Journal of Translational Medicine volume 23, Article number: 557 (2025) Cite this article
There is a dearth of population-based studies on the association between the diversity of the oral microbiome and the risk of chronic obstructive pulmonary disease (COPD). The study aims to investigate the association between oral microbiome diversity and COPD.
In this cross-sectional study, data from the National Health and Nutrition Examination Survey (NHANES 2009–2012) were analyzed. The association between the oral microbiome α-diversity and COPD risk was examined via multivariable logistic regression, with Restricted cubic splines revealing potential non-linear trends. The β-diversity disparities between COPD and non-COPD groups were delineated using Principal Coordinate Analysis (PCoA) and Permutational Multivariate Analysis of Variance (PERMANOVA).
A total of 6061 participants were included in this study. For α-diversity, the observed ASVs were significantly associated with COPD risk (OR = 0.964, 95%CI: 0.936–0.993, P = 0.016). Similarly, Faith’s phylogenetic Diversity showed a significant association with COPD risk (OR = 0.955, 95%CI: 0.919–0.993, P = 0.020). The Shannon-Weiner index was also associated with COPD risk (OR = 0.829, 95%CI: 0.702–0.981, P = 0.029). For β-diversity, PCoA and PERMANOVA analysis showed statistically significant differences in Bray–Curtis, unweighted, and weighted UniFrac distances (all P < 0.01) between the COPD and non-COPD groups.
Significant differences in oral microbiome α-diversity and β-diversity were found between COPD and non-COPD populations, with α-diversity (observed ASVs, Faith’s Phylogenetic Diversity, Shannon-Weiner index) being negatively associated with the risk of COPD.
Chronic Obstructive Pulmonary Disease (COPD) is a heterogeneous and progressive lung disorder characterized by persistent respiratory symptoms and airflow obstruction due to chronic inflammation of the airways and/or alveoli[1]. It is primarily caused by the complex interplay of genetic predispositions, such as SERPINA1 mutations, and environmental exposures (like smoking and air pollution from biomass fuels) [1, 2]. Additional risk factors, such as occupational hazards, gender disparities, socioeconomic status, early-life respiratory infections, and history of asthma, all contribute significantly to the global burden of the disease[3,4,5,6,7]. According to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines, an estimated 10.3% (equating to 391.9 million people) of individuals aged 30–79 years suffer from COPD, with a significant 80.5% residing in low and middle-income countries[8]. By 2050, a 23% rise in COPD patients is estimated among adults, reaching nearly 600 million patients.
The oral microbiome refers to the diverse community of microorganisms residing in the oral cavity, including bacteria, viruses, fungi, and protozoa. Emerging research has highlighted the significance of the oral microbiome in various diseases beyond the oral cavity, including respiratory diseases[9]. Oral dysbiosis is a significant contributor to pulmonary complications, primarily via the aspiration of oral microflora into the respiratory system, which incites host immune reactions and inflammation[10,11,12]. Some studies suggested that oral microbiome may play a role in chronic inflammation and the worsening of respiratory symptoms in COPD patients[13, 14], however, there is a dearth of population-based studies on the association between oral microbiome diversity and COPD. Our study aimed to examine the association between oral microbiome diversity (including α-diversity and β-diversity) and the risk of COPD by performing a cross-sectional analysis on a substantial, nationally representative dataset, to enhance the understanding of the potential role of oral microbiome in COPD prevention.
National Health and Nutrition Examination Survey (NHANES), with a complex, multistage probability sampling design, is a program conducted by the National Center for Health Statistics (NCHS), aiming to assess the health and nutritional status of adults and children in the United States. There are some methods to provide comprehensive information on various health conditions, including interviews, physical examinations, and laboratory tests. NHANES was approved by the NCHS Institutional Review Board, and informed consent was obtained from all participants.
Data were utilized from NHANES 2009–2010 and 2011–2012 cycles. Participants aged ≥ 20 years with detailed information on oral microbiome data and lung function were included. Demographic data, lifestyle factors, and comorbidities were also collected. The flowchart for participant selection was shown in Fig. 1.
We collected spirometry data including forced expiratory volume in 1 s (FEV1), forced vital capacity (FVC), and FEV1/FVC ratio. According to official documents, participants met the criteria were instructed to perform the spirometry test in a standing position. Testing continued until a reproducible spirogram was achieved, until a maximum of eight spirometry curves were obtained, or until the participant could no longer continue. The goal was for participants to achieve three acceptable exhalation maneuvers according to the American Thoracic Society (ATS)[15] criteria, where the two highest values for FVC and FEV1 (each from an acceptable forced expiratory maneuver) showed minimal variability: specifically, the two largest FVC values from two acceptable curves should agree within 150 ml, and similarly for the two largest values of FEV1. COPD was defined using the Global Initiative for the GOLD guidelines, with an FEV1/FVC ratio of less than 0.7[16]. In addition, if baseline spirometry indicated the presence of airflow obstruction, bronchodilator testing was performed, and positive responders (an increase of ≥ 12% and ≥ 200 mL as an absolute value compared with a baseline in either FEV1 or FVC) were excluded from the analysis.
Oral microbiome testing was conducted using oral rinse samples from NHANES participants in the 2009–2010 and 2011–2012 cycles. Data processing involved DNA extraction, PCR amplification, sequencing, and bioinformatics analysis to produce relative abundance/read count tables for taxonomic levels from phylum to genus. Microbial sequences were classified into amplicon sequence variants (ASVs) to generate α-diversity metrics (within-sample; observed ASVs, Faith’s Phylogenetic Diversity, Shannon-Weiner index, and Simpson Index) and β-diversity metrics (between-sample; unweighted UniFrac, weighted UniFrac, and Bray–Curtis dissimilarity). The α-diversity metrics were calculated using rarefaction values, with random subsampling from 2000 to 10,000 reads[17]. For the final analysis, we used datasets subsampled to 10,000 reads and averaged the 10 subsamples[18, 19]. The β-diversity was measured between all pairs of samples and represented in a distance matrix, which quantifies the dissimilarity between microbial communities.
The following covariates were included, such as age, gender, race, education level, marital status, and PIR (poverty income ratio, ≤ 1.3; 1.3–3.5; > 3.5), and BMI (body mass index, < 25 kg/m2, 25–30 kg/m2, ≥ 30 kg/m2). Household interviews were conducted to collect data on smoking and drinking behaviors. Diagnoses of hypertension and diabetes were based on self-reported information obtained through questionnaires. The diagnosis of periodontitis was based on the presence of probing depth (PD) ≥ 5 mm or attachment loss (AL) ≥ 3 mm at two or more non-adjacent sites[20].
The analysis utilized NHANES sampling weights to account for intentional oversampling and ensure the estimates are representative of the US population. For continuous variables, survey-weighted medians (Q1, Q3) and P-values (from survey-weighted linear regression) were reported. For categorical variables, survey-weighted percentages (95% CI) and P-values (from the survey-weighted Chi-square test) were reported. Differences in α-diversity metrics between the COPD and non-COPD groups were assessed, and Pearson correlation analyses were performed to explore associations between α-diversity and lung function (FVC, FEV1, FEV1/FVC). Weighted logistic regression models were used to examine the relationship between α-diversity indicators and COPD risk. Restricted cubic spline (RCS) curves were plotted to visualize the association of the α-diversity with COPD risk. Subgroup analyses were conducted to assess whether the relationship between α-diversity and COPD risk varied by group and interaction analyses were also performed. The β-diversity disparities between COPD and non-COPD groups were delineated using Principal Coordinate Analysis (PCoA) and Permutational Multivariate Analysis of Variance (PERMANOVA). All analyses were performed using R (version 4.2.3), and the P-value < 0.05 was considered statistically significant.
This study included a total of 6061 participants, comprising 5418 non-COPD individuals and 643 COPD individuals. Compared to the non-COPD group, participants in the COPD group were older (55 vs. 41 years), with a higher proportion of males (58.5% vs. 49.7%), a lower proportion of individuals with higher education (58.03% vs. 64.5%), and higher proportions of current drinkers (87.01% vs. 81.26%) and smokers (33.97% vs. 14.76%). Regarding lung function, there was no statistically significant difference in FVC between the two groups (P = 0.994), but the COPD group had significantly lower FEV1 (2833 ml vs. 3316 ml, P < 0.001) and FEV1/FVC ratios (0.68 vs. 0.81, P < 0.001) compared to the non-COPD group. Regarding α-diversity metrics, the COPD group exhibited significantly lower values in observed ASVs (117.30 vs. 126.10, P = 0.001), Faith’s Phylogenetic Diversity (13.53 vs. 14.22, P = 0.003), and the Shannon-Weiner index (4.51 vs. 4.67, P = 0.002), excluding the Simpson Index (0.91 vs. 0.92, P = 0.932), compared to the non-COPD group, as detailed in Table 1. Additionally, the prevalence of comorbidities such as hypertension (P < 0.001), diabetes (P = 0.008), and periodontitis (P < 0.001) were higher in the COPD group than in the non-COPD group.
In Supplementary Figs. 1–4, Pearson correlation analysis was used to evaluate the association between α-diversity indicators and lung function in the total, non-COPD, and COPD populations. These analyses revealed a positive association between α-diversity and lung function (FVC, FEV1, and FEV1/FVC), except for the Simpson Index, which was not associated with FEV1/FVC. Notably, the associations between α-diversity and lung function were stronger in the COPD population compared to the non-COPD population. In the COPD group, four α-diversity indices showed stronger associations with FEV1 (observed ASVs: r = 0.215, P < 0.001; Faith’s Phylogenetic Diversity: r = 0.202, P < 0.001; Shannon-Weiner index: r = 0.176, P < 0.001; Simpson Index: r = 0.136, P < 0.001).
Table 2 presented the association between four α-diversity metrics and the risk of COPD. The observed ASVs were significantly associated with COPD risk (unadjusted: OR = 0.938, 95%CI: 0.912–0.964, P < 0.001; model 3: OR = 0.964, 95%CI: 0.936–0.993, P = 0.016). Similarly, Faith’s phylogenetic Diversity showed a significant association with COPD risk (unadjusted: OR = 0.929, 95%CI: 0.896–0.962, P < 0.001; model 3: OR = 0.955, 95%CI: 0.919–0.993, P = 0.020). The Shannon-Weiner index was also associated with COPD risk (unadjusted: OR = 0.775, 95%CI: 0.672–0.894, P < 0.001; model 3: OR = 0.829, 95%CI: 0.702–0.981, P = 0.029). The Simpson Index did not show a statistically significant association with COPD through multiple logistic regression models (all P > 0.05). Additionally, we analyzed the association between α-diversity indicators and the risk of COPD in various subgroups. As shown in Supplementary Figs. 5–8, observed ASVs and Faith’s Phylogenetic Diversity were significantly associated with COPD risk, except in the 20–45 age group, the Mexican American population, and other ethnic subgroups. The Shannon-Weiner index did not show an association with COPD risk in these subgroups, nor other Hispanic populations or never-drinkers. For the Simpson Index, an association with COPD risk was observed in some subgroups, including those aged 46–69 years, with an education level lower than high school, with partner, former alcohol drinkers and smokers, and without a history of periodontitis.
We explored the relationship between different α-diversity indices and the risk of COPD by plotting the RCS curves. As shown in Fig. 2, a non-linear association between α-diversity indicators and the risk of COPD was found (observed ASVs: Pnonlinear = 0.021; Simpson Index: Pnonlinear = 0.003). In contrast, Faith’s Phylogenetic Diversity and the Shannon-Weiner index exhibited a linear relationship with COPD risk (P overall < 0.001).
Restricted cubic spline curves between alpha diversity metrics and COPD risk, a observed ASVs; b Faith’s Phylogenetic Diversity; c Shannon-Weiner index; d Simpson Index
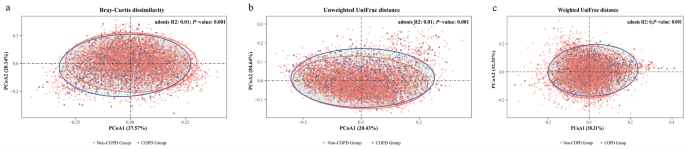
As shown in Fig. 3, we compared the differences in β-diversity metrics between the COPD and non-COPD populations using PCoA analysis. To quantify the extent of these differences in oral microbial β-diversity between groups, PERMANOVA analysis was conducted. The differences in β-diversity between the two groups were statistically significant (Bray–Curtis dissimilarity: R2 = 0.01; unweighted UniFrac distance: R2 = 0.01; weighted UniFrac distance: R2 = 0; all P < 0.01).
Comparison of oral microbial beta diversity in COPD and non-COPD populations, a Bray–Curtis dissimilarity; b unweighted UniFrac distance; c weighted UniFrac distance
In this study, there were significant differences in oral microbiome α-diversity and β-diversity between COPD and non-COPD populations. The α-diversity indicators (observed ASVs, Faith’s Phylogenetic Diversity, Shannon-Weiner index) were significantly lower in COPD than in non-COPD populations. The β-diversity metrics (Bray–Curtis dissimilarity, unweighted UniFrac distance, weighted UniFrac distance) also indicated significant differences in oral microbiome diversity between the two groups. Further analyses showed that α-diversity was negatively associated with COPD risk, with higher α-diversity associated with lower risk of COPD.
The observed ASVs reflect species richness, Faith’s Phylogenetic Diversity captures evolutionary diversity, and the Shannon-Weiner index accounts for both richness and evenness, providing a comprehensive measure of microbiome complexity. Our results indicated that higher α-diversity was associated with a reduced risk of COPD. Specifically, for every 10-unit increase in observed ASVs, the risk of COPD decreased by 3.6%, while each 1-unit increase in Faith’s Phylogenetic Diversity and the Shannon-Weiner index was associated with a 4.5% and 17.1% reduction in COPD risk, respectively. These findings suggested that a more diverse oral microbiome may better resist pathogenic invasion, potentially reducing COPD risk. Previous studies have shown that COPD patients with frequent exacerbation exhibit lower microbiome diversity, both in the oral cavity and sputum, compared to those with infrequent exacerbations[21]. Moreover, another study has indicated that reduced microbiome diversity has been associated with frequent acute exacerbations of chronic obstructive pulmonary disease (AECOPD)[22]. At the same time, a multicenter study found that the dysbiosis in microbiome during exacerbations was linked to increased exacerbation severity[23]. A meta-analysis also found that patients with AECOPD had a lower Simpson Index for airway microbiome compared to healthy controls, further suggesting reduced microbiome diversity during acute episodes[24]. Additionally, the sputum microbiome α-diversity was found to be an important predictor of one-year mortality in COPD patients, with non-survivors exhibiting significantly lower diversity. For each 1-unit increase in observed ASVs, the risk of COPD was reduced by 4%, while a 47% reduction in risk was associated with the Shannon Index and a 38% reduction with Faith’s Phylogenetic Diversity[25]. These results underscore a significant association between α-diversity and COPD risk, and emphasize the importance of maintaining a diverse and balanced oral microbiome to improve patient outcomes.
The significant difference in β-diversity was identified between COPD patients and non-COPD patients, which is consistent with previous findings[14, 26, 27]. The magnitude of this variation can be ascertained through the utilisation of diverse analytical approaches, including Bray–Curtis dissimilarity, unweighted and weighted UniFrac distance. This finding signifies that there is a substantial variation in the composition of the oral microbiome community between these two groups. For example, Haldar et al. identified a significant difference in the microbiomes of healthy individuals and COPD patients through the weighted UniFrac distances (P = 0.01)[26]. Similarly, several studies based on the Bray–Curtis distance have shown significant β-diversity differences between healthy controls, stable COPD patients, those with AECOPD, and those in recovery[14, 27]. We used PCoA and PERMANOVA analysis to assess the association between β-diversity and COPD, and assessed the models’ fit by comparing model R2 values. P-values were from the Adonis to test the significance of difference between the COPD and non-COPD groups[28]. We acknowledge that the R2 values from the PERMANOVA analyses are indeed low, despite being statistically significant. A plausible rationale for this discovery would be the significant between-sample differences in oral microbiome diversity between individuals with COPD and those without COPD[29]. Notably, the large sample size in our study guaranteed the statistical power to discern subtle variations in oral microbiome, especially those impacted by the fraction of a small number of taxa[18]. The between-sample variation observed in our study may reflect the complex nature of the oral microbiome, where overall microbiome diversity could be more indicative of COPD risk than the presence or absence of specific bacteria. For example, some studies have demonstrated differences in microbiome diversity between healthy individuals and COPD patients, with variations in microbiome diversity linked to disease status[14, 26, 30]. Therefore, these studies have demonstrated distinct oral microbiome diversity between COPD and non-COPD groups, supporting the significant differences in oral microbiome β-diversity observed in the present study.
The reduction in the diversity of the oral microbiome may be a contributing factor in the development of COPD, through mechanisms such as dysbiosis of the oral-lung axis and inflammation[12, 31,32,33]. The oral and lung microbiomes exhibit a high degree of compositional similarity, a phenomenon attributable to the anatomical connection between the oral cavity and the respiratory tract[34]. It is hypothesised that oral microorganisms may enter the respiratory system through clinical microaspiration and enter the respiratory tract directly, exacerbating lung flora imbalance and inducing lung injury[12]. The association between the oral microbiome and COPD extends beyond direct microbial transfer, encompassing potential host–pathogen interactions that may influence lung health by instigating systemic inflammation or modulating host immune defense mechanisms[12]. Dysregulation of the oral microbiome may be associated with the progression of COPD, especially during acute exacerbations, as changes in the oral microbiome may exacerbate lung inflammation[31, 35, 36]. Furthermore, oral microbiome have been demonstrated to contribute to the development of COPD by promoting the attachment of respiratory pathogens, influencing the production of inflammatory cytokines, and participating in biofilm formation[32, 37, 38]. Overall, the potential mechanistic pathways between oral microbiome and COPD remain unclear and require further investigation.
In addition, evidence suggests that probiotics and appropriate dental care can have a positive impact on oral and respiratory health; maintaining good oral hygiene and increasing intake of beneficial bacteria is an intervention strategy to reduce the risk of developing COPD[39, 40]. Therefore, healthcare professionals should consider monitoring patients’ oral health as an integral part of comprehensive COPD management. Meanwhile, larger prospective studies can be conducted in the future to further validate the association between oral microbiome diversity and the risk of COPD and its associated mechanisms, and to explore the role of specific microbial species in the pathogenesis of COPD.
This study has several strengths. The NHANES database provides a nationally representative dataset with extensive health information, ensuring broad representation and credibility, which enhances the external validity and generalizability of our findings. This study is among the largest population-based analyses examining the link between oral microbiome diversity and COPD risk, with a large sample size that enhances the statistical power and robustness. Our study employed multiple measures of α-diversity and β-diversity to thoroughly evaluate the oral microbiome, enabling us to explore its association with COPD risk from various perspectives and providing a comprehensive understanding of its potential role in COPD development. This study also has several limitations. Firstly, the cross-sectional design of the study, in conjunction with the collection of data at the same time points, resulted in difficulties in determining the direction of causality between variables. The recommendation for future studies is the implementation of prospective studies or longitudinal analysis, to enhance the comprehension of potential causal mechanisms. Secondly, the NHANES database lacks composition of oral microbiome, preventing us from analyzing the association between it and COPD risk. Thirdly, the cross-sectional nature of the study and the reliance on publicly available datasets, such as NHANES, make it challenging to account for all potential confounders. Although we tried to adjust covariates as much as possible, some variables could be further studied, including diet, exercise, air pollution, and use of inhaled glucocorticoids[1, 41,42,43,44].
In this study, we found significant differences in the α-diversity and β-diversity of oral microbiome between COPD and non-COPD populations, with α-diversity negatively associated with the risk of COPD. The findings provide a basis for further exploration of the role of the oral microbiome in COPD.
The dataset (s) supporting the conclusions of this article are available in the NHANES repository, with data accessible via the CDC’s website at http://www.cdc.gov/nchs/nhanes/index.htm.
- AECOPD:
-
Acute exacerbations of chronic obstructive pulmonary disease
- AL:
-
Attachment loss
- ASVs:
-
Amplicon sequence variants
- ATS:
-
American Thoracic Society
- BMI:
-
Body mass index
- COPD:
-
Chronic obstructive pulmonary disease
- FEV1:
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- GOLD:
-
Global Initiative for Chronic Obstructive Lung Disease
- NCHS:
-
National Center for Health Statistics
- NHANES:
-
National Health and Nutrition Examination Survey
- PCoA:
-
Principal coordinate analysis
- PD:
-
Probing depth
- PERMANOVA:
-
Permutational multivariate analysis of variance
- PIR:
-
Poverty income ratio
- RCS:
-
Restricted cubic spline
We sincerely thank NHANES for their invaluable data contribution to our research.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Approval was granted by the National Center for Health Statistics (NCHS) Research Ethics Review Board.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Xia, Xx., Li, Cx. & Guo, Hr. Association between oral microbiome diversity and chronic obstructive pulmonary disease in the US population. J Transl Med 23, 557 (2025). https://doi.org/10.1186/s12967-025-06553-9













:max_bytes(150000):strip_icc()/MSDPATR_EC035-51da748c0d984eb89c8dfd8d9a8ecb82.jpg)