Journal of Translational Medicine volume 23, Article number: 791 (2025) Cite this article
AbstractSection Background
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a critical therapeutic strategy for acute myeloid leukemia (AML). However, relapse remains a major challenge, with limited effective options for managing post-transplant recurrence. Tumor cells escape immune surveillance by establishing a complex, immunosuppressive tumor microenvironment (TME), which plays a central role in immune evasion and disease relapse. Understanding the TME’s impact on immune cells and their functional alterations is therefore of significant clinical importance.
AbstractSection Methods
Utilizing a non-irradiated AML mouse model and bioinformatic analysis, we systematically analyzed T-cell dynamics during AML progression and identified abnormal metabolic activation in CD8+ T cells at early disease stages, which correlated with leukemia cell immune evasion.
AbstractSection Result
We found that when major histocompatibility complex (MHC) molecules on AML cells were haplomatched with immune cells, CD8+ T cells exhibited robust cytokine and cytotoxic activation, retaining immune profiles similar to those of normal T cells. However, under the same tumor burden, T cells, particularly CD8+ T cells, showed dramatic metabolic gene activation in MHC-matched leukemic mice. This metabolic activation led to rapid exhaustion of CD8+ T cells, accelerating leukemia progression. Based on these activated metabolic genes, we established a metabolism-associated genes (MAGs) scoring system. Validation using public datasets revealed that a high MAG score is associated with poor prognosis in AML patients.
AbstractSection Conclusions
Our findings provide molecular evidence supporting the stronger graft-versus-leukemia effects observed in HLA haploidentical HSCT. Moreover, they highlight that abnormal metabolic activation in CD8+ T cells during early AML progression contributes to rapid disease advancement and poor prognosis.
Acute myeloid leukemia (AML) is a heterogeneous hematopoietic malignancy that represents a significant threat to human health [1]. Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a well-established and effective treatment for AML [2, 3]. In recent years, haploidentical stem cell transplantation (haplo-SCT) has achieved outcomes comparable to those of human leukocyte antigen (HLA)-matched sibling donor transplantation (MSDT). Notably, in high-risk AML, haplo-SCT recipients exhibit lower relapse rates, suggesting stronger graft-versus-leukemia (GVL) effects of haplo-SCT [4,5,6]. Previous studies have provided cellular-level evidence in haplo-SCT models, showing that when the MHC molecules on T cells were haplomatched with leukemia cells, immune cells exhibit enhanced proliferation and stronger cytotoxicity [7, 8]. However, the molecular characteristics and underlying mechanisms of this phenomenon remain unclear.
Relapse remains a major challenge in AML therapy, with limited treatment options for patients who relapse post-allo-HSCT. Immune escape is the primary cause of relapse [9, 10]. While the immunosuppressive tumor microenvironment (TME) has been extensively studied in solid tumors, research on the AML TME is still scarce [11, 12]. The clinical application of immune checkpoint inhibitors, which modulate T cell activity within the TME, has shown limited efficacy in AML [13, 14]. With advancements in multiomics technologies, the AML TME has been shown to exhibit an inflammatory signature, leading to immune dysfunction, including exhaustion and senescence. This contributes to poor treatment response and prognosis in AML patients [15,16,17,18,19]. However, due to the complexity of clinical settings, distinguishing the causal relationship between leukemia progression and immune dysfunction remains challenging. Moreover, most current studies utilize sequencing data from patient bone marrow (BM) and peripheral blood (PB) samples [18, 20]. Due to the difficulty of sampling, immune cell dynamics in secondary lymphoid organs remain largely unexplored. Unlike solid tumors, which remain localized with a confined TME, AML cells circulate through the bloodstream, influencing immune interactions across various tissues. The immune profiling dynamics in peripheral lymphoid organs, as well as the differences in immune signatures between PB and lymphoid organs, remain poorly understood.
Metabolism plays a crucial role in T cell activation by transmitting activation signals, regulating immune differentiation pathways, and influencing epigenetic remodeling, which ultimately determines the outcome of adaptive immune responses [21]. Naïve T cells exhibit a low-rate catabolic metabolism, primarily generating ATP through mitochondrial oxidative phosphorylation (OXPHOS) and fatty acid oxidation. Upon activation, T cells undergo metabolic reprogramming, shifting to aerobic glycolysis while maintaining OXPHOS to meet their increased energy demands [22, 23]. Tumor cells impose metabolic restrictions on T cells by depleting glucose, producing lactate, and releasing other immunosuppressive byproducts [14, 24]. Compared to the remission state, T cells isolated from AML patients relapsing after allo-HSCT exhibit reduced glycolytic activity and impaired oxidative phosphorylation [25]. Moreover, AML with isocitrate dehydrogenase 1 (IDH1) mutations significantly alters glycolysis in CD8+ T cells, and these metabolic changes correlate with poor prognosis [26, 27]. However, other studies indicate that AML progenitor cells actively shape the TME, enhancing the expression of metabolic genes in immune cells [28]. These findings suggest that the dynamics of metabolic programs during AML progression remain complex and controversial.
Our previous work established a non-irradiated AML mouse model, where haplomatching of the major histocompatibility complex (MHC) between leukemia cells and recipient T cells resulted in an indolent tumor progression pattern [7]. This model not only serves as a platform for investigating the molecular mechanisms underlying the stronger GVL effects observed in the haplomatched group, but also allows exploration of how the TME influences tumor development under the same tumor burden but in different progression contexts. In this study, we collected RNA sequencing (RNA-seq) data from CD4+ and CD8+ T cells isolated from PB and spleen (SP) in both MHC-matched and haplomatched leukemic models, to dissect the immune profile changes during AML progression. We observed significant immune alterations even at a low tumor burden in the PB, with these changes being more pronounced in SP. Notably, CD8+ T cells exhibited the most prominent alterations. In the haplomatched group, T cells at the early stage of AML progression remained in a state similar to that of healthy mice. However, in the MHC-matched group, CD8+ T cells from both PB and SP displayed an abnormally high metabolic state. RNA-seq data from different AML stages further confirmed that the heightened metabolic characteristics of CD8+ T cells could serve as predictive markers for AML progression. Based on the abnormally activated metabolic pathways associated with leukemia progression, we established a metabolism-associated genes (MAGs) scoring system. Validation using public datasets confirmed that elevated expression of these MAGs correlates with poor prognosis in AML patients.
C57BL/6 (H-2b) and CB6F1 (H-2b/d) mice (8–10 weeks old) were purchased from Beijing Vital Laboratory Animal Technology Company, Ltd. (Beijing, China). C57BL/6 (H-2b) mice were purchased from Peking University Health Science Center Department of Laboratory Animal Science (Beijing, China). All mice were maintained in the specific pathogen-free animal facility of Peking University People’s Hospital. All experiments were performed according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
Staining of surface markers, was performed as previously described [7]. Briefly, surface staining was carried out at 4 °C for 20 min using directly-conjugated monoclonal antibodies. After incubation, the cells were washed and resuspended in phosphate buffered saline before flow cytometry analysis. Detailed information on the antibodies used is presented in Supplemental Table 6.
RNA was extracted using the RNeasy Micro Kit (QIAGEN, 74004) and purified by NEBNext Oligo d(T)25 beads (NEB, E7490). An NEBNext Ultra II RNA Library Prep Kit (NEB, E7770S) was used to generate cDNA libraries. AMPure XP beads (Beckman Coulter, A63881) were used to purify cDNA libraries between 300 and 500 bp. Paired-end sequencing was performed on a NovaSeq6000 (Illumina) and produced between 28 and 35 million 150-bp paired-end reads per sample. Raw RNA-seq FASTQ files were processed according to a previously described method [29]. Low-quality reads and adapter sequences were cleaned up, and the remaining reads were quantified against the GRCh38 reference at the transcript level using Hisat2 software and gene expression was quantified by StringTie.
Differential analysis was performed using the DESeq2 R package (v.1.34.0). The definitions of DEGs at different stages are indicated in both the results and figures. GO, KEGG and GSEA were conducted using the clusterProfiler R package (v.4.12.6). The ggplot2 R package (v.3.5.1) was used for plot customization.
The BMMC scRNA-seq data was downloaded from the GEO database GSE223844 [30]. The clinical information of selected CR and NR patients was summarized in Supplemental Table 4. Raw gene expression matrices were imported and processed using the “Seurat” package (v.5.1.0) in R software (2024.09.1 + 394), with following the standard pre-processing workflow. Quality control was performed to filter low-quality cells with the criterion: nFeature_RNA < 4000 & nFeature_RNA > 200 & nCount_RNA < 25,000 & percent.mt < 20. Reference mapping was performed using the bmcite dataset. Batch effect correction was performed using the Harmony R package (v.1.2.1). The AUCell MAG scores were calculated by “AUCell” R package (v.1.26.0).
Data on AML (PBMC/BMMC) were downloaded from The Cancer Genome Atlas (TCGA) database, and 132 AML cases with complete mRNA expression profiles and survival data were obtained from the Xena website (https://xenabrowser.net/datapages/). The expression counts of MAGs were then processed using DESeq2, followed by log2 transformation and z-score normalization. The MAG scores were calculated by summing the values of the MAGs. Patients with MAG scores higher than the median were classified as the high group, while those with MAG scores lower than the median were classified as the low group. Kaplan-Meier curves were analyzed using the survival (v.3.7.0) and survminer (v.0.4.9) R packages.
All the results are shown as mean ± SEM. Student’s t test was used for two groups analyses. One-way analysis of variance (ANOVA) is used to compare the means of more than two groups. When significant differences were detected (p < 0.05), post-hoc analysis was performed using Tukey’s Honest Significant Difference test to identify which specific groups differed from each other. The absence of P values in the graphs indicates that there is no statistic difference between groups. Statistical analyses were performed on GraphPad 10.0 and R software (2024.09.1 + 394). Survival curves were generated by the Kaplan–Meier method and compared using the log-rank test.
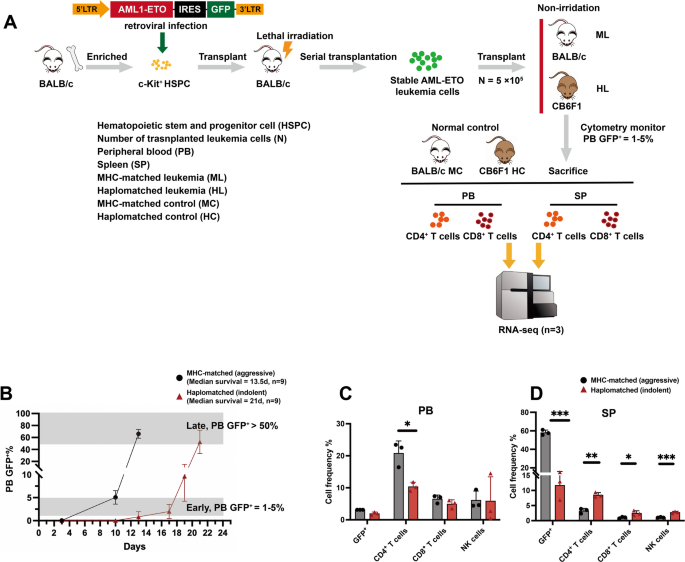
Recent clinical studies have demonstrated that haplo-SCT exhibits stronger GVL effects compared to MSDT [31,32,33]. The enhanced GVL effects and improved survival outcomes of haplo-SCT over MSDT have been preliminarily confirmed through both clinical cohort studies and basic research [8, 34, 35]. However, the immune profile changes and mechanisms underlying the enhanced GVL effects driven by MHC molecule differences in haplo-SCT remain to be further elucidated. To investigate these mechanisms, a non-irradiated AML-ETO leukemia mouse model was established by transferring the humanized AML-ETO fusion gene into BALB/c mice [36]. In this model, an MHC-matched group was generated by transplanting leukemia cells (H-2d) into BALB/c mice (H-2d), while the haplo-SCT group involved transplanting leukemia cells (H-2d) into CB6F1 (H-2b/d) mice (transplanted dose = 5 × 105) (Fig. 1A). This AML transplantation model offers a platform to observe the changes of immune cells during AML progression at different stages, without the confounding effects of graft-versus-host disease (GVHD).
Different progression patterns of AML and immune characteristics between the haplomatched and MHC-matched groups. (A) Graphic overview of the experimental settings. AML-ETO-GFP+ leukemia cells were generated by retroviral infection of the AML-ETO-GFP fusion gene into c-Kit+ hematopoietic stem and progenitor cells (HSPCs). These leukemia cells (5 × 10⁵ per mouse) were then transplanted into recipient mice. The haplomatched group exhibited significantly prolonged survivals compared to the MHC-matched group. When the peripheral tumor burden (TB) reached 1–5%, PB and SP CD4/CD8+ T cells were isolated for RNA-seq analysis. PB and SP CD4/CD8+ T cells from normal mice with corresponding immune backgrounds were also isolated and analyzed as controls (n = 3 per group). (B) Dynamics of AML-ETO-GFP+ leukemia cells in MHC-matched and haplomatched recipient mice after transplantation (n = 9 per group). (C) Flow cytometry analysis of the percentage of GFP+ cells and immune cell subsets in PB at a TB of 1–5% (n = 3 per group). (D) Flow cytometry analysis of the percentage of GFP+ cells and immune cell subsets in SP at a TB of 1–5% (n = 3 per group)
By monitoring the dynamics of AML cells (GFP+) after transplantation, we observed that the delayed leukemia progression in haplo-matched mice is primarily due to a prolonged period in which leukemia cells are detectable (1–5% GFP⁺) in peripheral blood (Fig. 1B). Moreover, the time interval required for leukemia cells to expand from 1 to 5% to over 50% is comparable between MHC-matched (days 10–13) and haplo-matched (days 17–21) mice. Therefore, analyzing the immune landscape at the early stage (1–5% GFP⁺ in PB) may provide important insights into the enhanced GVL effects observed in haplo-matched mice. Therefore, we collected CD4⁺ and CD8⁺ T cells from peripheral blood and spleen at the early stage and performed RNA-seq to define immune signatures (Fig. 1A). Flow cytometry analysis of PB and SP immune cell populations revealed that, despite having similar tumor burden (TB) in the PB at the early stage of leukemia progression, AML tumor cell infiltration in the SP was significantly higher in the MHC-matched group compared to the haplomatched group. Immune cell composition in the SP also exhibited notable differences, with increased ratio of CD3+CD4+ T cells, CD3+CD8+ T cells, and CD3−CD49b+ NK cells in the haplomatched group (Fig. 1C-D). This observation aligns with survival analysis [7], which showed that AML progression in the haplomatched group followed an indolent pattern, whereas the MHC-matched group exhibited more aggressive disease progression.
Overall, immune cell subpopulation changes in PB and SP suggest that MHC differences result in distinct immune profiles and AML progression patterns across different transplant models. Specifically, the MHC-matched group showed rapid, aggressive disease progression, while the haplo-SCT group exhibited an indolent progression pattern with significantly prolonged survival.
Compared to PB, the local TME exhibits significant spatiotemporal heterogeneity. The tumor ecosystem is composed of diverse cell types, forming a heterogeneous but highly organized community [37,38,39]. CD8+ T cells play a central role as effectors in anti-tumor immunity, and their characteristics show marked variations depending on the local microenvironment. Modulation of the TME has led to significant advances in immune therapies, particularly in restoring T cell-mediated immunity for tumor eradication [40, 41]. Furthermore, immune responses, particularly those targeting tumors, require intricate coordination among various cell types across distinct tissue compartment [42]. Understanding the site-specific characteristics of these immune responses is essential for elucidating tumor progression and the impact of tumor growth on local immune environments.
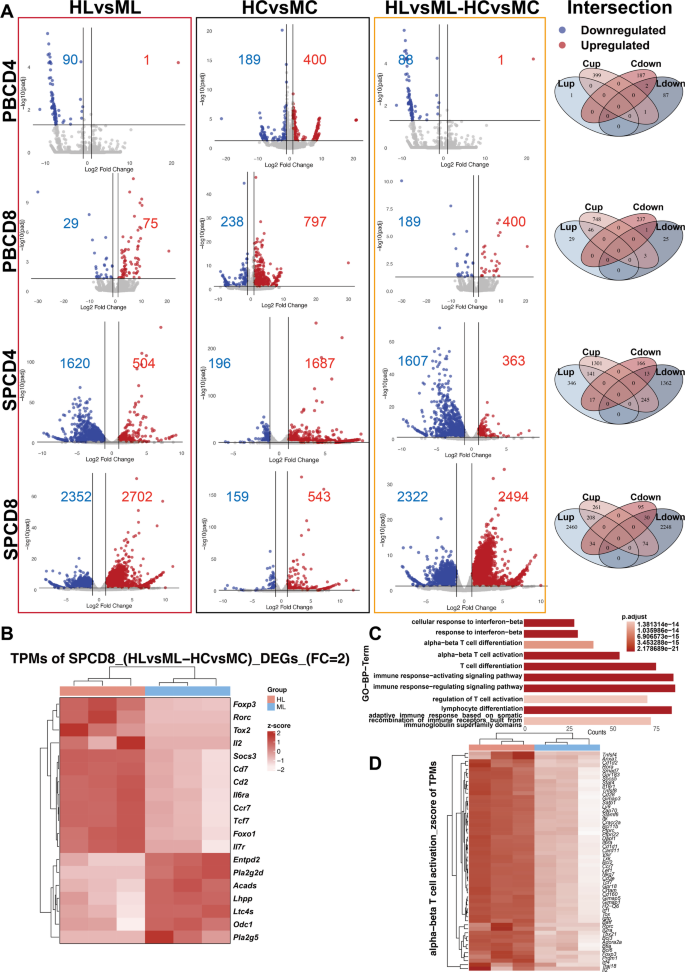
By monitoring the dynamics of AML cells and immune populations in both PB and SP, we observed significant differences in immune cell composition and leukemic cell distribution (Fig. 1C-D), suggesting distinct immune responses between circulating and lymphoid tissues. Therefore, we collected CD4+ and CD8+ T cells from both PB and SP of haplomatched and MHC-matched groups during the early stage of AML (when GFP+ cells in PB reached 1–5%) and performed RNA-seq analysis (Fig. 1A). As controls for baseline immune profiles, CD4+ and CD8+ T cells from the PB and SP of healthy BALB/c and CB6F1 mice were also sorted and subjected to RNA-seq. Investigating the contribution of MHC compatibility to differential GVL effects, differential analyses of PB and SP CD4+ and CD8+ T cells were performed between the haplomatched leukemia (HL) group and the MHC-matched leukemia (ML) group, as well as between the haplomatched control (HC) group and the MHC-matched control (MC) group. Genes with Log2FoldChange > 1 and adjusted p-value < 0.05 were considered differentially expressed genes (DEGs). The results showed that the number of DEGs in DEGs_PBCD4+ HLvsML and DEGs_PBCD8+ HLvsML was significantly lower than in DEGs_SPCD4+ HLvsML and DEGs_SPCD8+ HLvsML (Fig. 2A), indicating that the SP experienced more pronounced changes in immune profiles compared to the PB.
Distinct T cell activation features identified in the haplo group. (A) Volcano plot and Venn diagram comparing differential expression across groups. Differentially expressed genes (DEGs) with similar expression trends in HLvsML and HCvsMC were excluded, resulting in the final HLvsML-HCvsMC comparison. (B) Heatmap displaying selected T cell function-related genes upregulated in the HL group and genes significantly upregulated in the ML group (SPCD8_(HLvsML-HCvsMC)_DEGs, logFC > 2 and adjusted p-value < 0.05). (C) GO-BP results of SPCD8_DEGs (logFC > 2 and adjusted p-value < 0.05) enriched in haplo group. (D) Heatmap of SPCD8_DEGs in HLvsML-HCvsMC related to the “alpha-beta T cell activation” gene set
To eliminate potential confounding factors from differences in immunological backgrounds of the mouse strains, intersection DEGs showing consistent trends in both the HLvsML and HCvsMC groups were excluded. The remaining genes (DEGs_HLvsML-HCvsMC) were used for further analysis. Venn diagrams illustrating the intersections of DEGs are shown (Fig. 2A). The final results indicated that DEGs_SPCD8+ HLvsML-HCvsMC displayed the most significant differences, with 2494 upregulated genes and 2322 downregulated genes. When a stricter threshold (Log2FoldChange > 2 and adjusted p-value < 0.05) was applied, 1500 upregulated genes and 882 downregulated genes were identified. These findings suggest that, compared to PB T cells, SP T cells, especially CD8+ T cells, exhibit more differential gene expression and more pronounced changes in immune phenotypes.
A heatmap of key genes related to T cell function including Cd2, Cd7, Il2, Socs3, Foxp3, Tox2, Rorc, Tcf7, Ccr7, Il7r, Foxo1, and Il6ra, as well as other significantly upregulated genes in the ML group is shown (DEGs_SPCD8+ HLvsML-HCvsMC) (Fig. 2B). Gene Ontology (GO) Biological Process analysis of the upregulated genes in DEGs_SPCD8+ HLvsML-HCvsMC revealed significant enrichment in pathways related to T cell differentiation, activation, and immune response regulation (Fig. 2C). These findings, in addition to the altered percentage of immune cells, further elucidate the enhanced GVL effects in the haplomatched group. Notably, T cell activation related genes were significantly upregulated in haplomatched leukemia mice compared to MHC-matched leukemia mice (Fig. 2D). In summary, significant changes in SP CD8+ T cells were observed during the early stages of post-transplant AML progression. These changes were marked by activation in both differentiation and functional pathways, providing explanations for the stronger GVL effects of haplomatched group.
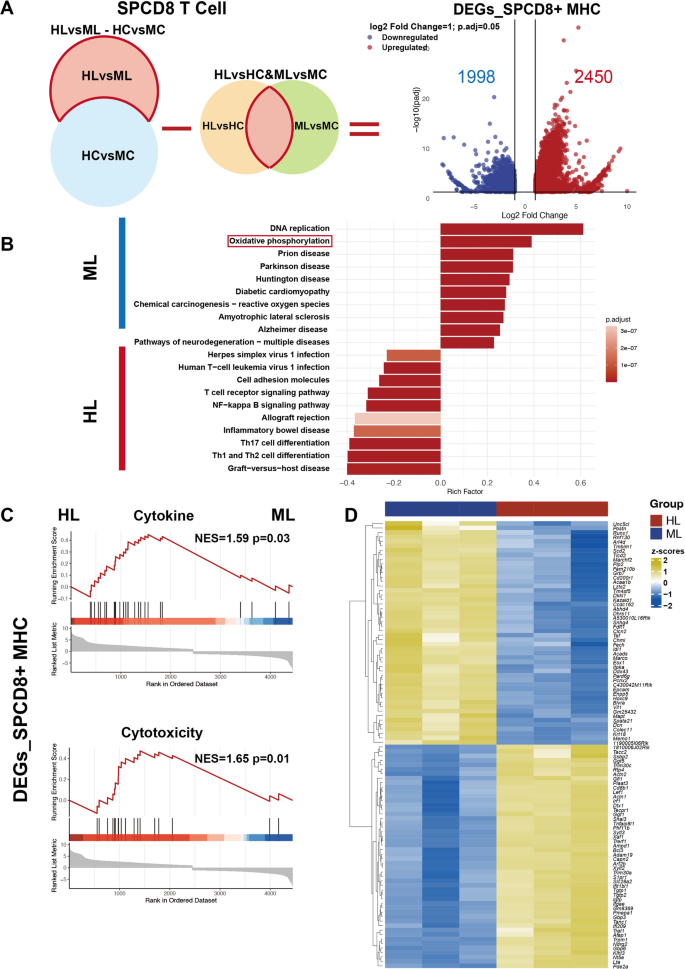
The pathological state induced by AML tumor cells leads to differential expression of immune profiles. To exclude potential interference caused by DEGs solely driven by differences in AML progression between MHC-matched and haplomatched leukemic mice, and to focus on DEGs arising from differences in MHC molecules under AML conditions, the overlapping genes between DEGs_SPCD8+ HLvsHC and DEGs_SPCD8+ MLvsMC were removed from the DEGs_SPCD8+ HLvsML-HCvsMC. This resulted in the final list of DEGs_SPCD8+ HLvsML-HCvsMC-[(HLvsHC)∩(MLvsMC)] (DEGs_SPCD8+ MHC), which included 2450 upregulated genes and 1998 downregulated genes (Fig. 3A). Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis of DEGs_SPCD8+ MHC revealed that pathways related to cell adhesion molecules, T cell differentiation, and inflammation were upregulated in the haplomatched group (Fig. 3B).
Enhanced cytokine production and cytotoxicity in SPCD8+ T cells from the haplomatched group. (A) Schematic overview of the workflow and volcano plot for identifying DEGs_SPCD8+ MHC. Genes overlapping between DEGs_SPCD8+ HLvsHC and DEGs_SPCD8+ MLvsMC were excluded, resulting in 2450 upregulated and 1998 downregulated genes in DEGs_SPCD8+ MHC. (B) KEGG pathway enrichment analysis of DEGs_SPCD8+ MHC. Top significantly enriched pathways are shown. (C) GSEA analysis of cytokine production and cytotoxicity-related gene sets on DEGs_SPCD8+ MHC. (D) Heatmap displaying normalized TPM values for the top 50 significant DEGs_SPCD8+ MHC in HL and ML subgroups, ranked by significance (adjusted p-value)
To further investigate the enhanced GVL effects observed in haplomatched group, we analyzed gene sets related to CD8+ T cell cytokines and cytotoxicity that were derived from previous studies (Supplemental Table 1) [43]. Gene Set Enrichment Analysis (GSEA) was performed on DEGs_SPCD8+ MHC (Fig. 3C). The results revealed significant enrichment of gene sets associated with cytokine production and cytotoxicity in the HL group, suggesting heightened T cell activation and effector function compared to the ML group. To further characterize these differences, a heatmap was generated to display the normalized TPM values of the top 50 significant DEGs_SPCD8+ MHC, clearly distinguishing the transcriptional profiles of HL and ML groups (Fig. 3D). Notably, key DEGs involved in cytotoxicity (e.g., Tgtp1, Tgtp2, Gbp3, Art2b) and cytokine production (e.g., Irf1, Tnfaip8l1, Lta, Igtp) exhibited subgroup-specific expression patterns, with pronounced upregulation in the HL cohort, further supporting their enhanced functional potential. In conclusion, by focusing on the DEGs induced by MHC molecular differences during AML progression (DEGs_SPCD8+ MHC), the haplomatched group CD8+ T cells exhibited enhanced cytokine and cytotoxicity characteristics, providing further insight into the stronger GVL effects observed in the haplomatched group.
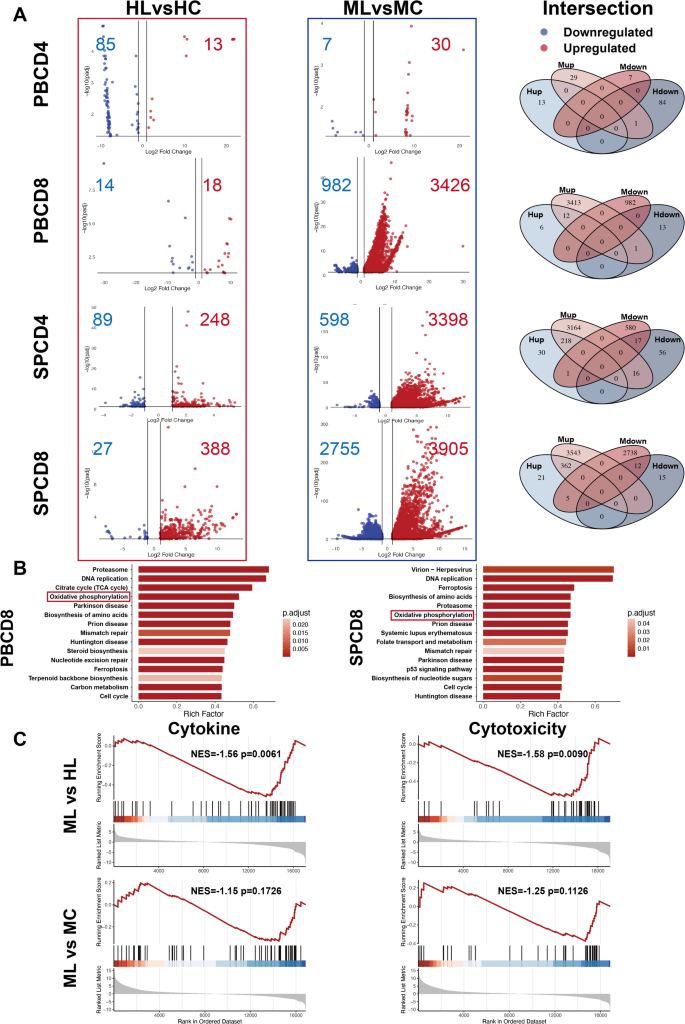
Due to the complexity of clinical settings, distinguishing the causal relationship between immune alterations and leukemia progression is challenging. Our MHC-matched and haplomatched leukemia models provide an ideal platform to investigate this relationship, as the same tumor type exhibits distinct progression patterns under different MHC compatibility conditions. To explore these differences, we performed differential analyses of CD4+ and CD8+ T cells from PB and SP between the HL and HC groups (HLvsHC), as well as between the ML and MC groups (MLvsMC). DEGs were selected using the criteria of Log2FoldChange > 1 and an adjusted p-value < 0.05. The results showed that CD4+ and CD8+ T cells in the indolent haplomatched group exhibited minimal DEGs compared to the healthy group, suggesting a preserved immune state. In contrast, the aggressive MHC-matched group displayed significantly more DEGs compared the healthy group, with this difference being more pronounced in CD8+ T cells than in CD4+ T cells, indicating a more substantial alteration in immune cell states (Fig. 4A).
The haplo group exhibits immune characteristics resembling normal physiological states. (A) Volcano plot and Venn diagram illustrating the comparison of differential gene expression between HLvsHC and MLvsMC. The number of DEGs were labeled. (B) KEGG pathway analysis of ML PB and SP CD8+ T cells revealed upregulation of multiple metabolism-related pathways in the ML group. (C) GSEA analysis of cytokine and cytotoxicity-related gene sets in MLvsHL and MLvsMC groups
Notably, KEGG analysis of the upregulated DEGs in the aggressive group revealed that both PB and SP CD8+ T cells were enriched in metabolism-related pathways. This suggests that CD8+ T cells in the aggressive group exhibit abnormally high metabolic activity during the early stages of AML progression. The KEGG pathways enriched include citrate cycle, oxidative phosphorylation, biosynthesis of amino acids, steroid biosynthesis, carbon metabolism and folate transport and metabolism (Fig. 4B). No statistically significant result was found in the KEGG analysis of DEGs in the indolent group. Moreover, GSEA was performed for MLvsHL and MLvsMC comparisons as well. The results showed that, compared to the MHC-matched group, CD8+ T cells from the haplomatched group exhibited stronger cytokine and cytotoxicity profiles. Additionally, although no statistically significant differences were observed, CD8+ T cells from the MC group showed a trend toward stronger functionality compared to those from the ML group (Fig. 4C). These results suggest that, under the same tumor type conditions, the immune cell state in the indolent MHC-haplomatched group is similar to that of healthy T cells. In contrast, CD8+ T cells in the aggressive MHC-matched leukemic mice exhibited abnormally activated metabolism, which failed to enhance T cell function and instead contributed to their dysfunction.
Given the surprising results of abnormal metabolic activation in the MHC-matched group during early AML stages, we further investigated how specific metabolic pathways regulate T-cell function during leukemia progression. To gain a deeper understanding of T-cell dynamics, we also collected SP CD8+ T cells from the late stage of AML in MHC-matched leukemia mice (PB GFP+ >50%, ML_late) and conducted RNA-seq. GSEA was performed on CD8+ T cells across the MLvsMC, MLvsHL and ML vs. ML_late groups. The results revealed that, regardless of comparison with the MC, HL or ML_late groups, CD8+ T cells in both PB and SP of the ML group showed enhanced metabolic activation (p-value cutoff = 0.05). Upregulated metabolic pathways in the ML group included carbon metabolism, biosynthesis of amino acids, glycolysis/gluconeogenesis, and OXPHOS (Fig. S1A-B). DEGs and KEGG analysis between ML and ML_late are shown in Fig. 5A and Figure S1C. Notably, OXPHOS emerged as a consistently enriched pathway across multiple KEGG and GSEA comparisons within the ML group (Figs. 3B, 4B and 5A and Fig. S1A-B), suggesting that it may serve as a core early warning signature of immune profile changes during the initial stage of aggressive leukemic progression.
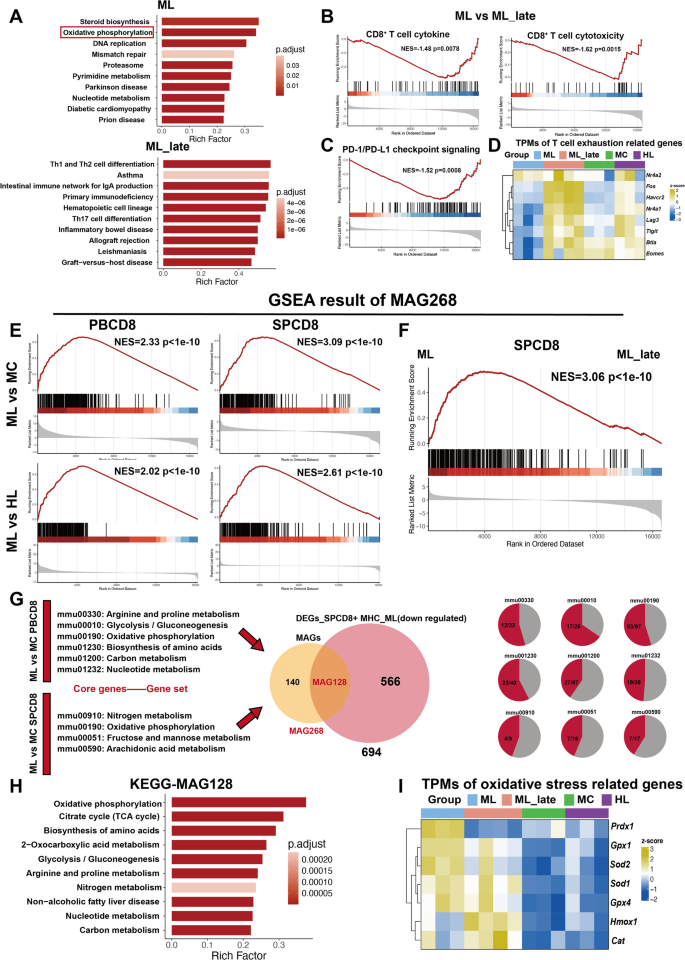
CD8+ T cells in the ML group exhibit abnormally high metabolic characteristics in early-stage AML, which can serve as a predictive marker for AML progression (A) KEGG pathway analysis of DEGs in ML vs. ML_late. (B) GSEA analysis of CD8+ T cytokine and cytotoxicity-related gene sets in ML and ML_late comparison. (C) GSEA analysis of PD-L1 expression and PD-1 checkpoint pathway in cancer in ML and ML_late comparison. (D) Heatmap showing TPM values of T cell exhaustion-associated genes across groups (ML, ML_late, MC, and HL). (E) GSEA results for MAG268 in PBCD8_MLvsMC, SPCD8_MLvsMC, PBCD8_MLvsHL, and SPCD8_MLvsHL. (F) Core genes from high metabolic pathways identified in GSEA analysis of PB and SP CD8+ T cells in the ML group were concluded into metabolism-related genes (MAG268). GSEA result of MAG268 geneset in SP CD8+ T cells between ML and ML_late. (G) Schematic overview of the workflow for identifying MAG128. The intersection of MAG268 and previously identified DEGs from SPCD8+ MHC (ML, downregulated) resulted MAG128. The proportion of MAG128 genes in various metabolic pathways was calculated. (H) KEGG pathway analysis of MAG128. (I) Heatmap showing TPM values of oxidative stress-associated genes across groups (ML, ML_late, MC, and HL)
GSEA between the ML_late and MC groups revealed persistent metabolic activation even in late-stage AML (Fig. S1D). However, this activation did not promote T cell activation, as there were no significant changes in cytokine or cytotoxicity-related pathways at either the early or late stages of aggressive leukemia progression (Fig. 4C and Fig. S2A). Interestingly, GSEA between ML and ML_late revealed that ML_late displayed enriched pathways related to both T-cell activation (cytokine production, cytotoxicity) and exhaustion (PD-1/PD-L1 checkpoint signaling) (Fig. 5B-C). Moreover, T-cell exhaustion markers were significantly upregulated in the ML_late group compared to both the ML and MC groups (Fig. 5D).
To further investigate whether the activation of metabolic genes in the ML group is a specific signature of early-stage aggressive leukemia, we extracted core genes from metabolic pathways identified in the PB_MLvsMC and SP_MLvsMC GSEA analyses. These genes were defined as MAGs, with a total of 268 identified (Supplemental Table 2, MAG268). GSEA revealed that MAG268 was significantly enriched in the ML group compared to ML_late, MC or HL (Fig. 5E-F), suggesting that metabolic activation could serve as an early warning marker for AML progression.
Furthermore, we intersected MAG268 with the previously identified DEGs_SPCD8+ MHC gene list (Fig. 3A) to identify genes specifically upregulated in CD8+ T cells from the ML group, regardless of whether they were influenced by MHC compatibility or leukemia progression. This analysis yielded 128 MAGs (Supplemental Table 3). KEGG analysis of MAG128 revealed that OXPHOS was the most significantly enriched pathway (Fig. 5G-H). The expression patterns of MAG128, particularly the 53 OXPHOS-related genes within MAG128, are shown in Figure S2B and S2C, respectively. Additionally, most genes in the OXPHOS pathway exhibited higher expression levels in the ML group compared to the ML_late, MC, and HL groups (Fig. S3). Genes involved in reactive oxygen species (ROS) scavenging, such as Sod2, Gpx1, and Prdx1 were significantly upregulated in the ML group (Fig. 5I), indicating an accumulation of oxidative stress during the early stage of aggressive leukemia. Notably, the specific upregulation of OXPHOS-related genes in the ML group did not enhance T cell activation (Fig. 4C and Fig. S3). Instead, it contributed to rapid T cell exhaustion and leukemia progression (Fig. 5C-D).
Taken together, pronounced metabolic abnormalities in both PB and SP CD8+ T cells is a specific signature in the early stage of the aggressive leukemic model (ML), whether compared to normal mice (MC), the indolent haplomatched leukemic model (HL), or the late stages of the aggressive leukemic model (ML_late). This metabolic activation does not promote CD8+ T cell functionality but rather suppresses their function, contributing to further exhaustion. The underlying mechanism of metabolic activation, particularly OXPHOS overactivation, and its role in CD8+ T cell exhaustion warrant further exploration.
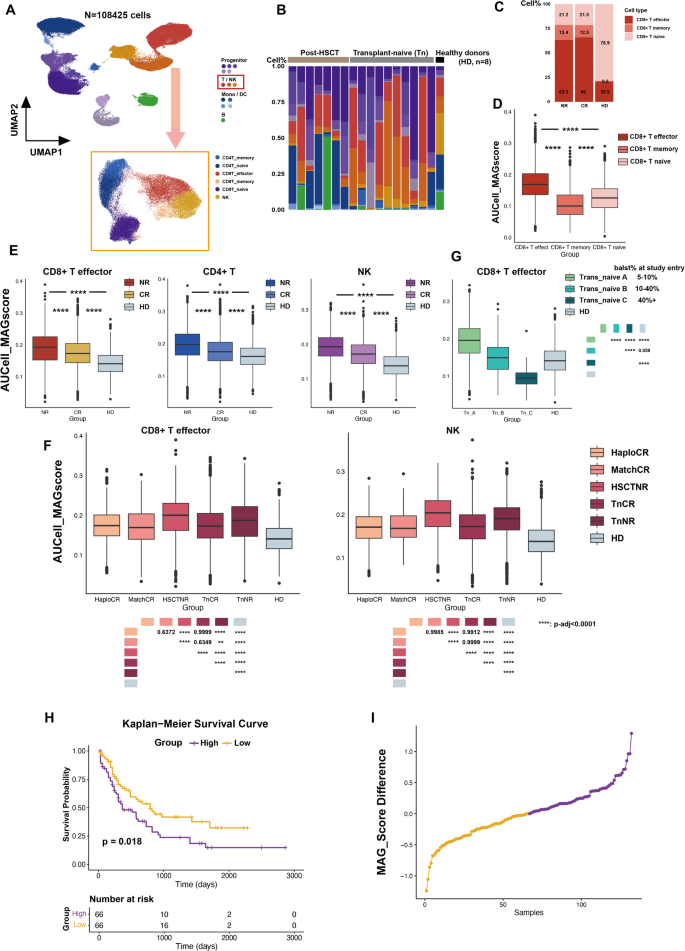
To further validate the clinical relevance of MAGs, we utilized public databases to explore the relationship between metabolic differences and AML prognosis. The abnormally high metabolic state identified in CD8+ T cells, which correlated with aggressive AML progression, was further confirmed in public single-cell RNA sequencing (scRNA-seq) data (GSE223844) [30]. We analyzed scRNA-seq data from bone marrow mononuclear cells (BMMCs) of AML patients with different clinical outcomes, categorized as complete remission (CR) or no response (NR), and included BMMCs from healthy donors (HD) as controls (Fig. 6A, Supplemental Table 4, Method). In total, 108,425 cells were analyzed. The distribution of cell types across AML samples with different HSCT backgrounds, as well as HD samples, is shown in Fig. 6B. A comparison of CD8+ T cell subsets revealed that naïve CD8+ T cells predominated in the HD group, whereas AML samples exhibited a significant increase in CD8+ T effector cells (Fig. 6C). Using the AUCell package, AUCell_MAGscores were calculated for each cell based on the MAG128 gene set (Supplemental Table 5). Across all samples, CD8+ T effector cells demonstrated significantly higher AUCell_MAGscores compared to CD8+ T naïve and memory cells (Fig. 6D). Further analysis of AUCell_MAGscores in CD8+ T effector cells across AML prognostic subgroups (CR, NR) and HD revealed significantly elevated scores in AML patients compared to HD, with NR patients showing higher scores than CR patients. These results confirm that the hypermetabolic state in CD8+ T effector cells is strongly associated with AML progression and poor prognosis. Notably, a similar pattern was observed in CD4+ T cells and NK cells (Fig. 6E). To exclude potential confounding effects of HSCT background and transplantation type, we further compared AUCell_MAGscores in CD8+ T effector cells across subgroups classified by treatment history: HaploCR, MatchCR, HSCTNR, Transplant-naïve (Tn) CR, TnNR, and HD. Regardless of HSCT background, CD8+ T effector cells in poor-prognosis subgroups consistently exhibited elevated AUCell_MAGscores. NK cells displayed a similar trend (Fig. 6F), further reinforcing the link between heightened metabolic activation and adverse AML outcomes.
Abnormally heightened metabolic states are associated with poor prognosis in AML. (A) Uniform manifold approximation and projection (UMAP) of single-cell transcriptomic profiles of bone marrow mononuclear cells (BMMC) samples from 17 AML patients at the screening point, alongside bmcite data (healthy donors). Annotated cell types are indicated by the color code. T and NK cells were subset and further clustered. (B) Distribution of cell types in screening samples from AML and healthy bone marrow. (C) Distribution of CD8+ T cell subsets in screening samples from AML patients and healthy bone marrow. (D) Statistical comparison of AUCell_MAG scores among CD8+ T effector cells, CD8+ T memory cells, and CD8+ T naïve cells. (E) Statistical comparison of AUCell_MAG scores between CD8+ T effector cells, CD4+ T cells, and NK cells among the complete remission (CR), no response (NR), and healthy donor (HD) groups. (F) Tumor burden classification based on the percentage of AML blasts in bone marrow at study entry: A = 5–10%, B = 10–40%, C = 40%+. AUCell_MAG scores of CD8+ T effector cells were compared across transplant-naïve patients in the A, B, and C groups. (G) Comparison of AUCell_MAG scores for CD8+ T effector cells and NK cells across different patient groups (patient information is provided in Supplemental Table 4). (H) Survival analysis based on bulk RNA-seq data of TCGA AML patients. High MAG expression was significantly associated with poor prognosis. (I) Distribution of MAG scores in TCGA AML patients. Statistical significance: **** P < 0.0001, ***P < 0.001, **P < 0.01, *P < 0.05
In AML mouse models, the hypermetabolic state of CD8+ T cells was predominantly observed during the early stage of aggressive AML (ML), whereas CD8+ T cells in haplomatched group exhibited profiles similar to those of normal T cells (HL). The comparable AUCell_MAGscores observed in the HaploCR and MatchCR subgroups may be attributed to differences in tumor burden. To further investigate the relationship between tumor burden and the metabolic state of CD8+ T effector cells, we analyzed the TnNR samples, which displayed elevated AUCell_MAGscores without transplantation background interference. These samples were classified into three subgroups based on bone marrow AML blast percentages: A (5–10%), B (10–40%), and C (40%+). Results revealed that CD8+ T effector cells in lower tumor burden samples exhibited a pronounced hypermetabolic signature, with AUCell_MAGscores progressively decreasing as AML advanced (Fig. 6G).
We further validated the correlation between MAG activation and poor prognosis using TCGA data. Samples with MAG_scores above the median were classified as the high group, while those below the median were categorized as the low group. Survival analysis revealed that the high MAG_score group had significantly poorer survival (p-value = 0.018) (Fig. 6H). Score distributions and a heatmap of MAG_scores are shown in Fig. 6I and Figure S4, respectively.
Taken together, these results confirm that the abnormally high metabolic state of CD8+ T effector cells is correlated with poor prognosis. Moreover, MAGs may serve as potential prognostic markers for precise AML risk stratification.
In this study, we characterized the immune profile under comparable AML burden but with distinct disease progression patterns. For the first time, we identified that CD8+ T cells exhibit abnormal metabolic activation during the early stage of AML progression. Despite the same tumor burden in peripheral blood, T cells displayed distinct immune profiles between the MHC-matched and haplomatched group. In haplomatched leukemic mice, immune cells maintained a state resembling that of healthy controls, with CD8+ T cells showing distinct activation features. This enhanced T cell function contributed to the indolent progression of leukemia in haplomatched mice. In contrast, CD8+ T cells in the MHC-matched group underwent substantial transcriptional changes, characterized by metabolic activation. Moreover, these immune alterations were more pronounced in CD8+ T cells from the SP compared to PB, suggesting dramatic changes in the TME within lymphoid organs during aggressive leukemia progression. Based on these dysregulated metabolic pathways, we established the MAG scoring system. Notably, a high MAG score was validated as a predictor of poor prognosis in AML at both single-cell and bulk transcriptomic datasets. Our findings suggest that abnormal metabolic activation of CD8+ T cells occurs early during AML progression and may contribute to their subsequent dysfunction and association with worse clinical outcomes. Furthermore, compared to single-gene biomarkers, which often have limited clinical utility due to tumor heterogeneity and the complexity of the TME [44], our MAG128 gene signature offers distinct advantages. By incorporating multiple genes across various metabolic pathways, the MAG128 score provides a more stable and comprehensive assessment of the metabolic state of CD8+ T cells. This multi-gene approach holds greater potential for clinical translation as a prognostic or predictive tool in AML.
Previous studies have demonstrated that haplo-SCT exhibits a stronger GVL effect compared to MSDT, characterized by enhanced immune cell cytotoxicity and lower relapse rates [7, 45]. Our study provides transcriptional evidence supporting this observation, showing that MHC haplo-matching between leukemia cells and recipient T cells enhances the cytotoxicity and activation of CD8+ T cells. Further investigation into the underlying reasons for the stronger GVL effect suggests that, in the haplo SCT mouse model, CD8+ T cells maintain metabolic profiles similar to those of normal T cells. This metabolic stability enables them to sustain effective antitumor activity during the early stages of AML progression.
Immune escape driven by the TME is a primary cause of AML relapse, and the TME exhibits high heterogeneity across different leukemia subtypes. By transplanting the same leukemic cells into MHC-matched or haplo-matched immune environments, we were able to trace the dynamics of the TME in the context of the same leukemia type but with differing progression patterns. We observed a significant activation of metabolic programs in the aggressive leukemia progression, suggesting a tight correlation between metabolic aberrations and the leukemia progression. The rapid growth of the tumor cells creates a hypoxic and nutrient-deprived TME [46]. Tumor-infiltrating T cell subsets rely on distinct metabolic features for survival and the exertion of antitumor immunity [47]. Under hypoxia, hypoxia-inducible factor 1-alpha (HIF-1α) enhances the expression of pyruvate dehydrogenase kinase 1 (PDK1), leading to reduced conversion of pyruvate to acetyl-CoA and impaired tricarboxylic acid cycle activity, thereby further compromising T cell anti-tumor function [48, 49]. Hypoxia also limits the infiltration of CD8+ T cells into the TME and induces their terminal differentiation [50]. Typically, the lack of glucose availability in the TME promotes tumor growth and hinders cytokine production by tumor-infiltrating lymphocytes (TILs) [51]. However, recent studies have shown that TILs can consume comparable amounts of glucose as tumor cells, indicating that T cells experience distinct metabolic stress depending on the TME [52, 53]. The accumulation of lactic acid resulting from enhanced aerobic glycolysis in the TME further impairs effector T cell function and proliferation [54, 55]. Moreover, it has been reported that T cells from patients relapsing after allo-HSCT exhibit reduced glycolysis, leading to decreased proliferation and impaired antileukemic activity [25]. Several amino acids, including arginine, tryptophan, and glutamine are linked to tumorigenesis and T cell fate [46]. Other factors in the TME, such as imbalanced metabolic activation leading to ROS overproduction and low pH values, directly induce T cell dysfunction [56]. Activated T cells exhibit adjusted metabolic pathways, consuming large amounts of glucose, amino acids, and fatty acids [57]. It is generally believed that the TME suppresses T cell metabolism through various intracellular and extracellular factors, thereby leading immune escape. However, we found that T cells in the early stages of aggressive AML progression (ML) exhibit abnormal metabolic activation compared to the late stages (ML_late) and indolent progression (HL) models, and this metabolic activation promotes exhaustion rather than functional activation with the leukemia progression.
Among the aberrant active metabolic signaling in the early stage of progressive leukemia progression, the OXPHOS pathway is widely enriched. OXPHOS is a key metabolic pathway for T cell activation, as well as an essential energy source for cancer cells to thrive under harsh TME conditions [58]. Researches have shown that impaired OXPHOS can suppress T cell proliferation and promote exhaustion, while enhancing OXPHOS can revitalize terminally exhausted T cells and improve responses to cancer immunotherapy, such as through IL-10 [59, 60]. IL-10-secreting chimeric antigen receptor (CAR) T cells possess preserved mitochondrial function and increased OXPHOS, thereby sustaining immune protection [61]. However, we found that the activation of OXPHOS in CD8+ T cells at the early stages of AML progression leads to T cell exhaustion rather than activation, which in turn promotes rapid leukemia progression. Mitochondria are the primary source of ROS. Both the electron transport chain and ATP synthesis processes within OXPHOS contribute to ROS generation, making them a major source of mitochondrial ROS [62]. In addition, the NADH/NAD+ ratio plays a critical role in modulating ROS production [63]. We observed that ROS scavenging-related genes, such as Sod2, Gpx1, and Prdx1, etc. were significantly upregulated in CD8+ T cells from the ML group, indicating that these cells are experiencing elevated oxidative stress. Consequently, the heightened oxidative stress and disrupted NADH/NAD+ ratios, resulting from excessive oxidative phosphorylation activation, may underlie the enhanced OXPHOS-induced T cell exhaustion.
T cells undergo significant metabolic reprogramming during activation and differentiation. Naïve T cells primarily rely on OXPHOS, whereas activated effector T cells typically shift to glycolysis and glutaminolysis to support their energetic and biosynthetic demands [64]. Impaired metabolic switching may contribute to the aberrant T cell metabolic upregulation observed in our study, leading to exhaustion and poor prognosis. In leukemia, OXPHOS is critical for the maintenance of leukemia stem cells (LSCs), and disrupting BCL-2-dependent OXPHOS has been shown to eliminate chemotherapy-resistant LSCs [65]. Therefore, targeting OXPHOS may offer dual benefits: eradicating LSCs and preventing T cell exhaustion. However, clinical trials using OXPHOS inhibitors in AML and other tumors have shown limited efficacy, with modest anti-tumor activity and dose-limiting toxicities, including neurotoxicity [66, 67]. Given the reliance of normal tissues on OXPHOS, a thorough evaluation of toxicity is essential before advancing these therapies clinically. We observed elevated OXPHOS activity during early aggressive leukemia progression, which correlated with poor prognosis of AML. This suggest that OXPHOS may serve as a predictive biomarker for risk stratification and therapeutic guidance. Supporting this, Li et al. identified a distinct CD8+ T cell subset with high OXPHOS activity (CD8+ TOXPHOS cells) in both the blood and tumors of melanoma patients. These cells were associated with resistance to immune checkpoint inhibitors (ICIs) and proposed as a blood-based predictive marker for ICI response [68]. Together, although therapeutic targeting of OXPHOS remains challenging, its potential as a predictive biomarker holds promise for near-term clinical application in guiding personalized treatment strategies.
While our study demonstrates a correlation between OXPHOS activation, T cell exhaustion and aggressive AML progression, the precise mechanisms by which OXPHOS activation drives T cell exhaustion and facilitates tumor immune escape during early AML progression remain to be fully elucidated. To accurately assess the metabolic state of CD8⁺ T cells within the dynamically evolving leukemic microenvironment at different stages of AML progression, direct measurements of oxygen consumption rate (OCR), extracellular acidification rate (ECAR), and ATP production are essential. In addition, the roles of individual respiratory chain complexes and the enzymatic activities driving OXPHOS should be further explored to uncover the intricate regulatory networks linking OXPHOS signaling to CD8⁺ T cell functions such as activation and differentiation. However, due to the dynamic nature of cellular metabolism, methodologies for targeted metabolic interventions in CD8⁺ T cells, particularly in vivo, are still under active development and have yet to be broadly established in the current literature. Another limitation of this study is the small sample size within each group. To enhance the translational relevance and overall robustness of our findings, future analyses should incorporate transcriptomic and metabolic profiling of immune cells from AML patients at different stages of disease progression, as determined by bone marrow aspiration or biopsy. Such studies would be instrumental in validating and extending our observations from the murine model to the human clinical context.
Overall, our study identifies, for the first time, an abnormally elevated metabolic state in CD8+ T cells during early AML progression, which may serve as a predictive marker for AML risk stratification. Notably, this heightened metabolic state does not enhance function but instead leads to T cell exhaustion. In the haplomatched group, despite having the same tumor burden, CD8+ T cells retained a normal metabolic state, preserving their activation and antitumor function. These findings provide deeper insights into TME dynamics in leukemia progression and highlight novel strategies for AML risk prediction.
The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/).
Gene Expression Omnibus (GEO; https://www.ncbi.nlm.nih.gov/geo/).
Bmcite data: https://explore.data.humancellatlas.org/projects/cc95ff89-2e68-4a08-a234-480eca21ce79. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The raw RNA-seq data generated by this project were deposited into the Genome Sequence Archive (https://ngdc.cncb.ac.cn/gsa-human) withaccession number CRA026671.
- AML:
-
Acute myeloid leukemia
- Allo-HSCT:
-
Allogeneic hematopoietic stem cell transplantation
- BM:
-
Bone marrow
- BMMCs:
-
Bone marrow mononuclear cells
- CR:
-
Complete remission
- DEGs:
-
Differentially expressed genes
- ECAR:
-
Extracellular acidification rate
- GO:
-
Gene Ontology
- GSEA:
-
Gene Set Enrichment Analysis
- GVHD:
-
Graft-versus-host disease
- GVL:
-
Graft-versus-leukemia
- Haplo-SCT:
-
Haploidentical stem cell transplantation
- HC:
-
Haplomatched control
- HL:
-
Haplomatched leukemia
- HD:
-
Healthy donors
- HLA:
-
Human leukocyte antigen
- ICIs:
-
Immune checkpoint inhibitors
- IDH1:
-
Isocitrate dehydrogenase 1
- KEGG:
-
Kyoto Encyclopedia of Genes and Genomes
- LSCs:
-
Leukemia stem cells
- MHC:
-
Major histocompatibility complex
- MSDT:
-
Matched sibling donor transplantation
- MAGs:
-
Metabolism-associated genes
- MC:
-
MHC-matched control
- ML:
-
MHC-matched leukemia
- NR:
-
No response
- OXPHOS:
-
Oxidative phosphorylation
- OCR:
-
Oxygen consumption rate
- PB:
-
Peripheral blood
- PDK1:
-
Pyruvate dehydrogenase kinase 1
- ROS:
-
Reactive oxygen species
- RNA-seq:
-
RNA sequencing
- ScRNA-seq:
-
Single-cell RNA sequencing
- SP:
-
Spleen
- TB:
-
Tumor burden
- TME:
-
Tumor microenvironment
- TILs:
-
Tumor-infiltrating lymphocytes
This work was partly supported by grants from the Major Program of the National Natural Science Foundation of China (No. 82293630), the National Natural Science Foundation of China (No. 82300243), the Beijing Municipal Health Commission project (No. BRWEP2024W134080115), the Beijing Natural Science Foundation General Project (No. 7252142).
This study did not involve human participants and human samples. All experiments were performed according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals.
All authors have read and approved the final manuscript for publication.
The authors declare no potential conflicts of interest.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Wang, B., Tang, J., Zhou, Y. et al. Abnormal metabolic activation of CD8+ T cells correlates with poor prognosis in acute myeloid leukemia. J Transl Med 23, 791 (2025). https://doi.org/10.1186/s12967-025-06833-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-025-06833-4