kolmogorov–Smirnov test. Continuous variables were reported as mean ± standard deviation (SD) for normally distributed data or median with interquartile range for non-normally distributed data. Group comparisons were made using Student’s t-test or the Mann–Whitney U-test, as appropriate. A P-value of < 0.05 was considered statistically significant.
The correlation between interstitial glucose values (measured by remote RT-CGMS) and capillary blood glucose values (measured by PBGM) was assessed using Spearman’s rank correlation. Measurement agreement and potential bias between paired readings were further evaluated using Bland–Altman plots, generated with GraphPad Prism 9.4.1 (GraphPad Software, San Diego, CA, USA).
The analytical and clinical accuracy of the system was assessed in accordance with ISO 15197:2013 standards [10]. Analytical accuracy was evaluated by calculating the mean absolute difference (MAD) for glucose values < 5.5 mmol/L and the mean absolute relative difference (MARD) for values ≥ 5.5 mmol/L [11]. Similar metrics have been adopted in prior veterinary studies to assess the accuracy of isCGM systems in healthy dogs [12]. To further evaluate agreement between interstitial glucose (RT-CGMS) and capillary blood glucose (PBGM), Bland–Altman analysis was performed on paired measurements. Clinical accuracy was determined using Parkes Consensus Error Grid Analysis (EGA), a validated tool for categorizing discrepancies in glucose measurements based on potential clinical risk. In this method, interstitial glucose values (y-axis) are plotted against reference blood glucose values (x-axis), with data points classified into five zones:
Additionally, glycemic characteristics in the diabetic dog model were comprehensively evaluated. Key metrics included mean blood glucose (MBG), mean of daily differences (MODD), mean amplitude of glycemic excursions (MAGE), coefficient of variation (CV), time in range (TIR; 3.3–11.1 mmol/L), time above range (TAR; >11.1 mmol/L), and time below range (TBR; <3.3 mmol/L).
The mean sensor wear time was longer in the group using protective garments (8.2 ± 6.7 days) compared to the group without garments (5.8 ± 3.1 days), although the difference was not statistically significant (P > 0.05).
Sensors integrated with the Osense™ Air device (Group 3) recorded a significantly higher volume of valid glucose data [95 (84, 96)] compared to Group 1 [67 (47, 78), P < 0.001] and Group 2 [76 (64, 80), P < 0.001] during the effective monitoring period.
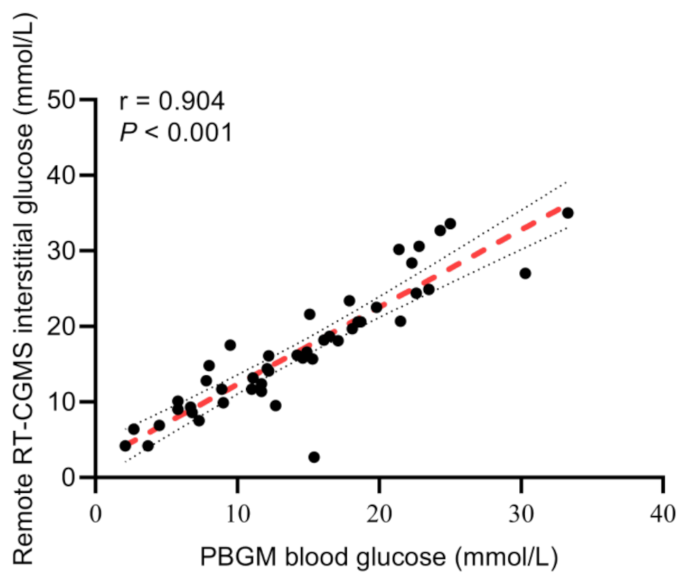
A total of 46 paired glucose readings obtained from the remote RT-CGMS and PBGM were analyzed. Spearman’s correlation analysis revealed a strong and statistically significant correlation between the two methods, with a coefficient of r = 0.904 (95% confidence interval: 0.829–0.947, P < 0.001), indicating high consistency in glucose measurement across platforms (Fig. 2).
Correlation between interstitial glucose measured by the remote RT-CGMS and blood glucose measured by PBGM in diabetic dogs (P < 0.001). r, correlation coefficient; RT-CGMS, real-time continuous glucose monitoring; PBGM, portable blood glucose meters
In the low glucose range (blood glucose < 5.5 mmol/L), the MAD was 2.2 mmol/L before calibration and 2.7 mmol/L after calibration. For the higher glucose range (blood glucose ≥ 5.5 mmol/L), MARD significantly decreased from 28.5 to 14.5% following calibration.
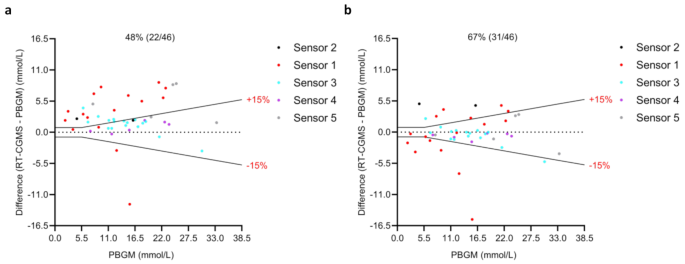
According to ISO 15197:2013 standards, acceptable accuracy is defined as being within ± 0.83 mmol/L of the reference value when the concentration was < 5.5 mmol/L, and within ± 15% of the reference value when the concentration was ≥ 5.5 mmol/L. Across the entire glucose range, the percentage of RT-CGMS readings that fell within these accuracy limits increased from 48% (22/46) before calibration to 67% (31/46) after calibration. These results indicate that calibration notably improved overall measurement agreement between RT-CGMS and PBGM. Bland–Altman plots illustrating these comparisons are shown in Fig. 3a and b.
Bland–Altman plots comparing interstitial glucose values measured by remote RT-CGMS (before and after calibration) with reference blood glucose values obtained using PBGM (Accu-Chek). (a) Differences prior to calibration. (b) Differences following calibration. Each point represents a paired glucose measurement (n = 46). Different colors correspond to different RT-CGMS sensors used during the study. The central solid black line indicates the mean bias, while the dashed black lines represent ISO 15197:2013 accuracy limits: ±0.83 mmol/L for glucose concentrations below 5.5 mmol/L and ± 15% for concentrations at or above 5.5 mmol/L. Percentages represent the proportion of samples falling within these limits (central % value). PBGM, portable blood glucose meters; RT-CGMS, real-time continuous glucose monitoring
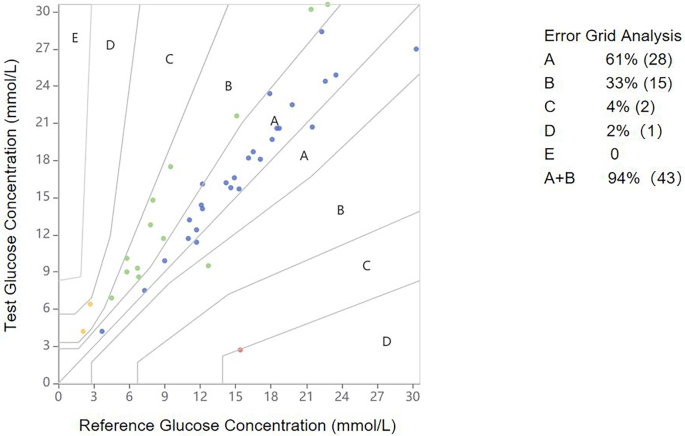
The clinical accuracy and safety of remote RT-CGMS interstitial glucose measurements were assessed in comparison with PBGM readings, as shown in Fig. 4. According to the Parkes Consensus EGA, 94% (43 out of 46) of RT-CGMS results fell within Zones A and B.
Parkes Consensus EGA illustrating the clinical risk distribution of glucose values measured by remote RT-CGMS compared to PBGM in diabetic dogs. Reference glucose values (PBGM) are plotted on the x-axis, and corresponding interstitial glucose values from RT-CGMS are plotted on the y-axis. The different zones represent the magnitude of clinical risk: no effect on clinical action (Zone A); altered clinical action with little or no effect on the clinical outcome (Zone B); altered clinical action likely to affect the clinical outcome (Zone C); altered clinical action that could pose a significant medical risk (Zone D); and altered clinical action that could have dangerous consequences (Zone E). EGA, error grid analysis; PBGM, portable blood glucose meters; RT-CGMS, real-time continuous glucose monitoring system
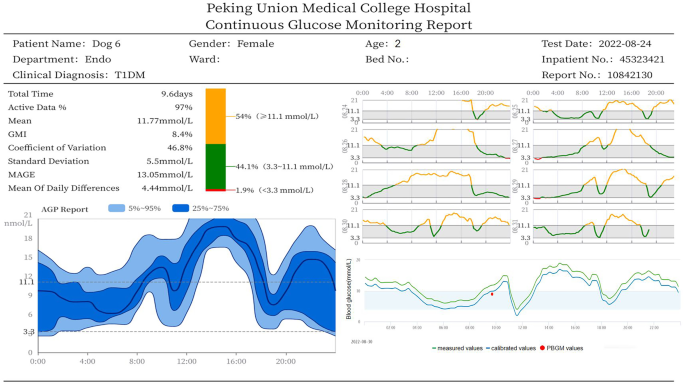
Figure 5 presents a representative CGM report for a 2-year-old female diabetic dog, capturing 9.6 days of monitoring data with a high active data acquisition rate of 97%. The report revealed marked glucose variability across key metrics. The MBG was 11.8 mmol/L, with a MODD of 4.4 mmol/L and a MAGE of 13.1 mmol/L. The CV reached 46.8%, indicating substantial glycemic instability. The TIR3.3−11.1 analysis showed that glucose levels remained within the target range (3.3–11.1 mmol/L) for only 44.1% of the monitoring period. The TAR was 54.0%, while the TBR was relatively low at 1.9%. These fluctuations were visualized through an ambulatory glucose profile (AGP), which displays the 25th–75th percentile range and 5th–95th percentile range to illustrate overall glucose variability. In addition to AGP curves, the CGM report includes real-time blood glucose trends before and after calibration, as well as PBGM reference values used for calibration. All data could be accessed remotely via a computer or smartphone interface.
CGM report displaying blood glucose trends before and after calibration, accessible via a computer or smartphone. Green lines represent glucose values before calibration; blue lines represent values after calibration; red dots indicate PBGM reference values used for calibration. CGM, continuous glucose monitoring; PBGM, portable blood glucose meters
This pilot study evaluated the feasibility and preliminary performance of a remote RT-CGMS system in diabetic dogs. Despite a small sample size (n = 7) and a limited number of paired glucose measurements (n = 46), the system demonstrated three key outcomes: (1) significantly increased valid data acquisition after Osense™ Air integration, (2) strong correlation with PBGM values (r = 0.904), and (3) improved analytical accuracy post-calibration in the hyperglycemic range. These findings establish a foundation for larger studies aimed at enhancing statistical power and validating clinical applicability across broader veterinary populations.
Diabetic dog models are essential for studying the pathophysiology of diabetes, its complications, drug development, and therapeutic interventions. Diabetic dog models were established using a validated STZ-based protocol [13, 14]. While effective, this approach resulted in variable onset of hyperglycemia and occasional severe events, such as hypoglycemic seizures 87 days post-induction. These clinical presentations underscore the limitations of PBGM for continuous glycemic monitoring and support the need for less invasive and more dynamic alternatives like RT-CGMS.
Sensor retention posed a challenge due to fur coverage, activity levels, and skin-related complications. Protective garments provided modest mechanical support but showed limited efficacy over time. Adverse effects such as erythema and scabbing were observed, aligning with similar dermatologic issues reported in both canine [8] and human CGM users [15, 16]. Most dogs tolerated the sensor and protective garments relatively well during routine daily activities, including feeding, walking, and social interaction. However, behavioral signs of discomfort—including scratching, rolling and removal attempts—increased with repeated use. While not formally assessed in this study, these observations suggest the importance of incorporating structured behavioral evaluation in future trials.
Osense™ Air integration substantially enhanced data continuity by enabling automated, real-time transmission and eliminating the need for manual scanning. This not only improved data completeness but also reduced labor and stress associated with frequent handling, contributing to more consistent glucose trend visualization. The valid data volume increased significantly from a median of 67 in Group 1 to 95 in Group 3 (P < 0.001).
In addition to enhanced data continuity, we assessed the agreement between RT-CGMS and PBGM glucose measurements. While previous studies have reported correlation coefficients of 0.81 and 0.73 in humans [17, 18] and 0.65 in diabetic dogs [19], our study demonstrated a stronger correlation (r = 0.904), indicating excellent agreement. As shown in Fig. 2, most glucose readings were predominantly skewed toward the hyperglycemic range, while values in the hypoglycemic range were limited. This underrepresentation was likely due to the absence of nocturnal PBGM measurements, which also restricted sensor calibration and sensitivity assessment during the night. Consequently, key metrics of glycemic variability-such as TBR and MAGE-may have been underestimated. Future studies should incorporate automated or minimally invasive nocturnal blood sampling and advanced CGM analytics to better capture overnight glucose fluctuations and enhance detection of hypoglycemic episodes in diabetic dogs.
In this study, we introduced capillary calibration to a remote RT-CGMS, addressing a key limitation of previous isCGM devices. Analytical performance was significantly enhanced in the hyperglycemic range (≥ 5.5 mmol/L), where calibration reduced MARD 28.5–14.5%, aligning with moderate CGM accuracy standards. In contrast, performance in the hypoglycemic range (< 5.5 mmol/L) remained suboptimal, with MAD values of 2.2 mmol/L before calibration and 2.7 mmol/L after calibration—both exceeding the ISO 15197:2013 threshold of ± 0.83 mmol/L and typical benchmarks in human CGM systems (< 1.0 mmol/L). This reduction in sensitivity under low glucose conditions is consistent with previous findings in both human patients [20] and diabetic dogs with ketoacidosis [21], likely due to physiological delays in interstitial glucose response compared to capillary glucose during hypoglycemia. Analytical accuracy was further supported by Bland–Altman analysis, which showed that 67% of values fell within the limits of agreement—an improvement over the 40.2% reported in prior canine studies [21], though still below the 60% target suggested by human CGM research [22]. Clinical accuracy, evaluated via Parkes Consensus EGA, revealed that 94% of paired values fell within Zones A and B, indicating a generally safe clinical profile. This calibration benefit was enabled by Bluetooth-based PBGM transmission and contextual labeling (e.g., pre/postprandial), facilitating physiologically relevant adjustments. While no systematic drift was observed during the 14-day monitoring period, the absence of overnight PBGM data limited assessment of long-term calibration stability. Further studies should include increased frequency of nighttime reference sampling and refine algorithms to improve sensor sensitivity under hypoglycemic conditions.
Moreover, several species-specific factors may limit the accuracy of human-designed glucose monitoring devices in dogs. First, glucose dehydrogenase-based PBGM systems may underestimate canine blood glucose due to interspecies differences in erythrocyte glucose levels [23], potentially contributing to deviations observed in the Parkes Consensus EGA (Fig. 4). Although glucose oxidase (GOx)-based CGM sensors catalyze the same reaction in humans and dogs [24], physiological differences—such as thicker canine skin (4–5 mm vs. 1.5–2.0 mm in humans) [25, 26] and denser subcutaneous tissue [27] —may affect insertion depth and signal stability. While dermis may enhance glucose exposure via its capillary density [7], inconsistent placement like reduced accuracy. Additionally, dogs’ higher core body temperature (38.5–39.2 °C) may alter GOx enzyme activity and sensor stability [28, 29]. Species-specific immune responses can also affect sensor lifespan and signal quality [30]. These findings highlight the need for species-adapted sensor design, calibration, and thermal compensation in veterinary applications. Despite these challenges, the system effectively captured real-time glycemic trends, enabling timely detection of critical events and informed adjustments to glucose management.
In addition to technical performance, the clinical adoption of remote RT-CGMS in veterinary settings depends on cost-effectiveness and user-friendliness. While the multi-component system may incur higher initial costs than PBGM or isCGM, these may be offset by improved glucose control, reduced manual sampling, and better long-term outcomes. From a usability standpoint, the system demonstrated favorable user-friendliness in this study. The Bluetooth-enabled data transmission platform supported automated glucose trend collection, while the calibration interface allowed for intuitive tagging of pre- and postprandial blood glucose values. These features reduced the operational burden for veterinary staff and may improve owner compliance in at-home care scenarios. To enhance practical viability, future RT-CGMS should simplify setup, lower costs, and integrate veterinary-specific interfaces. Rental or subscription models reduce financial barriers and expand access diverse clinical settings, supporting broader adoption in routine clinical monitoring, outpatient chronic disease care, and remote veterinary telemedicine platforms.
This study has several limitations. First, the small sample size and limited paired glucose data may have reduced the statistical power. As a feasibility study, the goal was on technical validation rather than inferential analysis. Second, the absence of consistent nocturnal capillary glucose monitoring limited the assessment of full glycemic variability. Third, behavioral responses were not systematically evaluated, though some dogs showed reduced tolerance over time, affecting sensor wearability. Forth, the use a human-designed CGM system may have introduced bias due to interspecies differences in skin structure, thermoregulation, and immune response. Lastly, environment humidity may have influenced sensor performance, differing from typical home-use conditions.
This study confirms the feasibility of using a remote RT-CGMS system with intelligent tracking in diabetic dogs. The approach improved data quality, analytical performance in hyperglycemia, and demonstrated strong PBGM correlation. With further development, this technology could serve as a valuable tool for routine veterinary care, chronic disease management, and integration into telemedicine platforms.
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
- AGP:
-
Ambulatory glucose profile
- CGM:
-
Continuous glucose monitoring
- CV:
-
Coefficient of variation
- EGA:
-
Error grid analysis
- GOx:
-
Glucose oxidase
- isCGM:
-
Intermittently scanned continuous glucose monitoring
- ISO:
-
International Organization for Standardization
- iVTS:
-
Intelligent vitals tracking-glucose management system
- MAD:
-
Mean absolute difference
- MAGE:
-
Mean amplitude of glycemic excursion
- MARD:
-
Mean absolute relative difference
- MBG:
-
Mean blood glucose
- MODD:
-
Mean of daily difference
- NFC:
-
Near-field communication
- PBGM:
-
Portable blood glucose meter
- PoE:
-
Power over ethernet
- RT-CGMS:
-
Real-time continuous glucose monitoring system
- STZ:
-
Streptozotocin
- TAR:
-
Time above range
- TBR:
-
Time below range
- TIR:
-
Time in range
Not applicable.
This study was supported by grants from the National Key Research and Development Program of China (Grant Number 2019YFC0118505) and the National High Level Hospital Clinical Research Funding (Grant Number 2022-PUMCH-B-015).
The animal experiment protocol was approved by the Committee of Welfare and Ethical Inspection in Animal Experimentation of Peking Union Medical College Hospital (No. XHDW-2020-047).
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Xi, J., Yang, H., Zhang, Q. et al. Glucose monitoring intelligent tracking system for remote glycemic assessment in diabetic dogs: a novel approach. BMC Vet Res 21, 354 (2025). https://doi.org/10.1186/s12917-025-04809-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12917-025-04809-6