BMC Public Health volume 25, Article number: 566 (2025) Cite this article
Impaired glycemic control, a sedentary lifestyle and diabetes related consequences are common challenges faced by experts and individuals with type 2 diabetes mellitus (T2DM). Although regular exercise has been shown to improve glycemic control in individuals with T2DM, conventional exercise recommendations are not always feasible for patients due to time constraints. Therefore, “exercise snacking’’, which involves short bouts of exercise interspersed throughout the day, has emerged as a potential alternative to traditional exercise regimes. However, optimal intensity, amount, frequency and/or type of “exercise snacking’’ for individuals with T2DM remains unclear. The purpose of this three-arm randomized crossover study is to examine the acute effects of different “exercise snacking” modalities on cardiometabolic factors and mental health.
Ten sedentary patients (five males and five females, age range: 18–45 years; body mass index (BMI) range: 25–35 kg/m2) diagnosed with T2DM without additional diseases will be recruited. In this crossover study, participants will receive all of the following interventions in a random order: (1) three short sessions of 6 × 1 min of cycling at 90% of maximal heart rate (HRmax) (2) three short sessions of 1 × 20 s of “all out’’ sprints at cycle ergometer and (3) no intervention. Glycemic parameters, blood pressure, rate of perceived extraction (RPE), adverse events, enjoyment and affect will be evaluated before and directly after each intervention.
The results of this acute study have great potential to inform future public health efforts designed to improve glycemic control, increase exercise rates and affect overall health in individuals with T2DM.
The study was registered on ClinicalTrials.gov (NCT06146036) registred on 18 December, 2023.
The prevalence of type 2 diabetes mellitus (T2DM) continues to increase alarmingly, with 642 million patients predicted by 2040, posing an enormous economic and social challenge [1]. In addition, previous studies [2, 3] have shown that most patients with T2DM continue to spend considerable periods of the day in hyperglycemia despite pharmacological interventions. Consequently, patients with T2DM face an increased risk of diabetes-related complications as well as cardiovascular disease attributed to both, long-standing hyperglycemia [2] and postprandial hyperglycemia [3]. Therefore, effective and accessible interventions are crucial for preventing and treating for diabetes-related complications and increasing the life expectancy of T2DM patients.
Exercise has been established as an essential nonpharmacological approach in the management of T2DM due to its multifaceted benefits on glycemic control including improved insulin sensitivity and enhanced whole-body glucose uptake [4, 5]. In addition, previous studies [6,7,8] reported that daily acute exercise is of greater clinical importance for long-term improvements in glycemic control compared to chronic exercise adaptations to different exercise modalities. However, there continues to be debate which exercise modality is the most effective and tolerable for T2DM patients. Specifically, both resistance [9] and moderate continuous training (MICT) [10] result in enhanced glucose uptake that persists for up to 48 hours [11]. Furthermore, recent studies [12, 13] suggest that higher glucose utilization and better glycemic control are strongly related to exercise intensity, meaning that traditional exercise programs may no longer be the “gold standard’’ for achieving better glycemic control. Unfortunately, within the field of investigation, several crucial questions remain unanswered and it is of great interest to focus on addressing these gaps.
First, despite numerous benefits, the majority of individuals with T2DM do not exercise regularly. Challenges are complex and numerous but the most common reason is the lack of time [14]. To address the lack of time and intensity issues, High-Intensity Interval Training (HIIT) and Sprint Interval Training (SIT) protocols have become enormously popular among T2DM population as time-efficient and effective solutions [15, 16]. The current literature on acute effects [17,18,19] suggests that the HIIT and SIT protocols are more effective than traditional exercise modalities for managing glycemic control with other clinically relevant benefits. Nevertheless, some authors [15, 20] voiced concern about the time efficiency of these interventions (up to 60 min), as well as the high perceived exertion responses and side effects associated with them. Consequently, poor adherence to these sessions is still a scientific and practical challenge. Finally, recent studies [21, 22] have provided concrete evidence that breaking the entire workout session into several shorter sessions throughout the day resulted in better glycemic control among patients T2DM. Specifically, Reynolds et al. [21] and Pahra et al. [22] examined the effects of three separated bouts compared to one continuous bout and concluded that accumulated exercise was more beneficial for blood glucose regulation. However, it remains unclear how breaking the HIIT and/or SIT protocols can affect glycemic responses and outcomes such as enjoyment and affect. Thus, there is a growing interest in investigating the health benefits of short-duration intense exercise interventions in individuals with T2DM [15, 23, 24].
Given that increasing the level of physical activity among the T2DM population requires time-efficient training protocols, that better glycemic responses strongly depend on the intensity of the sessions, and that exercise interventions accumulated in the multiple short and intense bouts spread throughout the day showed promising effects - the concept of “exercise snacking’’ has emerged as a potential modality to enhance physical activity among healthy and clinical populations. Briefly, “exercise snacking’’ involves brief, very intense bouts of exercise throughout the day, challenging the conventional idea of continuous, prolonged, and stressful sessions [25]. Furthermore, “exercise snacking’’ provides a time-effective alternative and induces lower perceived stress compared to HIIT, as demonstrated by Stork et al. [26]. Additionally, previous research [27] has established that “exercise snacking’’ ensures safety, and can be seamlessly incorporated into daily routines, making them more feasible for the TD2M population. To date, a few studies [12, 28, 29] found that “exercise snacking’’ can significantly improve several health aspects including cardiorespiratory fitness and body composition. Moreover, engaging in short bouts of intense exercise showed a 38%– 40% reduction in all-cause and cancer mortality risk and a 48%– 49% reduction in cardiovascular disease mortality risk [30]. However, equivocal results have been found regarding glycemic control [12, 31].
Thus, caution should be taken in interpreting the literature review regarding the efficacy of these modalities due to the study population and different methodological approaches. Moreover, there is a notable research gap in understanding the overall effects of different “exercise snacking’’ modalities on glycemic control in patients with T2DM. The type, intensity, and volume of these exercise bouts and how they acutely affect glycemic control are unknown and need further exploration. Therefore, the aim of this three-arm randomized crossover study is to examine the acute impact of different “exercise snacking” modalities in laboratory conditions on glycemic control in adults with T2DM. Further, we explore the effect of the “exercise snacking’’ on blood pressure, and mental health (i.e., enjoyment, and affect).
This study was approved by the Ethical Committee of the Faculty of Sport and Physical Education (ref. 04-1943/2; approval date: 5 December 2023), University of Niš, and will be conducted in accordance with the Declaration of Helsinki. Moreover, the study was registered on ClinicalTrials.gov (NCT06146036).
This study will be a randomized clinical trial with a crossover design. The protocol was written in accordanceith the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) guidelines. The complete copy of SPIRIT checklist is available in the supplementary material (additional file 1). Prior to the experimental protocols, participants will be asked to come to the laboratory on four different days, according to an individual schedule. During the first visit, the measurements will take place at the University Medical Centre (UMC) where an electrocardiographic stress test (ECG) will be performed to determine whether the participants can take part in the study. During the second, third and fourth visit, the measurements will take place in the laboratory of the Faculty of Sport and Physical Education. At the second, third and fourth visits, baseline testing will be conducted to examine anthropometric characteristics, cardiorespiratory fitness levels, and heart rate (HR) parameters, followed by the familiarization process. A detailed description of all assessments can be found in the pre-exercise interventions section.
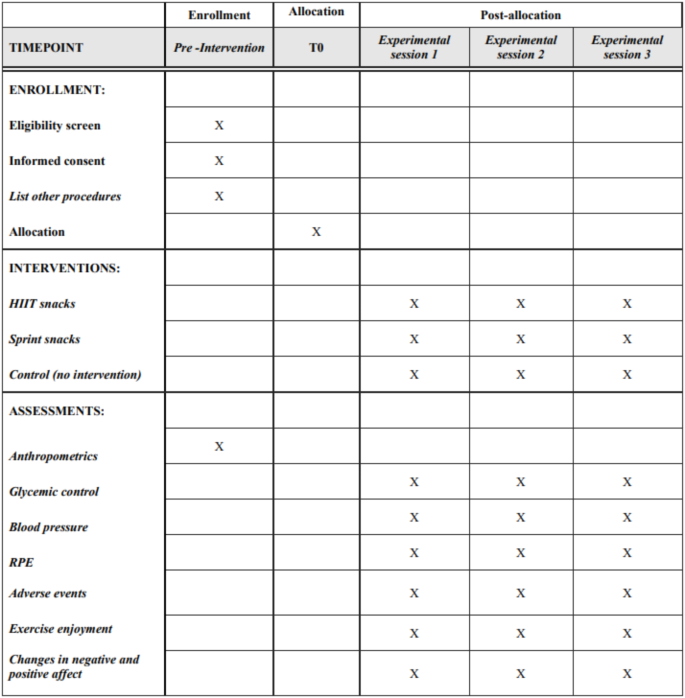
Regarding the main experimental protocol before each trial participants will be asked to avoid any strenuous exercise for at least 72 h. After 7 days of baseline assessment, participants will come to the laboratory. Each of these trials will be separated by at least 5–7 days. Blood pressure, as well as rate of perceived exertion (RPE), enjoyment, affect, and adverse events will be measured before and after each protocol using the standardized equipment, questionnaires and rating scales (Fig. 1). Moreover, HR will be measured continuously during each exercise session. A detailed description of all assessments as well as experimental protocols can be found in the exercise interventions and primary and secondary outcomes.
Standard protocol items: recommendations for interventional trials (SPIRIT). Legend: HIIT - high-intensity interval; RPE - rate of perceived exertion
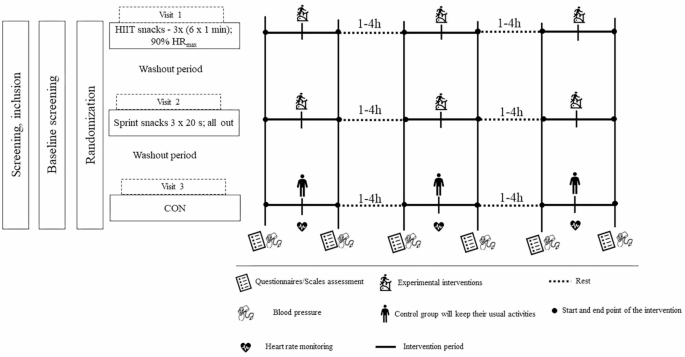
An open invitation will be sent to diabetic associations and to the UMC. Additionally, participants will be recruited through posters, social media, and word of mouth. Then, an initial meeting will be held to inform all interested participants about the study and future ideas. After the initial meeting, both a contact phone number and email addresses will be provided for the participants to contact the research team and obtain additional information. Participants who contact the research team will be able to ask questions regarding the study and if individuals decide to proceed, they need to provide basic information about themselves (age, height, weight and diabetes duration) and complete the Physical Activity Readiness Questionnaire-Plus (PAR-Q+). Participants will answer verbally, and each conversation will be recorded. Then, if the participant meets the eligibility criteria, we will invite them to an additional meeting where the research team will describe in detailed the study, objectives and safety of the participants. During this meeting, the participants will have to give their written approval for participation in the study. The complete graphical study design is shown in Fig. 2.
Graphical design of the study. Legend: HIIT - high-intensity interval training; HRmax– heart rate maximum; CON– control group;
The following inclusion criteria will be applied: (a) 18–45 year old participants (five males and five females) who have been diagnosed with T2DM at least 3 months prior to the start of the study according to standard criteria; (b) body mass index (BMI) between 25 and 35 kg/m2; (c) inactive participants according to the International Physical Activity Questionnaire (IPAQ) (< 150 min of moderate-intensity activities per week or < 75 min of vigorous-intensity activities per week or < 600 metabolic equivalent of task (MET) minutes per a week) [32]; (d) participants receiving medical therapy (no more than two medications) and not undergoing exogenous insulin therapy; (e) participants free of injuries and without any contraindications to exercise or chronic diseases that might be disruptive with vigorous activity; and (f) participants who are stable on their current diabetes medications, with no recent changes in their treatment regimen. The following exclusion criteria will be applied: (a) participants undergoing exogenous insulin therapy or/and receiving more than two glucose-lowering medicaments; (b) highly physically active participants according to IPAQ results (> 75 min per week of vigorous intensity activities achieving a minimum total physical activity at least 1500 MET minutes a week or ≥ 7 days of any combination of walking, moderate intensity or vigorous intensity activities achieving a minimum total physical activity of at least 3000 MET minutes a week); (c) participants suffering from any of the additional chronic diseases (renal diseases, liver diseases, neuropathy, cardiovascular diseases, uncontrolled hypertension [SBP > 160 mm Hg and/or DBP > 90 mm Hg after at least a 5-minute seated rest at the screening visit], history of respiratory disease including pulmonary hypertension or chronic obstructive pulmonary disease, history of musculoskeletal or neurological disorders and other metabolic diseases) [15]; (d) a significant resting and/or exercise ECG abnormality at the prescreening visit, which, in the opinion of the cardiologist exposes the participant to risk by taking part in the main trial; (e) participants who are strictly prohibited by medical professionals from engaging in vigorous exercise.
To determine the required sample size, an a priori power analysis using G*Power software (G*Power, Version 3.1, Heinrich-Heine University Düsseldorf, Germany) was used considering a crossover design with three groups. Sample size estimates were based on previous studies [7, 15, 33] with a similar design and the same primary outcome (24– h mean blood glucose). Therefore, a sample size of 10 participants was deemed sufficient to detect a medium effect size (η² = 0.06–0.13) with a statistical power of 0.80 (β = 0.80) and an alpha level of 0.05 (α = 0.05). This medium effect size on 24– h mean blood glucose concentration is further supported by findings from previous meta– analysis [34] and given that exercise has potent insulin-sensitizing and glucose-lowering effects in individuals with T2DM.
The randomization process will be performed through www.randomization.com. The order of the participants will be revealed upon of prescreening and baseline assessments.
Participants can withdraw from the research at any time without having to give reasons. However, it would be preferable to explain why because of the follow-up data. Participants can also withdraw before the start of the protocol if the cardiologist decides that performing intensive exercise based on ECG screening is dangerous. Finally, a doctor or the principal investigator may withdraw participants at any time for health reasons and terminate the exercise sessions.
Prior to the experimental protocols, participants will be asked to come to the laboratory on four different days, according to their individual schedules. At the first visit, an ECG test will be performed to determine whether participants can take part in the study. The test will be conducted, monitored and reviewed by a cardiologist. Written consent will be required for further inclusion in the study. Moreover, during the first visit, a flash glucose monitoring (fGM) device, FreeStyle, Libre 2 (Abbott Diabetes Care Inc, USA) will be inserted on the participant’s nondominant upper-arm (m. triceps) according to manual guidelines. The accuracy of the fGM devices will be validated using capillary vs. interstitial fGM measurements. Capillary measurements (Accu-Check; Roche Diagnostics, Basel, Switzerland) will be taken by each participant before each main meal and before sleep. During the second visit, participants will be tested for anthropometric characteristics (i.e., body height and body composition) and will undergo a maximal incremental test on cycle ergometer. Briefly, body height will be measured using a portable stadiometer (Seca 220; Seca Corporation, Hamburg, Germany) with a graduation of 0.1 m while body composition will be examined using an InBody 770 (InBody Co., Ltd., Cerritos, CA, USA). Body composition will be assessed in a fasted state and the testing will be conducted in the morning hours (from 8.00 a.m. to 10.00 a.m.). Following a light breakfast, which will adhere to the guidelines outlined in the 2008 American Diabetes Association (ADA) dietary recommendations for T2DM [35], the maximal incremental test on the cycle ergometer (Monark 894E Pike Bike, Sweden) will be conducted to assess maximal aerobic capacity at peak exercise (VO₂peak). Additionally, HR will be monitored continuously throughout the test using the Polar Team System H7 (Polar Electro Oy, Kempele, Finland), while HRmax will be utilized as a parameter to define the intensity of the exercise session which will not be performed in an all-effort manner. Participants will be also familiarized with wearing the HR monitors and using the RPE. Briefly, after a 5-minute warm-up at 50 W, the intensity will be increased by 15 W/min until the tempo could no longer be maintained at 50 rpm. Cardiorespiratory fitness will be measured using a portable gas analysis system (Quark b2, COSMED, Italy), VO2peak will be determined as the highest ten-breath rolling average and accepted if two or more of the following criteria were met: (1) voluntary exhaustion, (2) a plateau in VO2 despite increasing intensity; (3) HRmax within 10 beats of the age-predicted maximum. This protocol has already been used in patients with T2DM [15]. During the third visit and fourth visit, participants will be invited to attend to familiarization sessions to establish their capacity to execute both experimental protocols at prescribed intensity.
The diet will not be controlled, as the study will be based on participants’ everyday life habits. This study will exclusively focus on the effects of two “exercise snacking” modalities on acute glycemic responses. Furthermore, to minimize the risk of hypoglycemia, capillary blood glucose levels will be measured before and after each exercise session. Participants will consume carbohydrates (30 g of raisins or juice) if their blood glucose level prior to the session is < 5.6 mmol/L. However, if blood glucose exceeds 13.9 mmol/L, the session will be canceled [8]. Furthermore, if the blood glucose level at the end of the session is < 3.9 mmol/L, participants will consume 15 g of carbohydrates, and glucose levels will be checked again after 20 min. Finally, if blood glucose levels remain unchanged after 20 min, participants will consume an additional 15 g of carbohydrates [8].
The HIIT protocol utilized in this study met the criteria of a Guideline for Reporting HIIT protocols (AGReHIIT) [36].
After a short-term warm-up on the cycle ergometer (5 min, the intensity will be determined by the participants themselves), short-term, intense bouts of cycling 6 × 1 min at 90% HRmax with a minute of active break between bouts will be performed (at 50 W). Recovery will last 3 min, also at the self-paced intensity. The whole process will be repeated two more times during the day with intervals of 1–4 h. We will utilize the identical exercise protocol implemented by Francois et al. [12].
For the Sprint snacking (Ss) group training will consist of three separate sprints on the cycle ergometer separated by 1–4 h. The Ss exercise bouts will consist of a 2-minute warm-up followed by a 20-s sprint and then a 1-minute cooldown (three separate workout sessions each lasting 3 min and 20 s). The warm-up, cooldown, and recovery periods will all be performed at 50 W. Each sprint will be performed at a resistance of 0.21 N m/kg and include a 10-s period when participants will be advised to accelerate the number of rpm as fast as possible. This study will replicate the protocol established by Little et al. [28].
During CON, participants will come to the laboratory and remain sedentary throughout the entire period. Additionally, participants will be permitted to engage in reading or solving sudoku puzzles to minimize potential confounding stimuli that could influence blood glucose levels.
Glycemic control
Glycemic control will be assessed using the methodology described by Metcalfe et al. [15]. Glycemic parameters: The 24– h mean blood glucose (mmol/L), time spent in hyperglycemia (percent (%) of the day spent above 10 mmol/L), incremental area under the curve (AUC) and glycemic variability will be monitored using fGM for each 24– h period (baseline day, exercise day, and day following exercise interventions). More precisely, time spent in hyperglycemia will be measured by the percentage time of the day spent above 10 mmol/L; AUC will be determined from the fGM data three hours following each main meal. AUC will be calculated using trapezoidal model. Postprandial blood glucose will be defined as mean blood glucose levels measured 180 min after breakfast, lunch and dinner (3 h– postprandial blood glucose). Finally glycemic variability will be determined by Excel Macro (Easy GV 9.0; available at http://www.easygv.co.uk). The mean amplitude of glycemic excursions (MAGE) and standard deviation (SD) from the mean glucose [37] will be used for this purpose.
Blood pressure
Both systolic (SBP) and diastolic blood pressure (DBP) will be measured before and after exercise sessions using an automated device (Omron HEM-7130®). Briefly, participants will be in a seated position and the bracelet will be attached around the participant’s left arm, above the elbow, and in alignment with the brachial artery.
Rate of perceived exertion (RPE)
RPE will be examined using the Borg Scale (0–10 scale) with 1– being very light and 10 being– maximum effort [38]. The RPE will be examined before and after each exercise session during the main experimental period.
Adverse events
Adverse events will be evaluated and classified into four different categories: (1) minor and temporary, (2) serious symptoms (potential risk of severe injury or life threatening, 3) manifest injury or disease and 4) death. The adverse event rate will be calculated for each participant as the total number of sessions during which any adverse events occurred divided by the total number of attended sessions.
Mental health: exercise enjoyment
The Physical Activity Enjoyment Scale (PACES) will be used to determine the perceived enjoyment after each exercise session. The PACES is an 18-item physical activity/exercise enjoyment scale in which participants are asked to rate “how you feel at the moment about the physical activity/exercise you have been doing” on a 7-point bipolar Likert scale with a total score of 126. Higher scores indicate greater levels of exercise enjoyment [39]. The PACES has been widely used in patients with T2DM [40, 41]. Exercise enjoyment will be assessed immediately after each exercise session during the main experimental period.
Mental health: positive and negative affect
Positive and negative affect will be measured using the Positive and Negative Affect Scale (PANAS). The PANAS consists of 20 items, with 10 items measuring positive affect and 10 items measuring negative affect on a 5-point Likert scale (1 = not at all, 2 = a little, 3 = moderately, 4 = quite a bit, 5 = extremely) [42]. Positive and negative items are summed separately and both items range from 10 to 50. The total score is calculated by their sum. Higher scores in any of the positive and negative scales indicate higher levels of positive or negative affect, respectively. PANAS has been already employed in patients with T2DM [43]. Affect will be assessed before and after each exercise session during the main experimental period.
Statistical analysis will be performed using the IBM SPSS statistics program (version 26.0; Inc., Chicago, IL, USA). The mean ± standard deviation, Kolmogorov-Smirnov (normality of the distribution), and Levene’s (homogeneity of variance) tests will be determined for all outcome measures. Moreover, one-way repeated measures analysis of variance (ANOVA) will be performed to compare trial effects, and Bonferroni pairwise comparisons will be performed if any differences are identified. Sphericity will be analyzed with Mauchly’s test of sphericity followed by the Greenhouse–Geisser adjustment where required. In accordance with previous study [44] ordinal scale data will be analyzed by parametric statistical analysis. The significance level will be set at p < 0.05. Finally, effect sizes will be calculated using partial eta squared (η2) and reported as follows: small 0.01–0.05, medium 0.06–0.13, and large > 0.14 [45].
To the best of our knowledge, there are no comparative studies on patients diagnosed with T2DM. Therefore, we will conduct this study to determine the acute effects of two modalities of “exercise snacking’’ with different intensities and volumes with a particular emphasis on glycemic control. Moreover, this study will also examine blood pressure, individual responses to RPE, adverse events, exercise enjoyment and affect which is crucial for implementing protocols that contains intense exercise.
“Exercise snacking’’ is a relatively new exercise modality with a great potential among sedentary clinical population. The advantages of this form of exercise are demonstrated by the fact that the exercise can be conducted outside of the laboratory without equipment and/or supervision. Although “exercise snacking” can chronically affect cardiometabolic health [12, 28, 29], little is known about acute effects. Indeed, understanding the immediate impact of these short, high-intensity bouts on glycemic control is crucial for people with T2DM especially when taking into account that individuals with T2DM exhibit significant intraday fluctuations in glucose levels, raising concerns about the safety and feasibility of certain exercise interventions. Existing studies [12, 31, 46] investigating acute effects of new developed modality of exercise on glycemic control are limited and have provided contradictory findings. More precisely, Rafiei et al. [46] showed that intense bouts consisted of 15–30 s stair “climbing snacks’’ once per hour are effective for lowering insulin across the day in overweight and obese individuals. These findings are congruent with work of Francois et al. [12] which found reduced postprandial blood glucose levels and subsequent 24– h mean blood glucose concentrations after two different “exercise snacking’’ modalities in individuals with insulin resistance. Conversely, Godkin et al. [31] that 3 × 1-minute bouts of stairs climbing did not affect changes in glycemic control (24– h mean blood glucose levels, postprandial blood glucose levels and time spent in hyperglycemia) among T2DM population. However, the different study designs, intervention characteristics (i.e., activity, duration, and intensity) and populations assessed within studies make it difficult to compare the results between studies. Despite the limited and contradictory findings regarding glycemic control, it is noteworthy to highlight several mechanisms that may explain how “exercise snacking” has the potential to acutely improve glycemic control. Specifically, these mechanisms include increased muscle glycogen utilization and the activation of insulin-related signaling pathways within skeletal muscle, which ultimately enhances insulin sensitivity [47]. Furthermore, these short but intense exercise bouts can result in a 20–30% reduction in muscle glycogen levels and contribute to a decrease in blood glucose and a reduction in insulin resistance [48]. To induce these significant changes, it is crucial for participants to maintain a high-intensity effort during the exercise, aligning with the principles of “exercise snacking’’[49]. Finally, brief but intense exercise bouts activate intracellular signaling pathways associated with the translocation of glucose transporter type 4 (GLUT4), including mitogen-activated protein kinase (MAPK), AMP-activated protein kinase (AMPK), and calcium/calmodulin-dependent protein kinase II (CaMK II) [50]. These pathways collectively may contribute to the temporary hypoglycemic effects.
Furthermore, existing research on “exercise snacking” has not investigated psychological and affective responses, especially within clinical population. These facts are supported by the work Islam et al. [25] which highlighted patient acceptance as a crucial factor for wider adoption of “exercise snacking” approach. To address this gap, our study will specifically examine these crucial psychological and affective aspects within a clinical context, providing valuable insights into the feasibility of “exercise snacking’’. Thus, it is necessary to determine how the different exercise intensity, number of bouts and specific activity affect the intended effects. Finally, this study could provide a strong and promising clinical basis for the future use of “exercise snacking’’ modalities among patients with T2DM and prescribed guidelines. Additionally, it would be interesting to examine how these “exercise snacks’’ affect glycemic control before or/and after meals and other cardiometabolic outcomes in sedentary patients with T2DM. Therefore, our results will lead to potential new questions and new ideas for randomized and follow-up studies in patients with T2DM.
Data will be accessible upon request to the corresponding author.
- T2DM:
-
Type 2 diabetes mellitus
- RPE:
-
Rate of perceived excretion
- HIIT:
-
High intensity interval training
- SIT:
-
Sprint interval training
- MICT:
-
Moderate continuous training
- VO2peak :
-
Peak oxygen uptake
- HR:
-
Heart rate
- HRmax :
-
Maximal heart rate
- BMI:
-
Body mass index
- Ss:
-
Sprint snacks
- fGM:
-
Flash glucose monitoring system
- PACES:
-
The physical activity enjoyment scale
- PANAS:
-
Positive and negative affect scale
- IPAQ:
-
International physical activity questionnaire
- PARQ+:
-
Physical activity readiness questionnaire
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
This project has been supported by the Serbian Ministry of Education, Science and Technological Development. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
This randomized controlled crossover study was approved by the Ethical Committee of the Faculty of Sport and Physical Education (ref. 04-1943/2; approval date: 5 December 2023), University of Niš and it will be conducted in accordance with the Declaration of Helsinki. Moreover, the study was registered on ClinicalTrials.gov (NCT06146036). Informed consent will be obtained from all participants and/or their legal guardian(s).
Written informed consent will obtained from the participants for publication of their individual details. The document is available upon request to the corresponding author.
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Lazić, A., Danković, G., Korobeinikov, G. et al. Acute effects of different “exercise snacking’’ modalities on glycemic control in patients with type 2 diabetes mellitus (T2DM): study protocol for a randomized controlled trial. BMC Public Health 25, 566 (2025). https://doi.org/10.1186/s12889-025-21669-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-025-21669-9