Health and Quality of Life Outcomes volume 23, Article number: 30 (2025) Cite this article
Research on health-related quality of life (HRQOL) using minimally important differences for cancer care in Asian settings are sparse. This study aimed to describe functional HRQOL trajectories among Colorectal Cancer (CRC) patients undergoing adjuvant chemotherapy (AC) compared to those who did not (No AC), evaluate if AC was associated with change in HRQOL prospectively, and examine QOL differences between elderly and non-elderly CRC patients requiring AC.
CRC patients diagnosed between February 2018 to August 2021 were recruited from three Singapore public hospitals. Participants completed the EORTC QLQ-C30 over seven timepoints (diagnosis, predischarge, 1-, 3-, 6-, 9-, 12-months post-surgery). Clinical characteristics were collected from electronic medical records.
The sample comprised 251 participants (102 in AC group; 40.64%). Clinically relevant deteriorations in functional HRQOL were observed in both groups between baseline and predischarge. These returned to baseline by 12-month. AC was associated with poorer physical (β = -35.34, p < 0.05) and role functioning (β = -71.17, p < 0.05) over time. Being elderly was associated with poorer physical functioning (β = -0.44, p < 0.05) over time. However, the non-elderly AC subgroup tended to experience poorer HRQOL in general compared to elderly.
Functional recovery remains a challenge for CRC patients in general. However, non-elderly AC patients may experience more severe impacts to role and social functioning.
Colorectal cancer (CRC) is one of the most prevalent cancers, with nearly two million incident cases globally in 2018 [1]. Surgery remains the primary treatment for most patients presenting with potentially curable CRC [2, 3]. Following surgery, patients with more advanced cancers usually undergo adjuvant chemotherapy (AC) to reduce the risks of recurrent disease, which typically lasts around six months [4]. Undergoing AC for CRC using typical regimens – such as oxaliplatin or fluoropyrimidine – is known to impact the patient in various physiological ways, including side effects such as appetite loss, nausea, pain, and fatigue [5].
Health-related quality of life (HRQOL) is an important patient-reported outcome (PRO) in cancer care as it commonly encapsulates – and is strongly associated with – functioning and disability [6]. Within the clinical setting, HRQOL has traditionally been measured using quantitative instruments, which can either be generic (e.g., EuroQol’s EQ-5D-5L) or cancer-specific (e.g., the European Organisation for Research and Treatment of Cancer’s QLQ-C30) [7, 8]. There is literature to suggest that cancer patients in general who undergo AC tend to experience poorer functional HRQOL than those who do not, due in part to the side effects across their chemotherapy cycles [9,10,11,12]. More specific to CRC, Orive and colleagues highlighted AC was a statistically significant predictor of HRQOL prospectively over a 5-year period; patients who required AC were more likely to have poorer HRQOL score trajectories over time compared to those who did not [13]. Some studies have also suggested that the impact of CRC treatment on HRQOL is more severe in older patients. For example, Cabilan and Sonia’s systematic review found that being elderly (aged 65 years and above) and experiencing chemotherapy-related side effects were both independently associated with lower physical and functional HRQOL [14]. However, other studies suggested that younger cancer patients may experience poorer HRQOL instead [15, 16]. Moreover, research examining the impact of CRC diagnosis and treatment on patients’ HRQOL trajectories has been relatively sparse in Asian urban settings.
There were therefore three objectives to the present research. Firstly, we sought to describe the functional HRQOL trajectories of CRC patients who underwent elective surgery and subsequently required AC compared to those who did not (No AC) in an Asian urban setting. Secondly, we wanted to evaluate if requiring AC was significantly associated with CRC patients’ change in functional HRQOL over time. Lastly, we aimed to examine differences in functional HRQOL scores between elderly and non-elderly CRC patients who required AC.
This longitudinal observational study comprised a prospective cohort of patients diagnosed with CRC between February 2018 to August 2021 in Singapore. Patients were eligible to participate if they were 1) diagnosed with CRC and 2) underwent elective surgery for their CRC in one of the three public tertiary hospitals that served as study sites (National University Hospital, Ng Teng Fong General Hospital, Sengkang General Hospital). The study cohort was designed to be representative of the typical CRC patient population in Singapore – as public healthcare institutions, all three study sites serve patients of all indications (i.e., symptomatic or via the national CRC screening programme) regardless of socioeconomic status. Patients who did not undergo surgery for their CRC were excluded as most of such patients would have had unresectable cancer [2, 3]. This subpopulation is likely to be on extended therapeutic regimens with or without palliative care and tend to have a very different cancer experience compared to the “typical” CRC journey [17, 18].
Informed consent to participate was obtained at the point of diagnosis – the baseline. After consenting, participants were administered the study measures at seven timepoints – baseline, 1–2 days post-surgery before discharge from hospital (i.e., predischarge), and 1-, 3-, 6-, 9-, and 12 months after surgery. These timepoints encapsulated the typical CRC patient’s treatment pathway after diagnosis, including elective surgery and adjuvant chemotherapy.
Potential losses to follow up were mitigated by providing each participant several alternatives for questionnaire administration. These consisted of face-to-face (during the participant’s regular follow-up consultations), via telephone, or emailing of the relevant study instruments. For each scheduled timepoint, up to three separate attempts to contact the participant were made over a one-week period. The timepoint was considered missed only upon failure of all three attempts. Participants were only considered lost to follow up if three timepoints were missed consecutively.
QOL scores derived from Reudink and colleagues were used as a basis for sample size estimation due to similarities in eligibility criteria, use of the EORTC QLQ-C30, and prospective observational design [19]. The actual sample size estimation was calculated using GLIMMPSE version 3.1.3 [20]. GLIMMPSE is an internet-based open access program primarily meant to aid researchers in estimating sample size or power needed for repeated measures designs [21, 22]. We used 80.0% power and an alpha level of significance of 0.05, with expected outcome values ranging from 72.9 (at baseline) to 79.9 (at final timepoint) and their respective standard deviations based on the EORTC QLQ-C30’s global health status domain scores presented by Reudink and colleagues [19]. Means and variability scale factors were set to a default value of 1 as there was no evidence of exponential changes in QOL longitudinally. This generated a minimum sample size of N = 152.
Approval to proceed with this study was provided by the National Healthcare Group’s Domain Specific Review Board (NHG DSRB Ref: 2017/00518).
The primary instrument administered at each timepoint was the 30-item EORTC Core Quality of Life Questionnaire, version 3.0 (EORTC QLQ-C30). The EORTC Core Quality of Life Questionnaire (EORTC QLQ-C30) is a globally validated instrument designed to assess the QOL of cancer patients via functional health and perceived symptom burden [23]. Central to the EORTC QLQ-C30 is its global health status scale and five subscales representing the patients’ level of physical, role, emotional, cognitive, and social functioning. As PROs continue to rise in importance as a clinical indicator, an ongoing discussion in the health services research community is on how healthcare professionals can interpret changes in PRO scores [24, 25]. For the EORTC QLQ-C30, recent work by Musoro and colleagues has resulted in the development of minimally important differences (MID) as a framework for such interpretation of score changes specific to various cancer types [26]. An MID is defined as the minimum difference in scores either between patient groups or within-group measurement timepoints to be of relevance clinically and represents established guidelines by which healthcare providers can interpret HRQOL scores.
For the purposes of this study, we utilised the global health status (GQOL) score and the five functioning subscales – physical, role, emotional, cognitive, and social – of the QLQ-C30. Each item was presented as a statement or question with Likert-type response categories (e.g., “not at all” to “very much”). Using the EORTC QLQ-C30 scoring manual, participant responses were then transformed into a score for each subscale ranging from 0–100 [23]. Higher GQOL and functioning scores denoted better perceived QOL. Within- and between-group MIDs for EORTC QLQ-C30 were derived from Musoro and colleagues, who presented clinically relevant anchor-based values for improvements and deterioration in GQOL, physical, role, and social functioning [26] (see Supplementary Table 1). MIDs were not available for emotional and cognitive functioning in the CRC literature.
Demographic and clinical characteristics were also collected at the baseline timepoint using a standardised data collection form. For demographic data, this included the patient’s age at diagnosis, sex, and race (Chinese, Malay, Indian, Others). Clinical data was extracted from the patient’s electronic medical records, including tumour site (colon or rectal), whether the patient underwent laparoscopic or open surgery, if an ostomy was required, cancer staging (I to IV), pre-operative American Society of Anesthesiologists (ASA) classification, intra-operative estimated blood loss, post-operative morbidity (using the Comprehensive Classification Index; CCI), post-operative length of stay (LOS), and readmission within 30 days post-surgery. The main grouping variable of whether the patient required adjuvant chemotherapy was also retrieved from electronic medical records – for CRC in Singapore, this clinical decision is typically made by the treating team of surgeons and oncologists, and is determined by histological staging, evidence of metastasis, and risk of recurrence.
All analyses were performed using Stata/BE 17.0 (StataCorp). Descriptive statistics were presented using frequencies and proportions for categorical data (e.g., tumour site). Means and standard deviations were used to present continuous data (e.g., intra-operative estimated blood loss). Age was analysed as both a continuous variable as well as recoded into a categorical variable consisting of elderly (≥ 65 years at diagnosis) and non-elderly. Bivariate analyses were performed using chi-square for categorical variables and independent samples t-tests for continuous variables. In all instances, the grouping variable applied to the sample was whether the patient required AC.
Due to ceiling effects in several of the EORTC QLQ-C30 functioning subscales, we used multilevel mixed effects tobit regression to analyse the fixed main effect of requiring AC on the change in QOL outcome scores over the study period. The tobit regression models used robust standard errors, an unstructured covariance matrix, and included the addition of a random intercept and slope to account for variations in participants’ outcome scores across time. Covariates were included as fixed effects if they were statistically significantly different between the AC versus No AC group at baseline. Interaction effects between age and requiring AC were tested by fitting an interaction term into each tobit regression model. Descriptive subgroup analysis was performed to further examine how change in QOL outcome scores over time differed between elderly and non-elderly participants who required AC.
All available data was used, including participants who were lost to follow up (up to the latest completed timepoint), although participants who predeceased the end of the study period were excluded from the longitudinal analysis. No missing data imputation was performed as this required the condition of data to at least be missing at random, which was unlikely in our study’s context. Missing data was tabulated as the proportion of completed EORTC QLQ-C30 subscales per timepoint over the initial baseline sample size. A p < 0.05 was considered statistically significant for all analyses.
A total of 463 potential patients were screened for eligibility, of whom 77 (16.63%) were deemed ineligible, and 121 (26.13%) declined to participate. The remaining 265 patients were enrolled in the study. Some participants were lost to follow-up during the study period due to non-response, and an additional 14 patients were excluded from the final analysis. A flowchart illustrating the enrolment and follow-up process is presented in Supplementary Fig. 1. The final sample comprised 251 participants, of which 102 (40.64%) required adjuvant chemotherapy. The AC group was significantly younger than the No AC group (64.50 versus 70.21 years). The AC group also had a significantly higher proportion of patients with Stage III CRC (52.94% versus 18.80%) and a correspondingly lower proportion of Stage I (2.94% versus 25.56%). A comparison of baseline characteristics between the two groups is presented in Table 1.
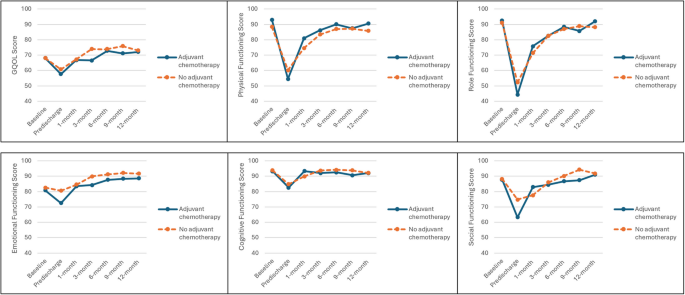
Table 2 and Fig. 1 descriptively present mean scores reported by both groups for GQOL and the five functioning subscales at each timepoint. Both groups experienced similar patterns of initially deteriorating outcome scores before a generally consistent recovery until the end of the study period.
Mean GQOL and functioning scores across study timepoints between patients in the AC and No AC groups
Within-group MIDs suggested that both AC and No AC patients suffered clinically relevant deteriorations between baseline and predischarge timepoints for GQOL (≥ 6 points), physical (≥ 7 points), role (≥ 11 points), and social functioning (≥ 6 points). These were likewise accompanied by clinically relevant improvements by 1-month in both groups for physical (≥ 8 points) and role functioning (≥ 14 points), although only the AC group experienced a significant recovery in social functioning (≥ 9 points) while recovery in the No AC group was more gradual. For all four subscales, QOL scores had returned to, or even exceeded, baseline levels by the end of the study period. Within-group trends were not as pronounced in the emotional and cognitive functioning subscales. Scores for both subscales generally recovered beyond baseline levels for both groups by 3-month and remained relatively stable throughout the study period.
Using between-group MIDs, no clinically relevant differences between AC and No AC groups were observed for physical, role, emotional and cognitive functioning subscales across the study period. In GQOL, the AC group only experienced significantly lower scores than No AC at the 3-month timepoint (66.48 versus 73.99). For social functioning, significantly different scores were observed at predischarge (63.30 versus 74.73) and 9-month (87.35 versus 94.33). The proportion of missing data differed between groups, ranging from 4.90 – 17.65% in the AC group versus 18.79 – 38.26% in the No AC group.
All multilevel mixed effects tobit regression models were fitted with adjuvant chemotherapy grouping and the baseline score for that respective outcome subscale as the main fixed effects. Continuous age and cancer staging were included into each model as covariates as they were significant between AC and No AC groups at the bivariate level (Table 3).
All six models significantly explained the variance in outcome scores of GQOL (Wald χ2 = 32.28, p < 0.001), physical (Wald χ2 = 58.68, p < 0.001), role (Wald χ2 = 30.85, p < 0.001), emotional (Wald χ2 = 62.00, p < 0.001), cognitive (Wald χ2 = 28.78, p < 0.001), and social (Wald χ2 = 59.67, p < 0.001) functioning. However, the main effect of requiring AC was only significantly associated with poorer physical (β = −35.34, 95% CI: −61.33, −9.36, p < 0.05) and role functioning (β = −71.17, 95% CI: −127.16, −15.19, p < 0.05) scores over time. Being elderly was significantly associated with poorer physical functioning scores over time (β = −0.44, 95% CI: −0.72, −0.16, p < 0.05). Significant interaction between age and requiring AC was observed for physical and role functioning (Table 3).
The AC group consisted of 57 elderly and 41 non-elderly patients. The elderly subgroup had a significantly higher proportion of Chinese patients (92.98% versus 73.17%). All other baseline demographic and clinical factors were similar between the two subgroups (Table 4).
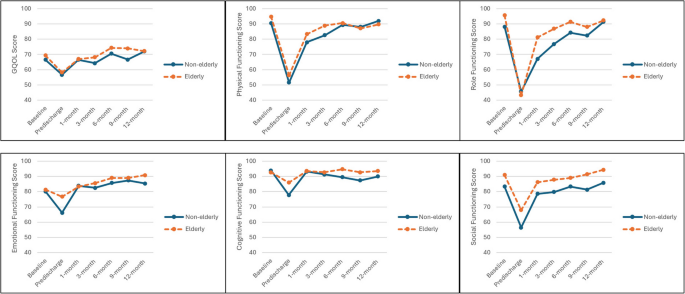
Trends in QOL outcome scores were broadly similar between elderly and non-elderly subgroups (Fig. 2). The non-elderly subgroup had generally lower scores across the subscales, with clinically relevant differences in GQOL at 9-month (66.67 versus 74.00), physical functioning at 3-month (82.63 versus 88.85), and role functioning at 1- (67.09 versus 81.17) and 3-month (76.75 versus 86.86). The non-elderly subgroup consistently experienced significantly poorer social functioning (≥ 6 points difference) compared to the elderly subgroup across the entire study period.
Mean GQOL and functioning scores across study timepoints for elderly and non-elderly patients in the AC group
Contrary to what prior literature may suggest, mean QOL scores amongst our local cohort did not significantly differ between AC and No AC groups across the study period. Few between-group timepoint differences were larger than the respective MID thresholds, and results from our multilevel tobit regressions suggested that requiring AC was only significantly associated with poorer physical and role functioning over time. These findings were interesting and appeared to contradict current knowledge [9,10,11,12]. We can think of two possible reasons for this discrepancy. Firstly, there appear to be few prospective longitudinal studies focusing on QOL and AC in CRC patients – most literature highlighting impaired QOL during or after chemotherapy have traditionally focused on breast cancer patients or samples with a mix of cancer types [11, 12]. Moreover, a recent meta-synthesis by Rutherford and colleagues highlighted that although systematic reviews investigating the impact of adjuvant treatment on CRC patients exist, these did not evaluate QOL [27]. The prospective QOL trajectories in our study’s AC group therefore represent new knowledge relating specifically to the CRC population, especially within the context of an Asian health system. Secondly, Singapore is an urbanised society with a highly developed healthcare infrastructure [28]. In the context of CRC patients requiring AC, this includes an extensive suite of supportive care encompassing palliative, psychosocial, rehabilitation and other domains [29]. It is possible that the ease of access to such adjunct services could have helped “close the gap” in QOL between the AC and No AC groups.
Within-group changes were more pronounced. Both groups experienced clinically relevant deteriorations to GQOL, physical, role and social functioning between baseline and the predischarge timepoint. This intuitively highlights the significant effect of surgical resection on our sample, although recovery was observed as soon as one month after surgery (for physical and role functioning). These trends could perhaps be qualitatively corroborated in recent work by van Kooten and colleagues, in which CRC patients expressed that it took time to cope with the initial challenges of surgery (e.g., poor bowel function, uncontrolled flatus, anxiety from complications) but also that they eventually resumed their day-to-day lifestyles [30]. QOL for patients in both groups recovered to – or in some cases exceeded (for emotional and social functioning) – baseline levels by the end of the study period, suggesting that AC may not inflict longer term effects on CRC patients. However, we notably observed that physical and role functioning scores only returned to baseline levels for both groups around the 12-month timepoint. This was in stark contrast to the other QOL subscales in which return to baseline was achieve by 6-month (GQOL, social) or even earlier (cognitive at 3-month; emotional at 1-month). Our findings suggest that regardless of whether patients require AC or not, being diagnosed with CRC and having to undergo treatment most heavily impacts their ability to resume pre-diagnosis lifestyle; these include activities of daily living (ADL) or familial and professional roles [31]. de Wind and colleagues, for example, found that CRC survivors in the Netherlands Cancer Registry had a 56% higher risk of losing paid employment when age- and sex-matched with a general population reference group [32].
Owing to statistical interaction effects observed between age and requiring AC in some of the QOL outcome models, descriptive subgroup analyses were performed. These suggested that elderly patients requiring AC experienced similar or better QOL compared to non-elderly patients. This was especially notable in role functioning with clinically relevant between-group differences at 1- and 3-month, and for social functioning over all timepoints. This is perhaps unsurprising; Waddel and colleagues’ systematic review found that younger adult cancer patients experienced more severe impacts to interpersonal social relationships and career prospects [33]. Similarly, our prior scoping review also found that young-onset CRC patients experienced more difficulties relating to performing their social responsibilities (e.g., caring for children), sexual dysfunction, and decline in performing prior occupational roles [34]. A separate study by Bailey and colleagues highlighted that CRC patients aged 50 and below at diagnosis tended to have poorer body image than older individuals – likely relating to difficulties in bowel control and stoma bag care within social settings – which is also known to impact social well-being [35, 36].
For patients who do require AC, it would therefore seem that elderly patients may be better off than their younger counterparts. This makes sense in the Singaporean context, where families tend to be small and nuclear with both spouses employed, and with the retirement age set at 63 years [37]. Non-elderly CRC patients requiring AC could be far more likely to experience financial toxicity from the indirect cost of work stoppages and/or changes in job scope due to cancer care [38]. Although outside the scope of the present study to explore, challenges related to sexual wellbeing – such as erectile dysfunction – are intuitively more impactful to younger patients for issues such as family planning [39]. Conversely, elderly CRC patients are more likely to be retired, with adult children who often function as informal caregivers providing functional, financial, and social support [40].
Taken as a whole, the present study’s findings highlight that requiring AC may not inflict as severe of a longitudinal impact to CRC patients’ QOL. CRC patients in our sample universally seemed to experience more severe difficulties with functional status across the treatment journey compared to other domains of QOL – with potential impairments to their physical abilities and ability to resume pre-diagnosis life roles. This does not imply that CRC patients requiring AC do not experience treatment-related side effects or discomfort. Moving ahead however, it may be worthwhile for allied health services and other support agencies to focus on getting CRC patients “back on their feet”, especially when it comes to younger individuals who require adjuvant treatments. Such efforts will inevitably go beyond the ability of healthcare institutions alone and should also involve flexibility and empathy from employers to recognise the challenges faced by cancer survivors [41, 42].
There are three limitations in our study that should be considered. Firstly, while quantitative domains of HRQOL and potential clinical and demographic baseline factors that may be associated with HRQOL were measured, we recognise that this may be non-exhaustive. HRQOL is a complex concept that can involve qualitative nuances such as coping strategies and interpersonal relationship dynamics [43]. Future studies should therefore attempt to incorporate mixed methods approaches in understanding the comparative lived experience of CRC patients who require, and do not require, AC. Secondly, we acknowledge that the longitudinal nature of our study could have introduced a selection bias – patients who were experiencing poorer baseline HRQOL might have declined participation, and similarly those whose HRQOL deteriorated over the study period could have been more likely to drop out. This was especially evident in the differential missing data proportions between the AC and No AC groups. As the No AC group were less likely to require face-to-face touchpoints involving treatment procedures compared to the AC group, it is possible that those who missed study timepoints were also patients who missed or elected to skip their scheduled follow-up appointments. Although difficult to address this problem in scientific research, healthcare institutions can attempt to mitigate this problem and acquire a “truer” picture of their CRC patients’ HRQOL by incorporating PROs into each routine cancer care visit. Lastly, our findings may not represent the experience of CRC patients with advanced disease who did not undergo surgery for their cancer. Care should be taken not to generalise our findings to CRC patients scheduled for palliative or end-of-life care.
To conclude, the present study represents an effort to understand the longitudinal impact of AC in CRC patients within in a highly developed Asian public healthcare system. Our findings suggest that in terms of clinical relevance, HRQOL trajectories are largely similar regardless of whether they required AC or not, although recovery of functional status remains a challenge for CRC patients in general. Nonetheless for CRC patients who do require AC, non-elderly individuals are likely to experience a more severe impact to their role and social functioning, and further research should be conducted to understand how the cancer care experience may be qualitatively different for younger patients.
The data that support the findings of this study are available on request from the corresponding author, KKT. The data are not publicly available due to potentially identifiable information derived from participants’ electronic medical records.
N/A.
The Singapore Colorectal Cancer Research Groupcomprising, in no particular order of precedence:
• Bettina Lieske
• Wai-Kit Cheong
• Jing-Yu Ng
• Dedrick Kok-Hong Chan
• Ian Jse-Wei Tan
• Kai-Yin Lee
• Bryan Buan
• Jarrod Kah Hwee Tan
• Choon-Sheong Seow
• Christopher Hang-Liang Keh
• Min-Hoe Chew
• Fung-Joon Foo
• Frederick Koh
• Sharmini Su Sivarajah
• Winson Jianhong Tan
This work was supported by the Singapore National Medical Research Council Clinician Scientist Award [MOH-000333–00].
Approval to proceed with this study was provided by the National Healthcare Group’s Domain Specific Review Board (NHG DSRB Ref: 2017/00518) in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients participating in this study.
N/A.
The authors declare no competing interests.
A list of authors and their affiliations appears at the end of the paper.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Cite this article
Lau, J., Peh, C.H., Ng, A. et al. Does adjuvant chemotherapy result in poorer health-related quality of life among colorectal cancer patients? A longitudinal multisite observational study in Singapore. Health Qual Life Outcomes 23, 30 (2025). https://doi.org/10.1186/s12955-025-02363-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12955-025-02363-1