Cancer Imaging volume 25, Article number: 91 (2025) Cite this article
The tumour sink effect is a phenomenon whereby the sequestration of a radiopharmaceutical in cancer lesions leads to decreased activity concentration in the blood stream and organs. The aim of this sub-analysis of the prospective 3TMPO study (NCT04000776) was to investigate the tumour sink effect on prostate-specific membrane antigen (PSMA) PET imaging in a population of patients with metastatic castration-resistant prostate cancer (mCRPC).
Ninety-seven participants underwent 68Ga-PSMA-617 PET/CT imaging. The activity concentration in the kidney, parotid, spleen, liver and blood was expressed as a percentage of injected activity per cubic centimetre (%IA/cm3). The total tumour volume was delineated, and the total lesion fraction (TLF), i.e., the percentage of injected activity sequestered in the tumour, was computed. Participants were stratified into three tumour burden groups: small (TLF < 10%), moderate (10% ≤ TLF < 25%), and large (TLF ≥ 25%). Weight, lean body weight, body surface area, and estimated glomerular filtration rate (eGFR) were investigated as additional factors affecting biodistribution.
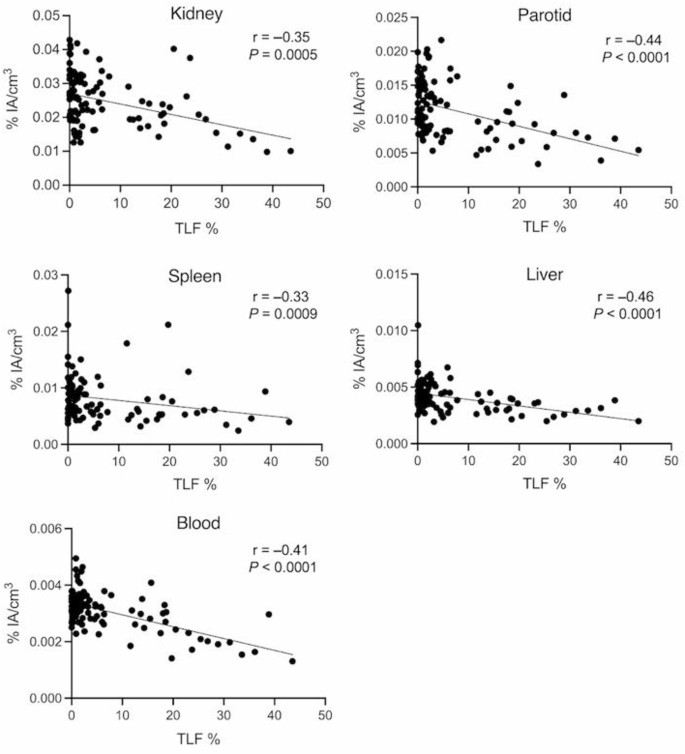
The TLF ranged from 0.0 to 43.5%. For all healthy tissues, the %IA/cm3 was negatively correlated with TLF (r ranging − 0.33 to − 0.46; P < 0.001). Patients with a large TLF had significantly lower uptake in all organs when compared to those with a small TLF (P < 0.05). Body habitus indices and/or eGFR were negatively correlated with the %IA/cm3 of the parotid, liver and blood (r ranging − 0.23 to − 0.33; P < 0.05). Combining predictive variables, the term [BSA / (1–TLF)] tended to yield the strongest negative correlations with healthy tissues %IA/cm3 (r ranging − 0.33 to − 0.63; P < 0.001).
The tumour sink effect was observed in a cohort of mCRPC patients scanned with 68Ga-PSMA-617. This finding strongly suggests that patients with a large TLF are likely to receive lower absorbed doses to organs at risk – i.e., be undertreated from a dosimetry perspective – following a fixed-activity regime of 177Lu-PSMA-617 radiopharmaceutical therapy, as commonly practiced. Individual factors such as body habitus and renal function further impact the biodistribution of PSMA radiopharmaceuticals.
NCT04000776, registered on 2019-06-27.
Radiopharmaceutical therapy (RPT) of metastatic castration-resistant prostate cancer (mCRPC) based on targeting prostate-specific membrane antigen (PSMA) utilizing 177Lu-PSMA-617 has been shown to prolong progression-free, quality-of-life deterioration-free, as well as overall survival, and has gained regulatory approval in many jurisdictions [1, 2]. However, the complete remission rate is dismal, and many patients do not experience significant anti-tumour effects, e.g., prostate-specific antigen continuously rises under treatment and/or pain is not relieved. In our experience, this tends to happen more often in cases of extensive metastatic disease, and others have shown that patients with large tumour volume have poor survival despite receiving PSMA RPT [3]. In these patients, the sequestration of a radiopharmaceutical by the tumour may contribute to reduce the uptake and thus the absorbed doses to healthy tissues, a phenomenon commonly referred to as the tumour sink effect [4]. Furthermore, it has been shown that for 177Lu-based PSMA RPT, the specific absorbed dose (i.e., Gy/GBq) to organs at risk varies considerably between patients, often by one order of magnitude or more [5, 6]. The currently approved fixed-activity 177Lu-PSMA-617 regime (i.e., six cycles of 7.4 GBq) being well tolerated implies that most patients receive absorbed doses below the tolerance threshold, and those affected by the tumour sink effect would tend to be further undertreated with such a one-size-fits-all regime.
The 3TMPO study was a multicentre, prospective study in patients with mCRPC investigating intermetastasis heterogeneity using 68Ga-PSMA-617, 18F-fluorodeoxyglucose (FDG) PET/CT and, in select participants, 68Ga-DOTATATE PET/CT (NCT04000776) [7]. Thus, this cohort constituted a unique opportunity to study the tumour sink effect on PSMA PET/CT. While other groups have reported on this topic, their analyses were limited to traditional PET metrics, such as molecular tumour volume (MTV), which only partially reflect tumour sequestration; as well as total lesion activity (TLA) and standardized uptake value (SUV), both of which are biased by body weight [8,9,10,11,12,13]. In an approach faithful to the sink effect concept as we originally introduced it for modern theranostics, we conducted this sub-analysis of the 3TMPO study using metrics that are more directly relevant to RPT—the total lesion fraction (TLF) and percent injected activity per cm3 (%IA/cm3)—and taking into account the influence of other patient factors known to affect PSMA radiopharmaceutical biodistribution [4, 5, 14, 15].
The 3TMPO study was approved by a central ethics committee and all participants signed a written consent form. Key eligibility criteria included a history of progressive mCRPC and the presence of at least three metastases on conventional imaging (CT and bone scan) [7]. This sub-analysis is limited to the 68Ga-PSMA-617 PET/CT. In brief, the latter was performed from at least mid-skull to proximal thighs 60 min after the injection of 1.8–2.2 MBq/kg of 68Ga-PSMA-617, with a low-dose CT scan. Using NEMA phantom acquisitions, the multiple PET/CT scanners of various makes and models that were used across the five participating centres were assessed for quantitative accuracy, and reconstruction parameters were standardized to reduce variability in image quality.
Using Encore software (MIM Software Inc., Cleveland, Ohio, USA), 3-cm circular volumes of interest (VOIs) were placed in the centre of the right liver lobe, avoiding any metastases, and in the ascending aorta. For the spleen, kidney, and parotid gland, 2-cm VOIs were centered on the location of the SUVpeak of each organ (highest SUVpeak across both sides for the kidneys and parotids). Activity concentration was primarily expressed as %IA/cm3, and secondarily as SUVmean. Whole-body MTV was segmented using an SUV threshold of 1.5x the liver SUVmean. Then, sub-VOIs, each < 1 cm3 in volume, as well as any area of uptake classified as physiological or likely benign, were removed. In addition to MTV and tumour SUV (max, peak, and mean), TLA and TLF (TLA divided by body weight in grams, expressed as a percentage) were computed, the latter constituting our primary tumour burden metric [15]. All reported SUV and TLA data were normalized for body weight. Participants were stratified into three tumour burden groups: small (TLF < 10%), moderate (10% ≤ TLF < 25%), and large (TLF ≥ 25%). We also conducted the same analysis as Gafita et al. [11], stratifying participants by MTV quintile.
The body habitus variables (i.e., surrogate volumes of distribution affecting uptake in healthy organs) of weight, lean body weight (LBW; James formula), and body surface area (BSA; Mosteller formula) were considered [16, 17]. Estimated glomerular filtration rate (eGFR; mL/min./1.73 m2) was determined from the serum creatinine level (µmol/L), using the CKD-EPI and MDRD-4 formulas [18, 19]. The creatinine clearance rate was computed using the Cockroft-Gault equation [20].
Continuous variables were reported as median, interquartile ranges (IQRs) or mean and standard deviation (SD) range. The relationship between organ uptake and tumour burden was assessed using Spearman’s correlations. We also examined the differences in organ uptake among categorized tumour burden groups using the Kruskal-Wallis test followed by Dunn’s post-hoc test with Bonferroni correction. Statistical analyses were performed using Prism (v. 9.4.1; GraphPad Software, La Jolla, CA, USA). P values of 0.05 or less were considered statistically significant.
Ninety-eight eligible mCRPC participants underwent a 68Ga-PSMA-617 PET/CT as part of the 3TMPO study. One participant was excluded because of missing data (creatinine to calculate eGFR); thus, 97 participants were included in this analysis (Table 1).
There was a very high interpatient variability in tumour uptake (SUV and %IA/cm3; Table 2, Supplemental Fig. 2) and tumour burden (MTV, TLA, and TLF; Table 2, Supplemental Fig. 1). When stratified according to their TLF, there were 72 participants with a low tumour burden, 17 with a moderate tumour burden, and eight with a large tumour burden. Notably, up to 43.5% of the PSMA radioligand was sequestered in the tumour; thus, it was not available for healthy tissue uptake. The latter also varied considerably among participants, with %IA/cm3 and SUVmean ranging by a factor of four to more than tenfold between individuals (Table 3, Supplemental Fig. 2).
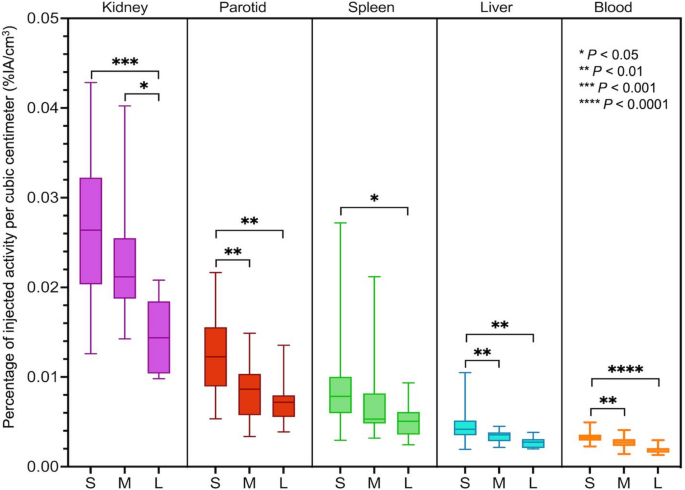
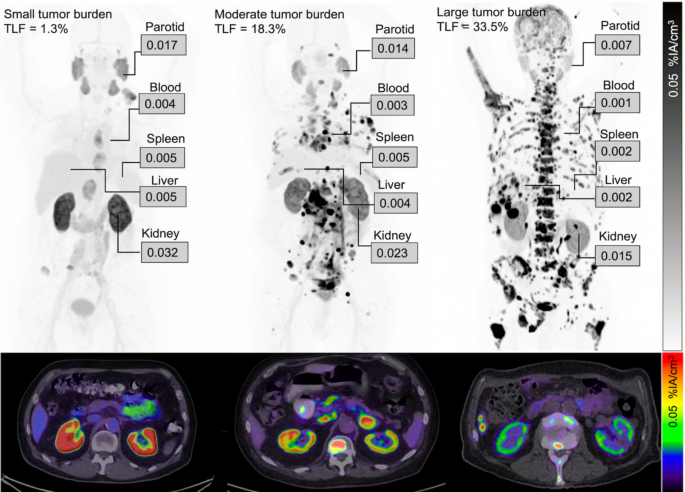
There were significant negative correlations between heathy tissue uptake (%IA/cm3) and TLF (Fig. 1; Table 4). When grouping participants according to their TLF, there was a statistically lower uptake in participants with a large tumour burden than in those with low tumour burden (Fig. 2). Representative cases are illustrated (Fig. 3).
Spearman’s correlations between healthy tissue uptake (mean percent injected activity per cm3; %IA/cm3) and total lesion fraction (TLF). Linear regression lines are shown for illustrative purposes (n = 97)
Healthy tissue uptake in participants with small (S: < 10%), moderate (M: 10–25%), and large (L: ≥ 25%) total lesion fraction (TLF). Median, interquartile range (box), and range (whiskers) are shown
Maximum intensity projections (top row) and fused axial slices (bottom row) of three patients with different total lesion fractions (TLF). Uptake values are shown as mean percentage of injected activity per cubic centimetre (%IA/cm3)
For the purpose of comparison with other studies, we assessed the correlations between healthy tissue SUVmean and MTV, which also proved to be significantly negative for all tissues: kidney (r = − 0.34; P < 0.001), parotid (r = − 0.45; P < 0.001), spleen (r = − 0.33; P = 0.001), liver (r = − 0.58; P < 0.001) and blood (r = − 0.41; P < 0.001). To replicate the analysis conducted by Gafita et al. [11], we stratified participants as controls (when MTV = 0; n = 7) and per MTV quintile (n = 90) (Supplemental Fig. 3). Trends towards lower tissue SUVmean in participants from the highest MTV quintile were observed, although not reaching statistical significance for the spleen.
Uptake by healthy tissues (%IA/cm3) was inversely related to weight, LBW, and BSA, with significant to near-significant correlations for parotid, liver, and blood (Table 4). Apart from the kidney, correlation coefficients were negative between other tissues’ uptake and renal function expressed as eGFR (CKD-EPI formula), and statistical significance was reached for liver and blood. Conversely, the kidney uptake at this imaging time point (60 min.) did not seem dependent on the renal function. We explored other indices of renal function as explanatory variables (Cockcroft-Gault creatinine clearance and eGFR using the MDRD-4 formula) but none yielded meaningfully different results (data not shown). When combining indices of body habitus and/or renal function with TLF, we found the strongest relationships with BSA/1–TLF, i.e., the BSA divided by the non-tumoral fraction. Notably, when expressing tissue uptake using SUVmean corrected for body weight, as others have done, the correlations with all body habitus indices turned positive, suggesting that body weight is a biased volume of distribution for SUV calculation with 68Ga-PSMA-617 (Supplemental Table 1). Regardless, SUVmean of all tissues was still significantly negatively correlated with TLF.
In a prospective observational cohort of patients with mCRPC at various stages of the disease who underwent 68Ga-PSMA-617 PET/CT, we observed a significant tumour sink effect in healthy tissues, i.e., the more the tracer is sequestered by the tumour, the less it is available to accumulate in these tissues. As introduced earlier, we believe that the most significant clinical implication of the tumour sink effect is that, in PSMA RPT, patients with a large tumour burden will likely be undertreated, from a dosimetry perspective, when a fixed activity is administered, such as per the currently approved regime of 177Lu-PSMA-617 [2]. Indeed, the tumour sequestration spares organs at risk (i.e., the parotid, the kidney, and the bone marrow, for which blood is often considered a dosimetric surrogate) from radiation exposure by removing a portion of the radiopharmaceutical from the blood stream. Consequently, when they are treated with a one-size-fits-all RPT regime, patients with a larger tumour burden, who tend to be more symptomatic and in greater need of therapeutic efficacy, may not receive the maximum injected activity – hence tumour absorbed dose – that they could tolerate. However, these are the patients who could potentially benefit the most from dosimetry-based, personalised RPT, especially since they have a poor prognosis [3, 21]. Further research validating the tumour sink effect on healthy tissues dosimetry in patients treated with PSMA RPT is warranted, paving the way to clinical trials of personalised PSMA PRT.
The sink effect is not limited to PSMA theranostics. We previously reported on the tumour sink effect in patients with neuroendocrine tumour undergoing 68Ga-DOTATATE PET/CT [4]. Our initial results, using a renal dosimetry-based regime of peptide receptor radionuclide therapy with 177Lu-DOTATATE, showed that it is possible to safely escalate administered activity and tumour absorbed dose in most patients, including those with larger tumour burden [22]. This approach could be translated to PSMA RPT. However, caution is advised in cases of sink effect resulting from extensive bone metastasis, which is more prevalent in prostate cancer than in neuroendocrine tumour patients. Indeed, while in such cases the bone marrow would receive lower self-dose and cross-dose from the blood, the cross-dose from bone tumour infiltration may be high, counteracting the bone marrow sparing by the sink effect. On the other hand, patients with extensive soft-tissue metastasis (with or without limited bone disease) clearly have the potential to draw more benefits from personalised RPT without risking undue toxicity. Further research is warranted on how to best use PSMA PET (e.g., TLF and/or healthy tissue uptake) to predict specific absorbed doses and, ultimately, to personalise the first cycle of PSMA RPT [23].
Others have reported on the sink effect on PSMA PET and we have summarized key features and results of their studies in Table 5. Unlike other researchers who analysed retrospective series, we derived our results from a prospective cohort. We used 68Ga-PSMA-617 in the 3TMPO study, a PSMA radioligand that is less commonly used in the clinics than 68Ga-PSMA-11 and 18F-DCFPyL, but which has a similar biodistribution [24, 25]. Thus, our results supplement those of others, confirming that the sink effect can be observed with all these urea-based radioligands, including 68Ga-PSMA-617, which shares the same pharmaceutical moiety as 177Lu-PSMA-617. Others have mainly used the MTV metric, or a visual categorical equivalent, to quantify or stratify tumour sequestration. While MTV is likely the most significant driver of the sink effect, intensity of uptake is the other defining dimension thereof. In this respect, Burgard et al. used TLA, the product of MTV and SUVmean, which is a more comprehensive metric for the purpose [13]. However, both MTV and TLA are contingent on knowledge of body weight to determine the relative magnitude of the tumour burden (e.g., for a given MTV or TLA, the tumour is twice as burdensome for a 50 kg patient than for a 100 kg patient). Instead, we propose TLF as a simplified, more interpretable, and direct metric of the tumour sink effect that is independent of weight and may facilitate interpatient comparisons [15]. In principle, TLF directly represents the proportion by which the healthy tissue uptake would be decreased because of cancer load. As for the metric of organ uptake, we primarily used %IA/cm3 rather than SUVmean, as others did, because the former is more directly related to the specific absorbed dose (i.e., Gy/GBq, or Gy per a fixed activity) when extrapolating to RPT. Indeed, assuming equal biokinetics, the specific absorbed doses in the tissues of patients of varied body weight will be proportional to %IA/cm3 but not to SUV. Notably, all but one group observed a statistically significant relationship describing a tumour sink effect in at least one healthy tissue of interest with direct relevance to PSMA RPT. Werner and al. were the exception and this is likely because their analysis was restricted to patients with low-volume disease (< 100 cm3) [12]. Observation of a meaningful sink effect obviously requires inclusion of patients with a large tumour burden.
A larger volume of distribution and a faster excretion from the body of virtually any radiopharmaceutical will inevitably contribute to lowering its concentration in blood and tissues. Accordingly, in patients with neuroendocrine tumours, we have found that body habitus and renal function were factors affecting the biodistribution and dosimetry of DOTATATE compounds in tissues of interest including the kidney [4, 26, 27]. In prostate cancer patients, using quantitative SPECT at ~ 48 h after 177Lu-PSMA-I&T RPT, we found that body habitus and renal function were both significantly and negatively correlated with specific absorbed dose estimates for the bone marrow and the kidney [14]. In accordance with our data, Violet et al. showed a significant absorbed dose reduction in parotid glands with increasing BSA [5]. In this study, we observed a strong correlation between body habitus indices or eGFR and activity concentration of 68Ga-PSMA-617 in the blood (as a surrogate for bone marrow), but not in the kidney. We hypothesize that at an early imaging timepoint (~ 60 min. for PET/CT), more complex and competing rapid kinetics at the renal cell level, superimposed over excreted activity into nephron tubules, may blur the relationships between apparent renal activity concentration and these patient characteristics. Moreover, the correlation between body habitus indices and renal SUVmean was revealed as significantly positive (Supplemental Table 1), pointing at body weight as an overestimated volume of distribution for 68Ga-PSMA-617, which does not exhibit a particular tropism for fatty tissues. This also raises concerns about the use of tumour SUV cutoffs for patient selection for RPT (e.g., SUVmax ≥ 20 in at least one lesion and ≥ 10 in all other lesions in the TheraP trial) [28], as overweight patients would technically be favoured over underweight patients in terms of access to the RPT. Normalizing SUV by LBW or BSA may help to reduce this bias [29, 30], although the use of a SUV ratio (e.g., uptake relative to the liver) may be preferable as it is independent of body habitus. We are currently conducting more in-depth analyses regarding these issues, which will be the topic of upcoming publications.
Patients with mCRPC have a wide range of PSMA-expressing tumour burdens, causing a tumour sink effect on 68Ga-PSMA-617 PET/CT, which manifests as a decreasing uptake in healthy tissues as the TLF increases. Other factors such as body habitus and renal function further affect biodistribution. This actionable knowledge is highly relevant for PSMA RPT, to which most of these patients are candidate. With the currently approved fixed-activity regime of 177Lu-PSMA-617, patients with a large tumour burden are likely undertreated from a dosimetry perspective, while being the most in need of therapeutic relief.
No datasets were generated or analysed during the current study.
- BSA:
-
Body surface area
- eGFR:
-
Estimated glomerular filtration rate
- IQR:
-
Interquartile range
- LBW:
-
Lean body weight
- mCRPC:
-
Metastatic castration-resistant prostate cancer
- MIP:
-
Maximum intensity projection
- MTV:
-
Metabolic tumour volume
- NEMA:
-
National Electrical Manufacturers Association
- PET/CT:
-
Positron emission tomography/computed tomography
- PSA:
-
Prostate-specific antigen
- PSMA:
-
Prostate-specific membrane antigen
- RPT:
-
Radiopharmaceutical therapy
- SD:
-
Standard deviation
- SPECT:
-
Single photon emission computed tomography
- SUV:
-
Standardized uptake value
- TLA:
-
Total lesion activity
- TLF:
-
Total lesion fraction
- VOI:
-
Volume of interest
We are grateful for the involvement of all who contributed to the 3TMPO study, including the research teams at each participating centre (sub-investigators and collaborators, research coordinators and nurses, nuclear medicine technologists), the cyclotron lab staff at the Centre de recherche du CHUS, the patient partners and, most importantly, the participants and their caregivers.
The 3TMPO study was supported by Oncopole EMC2, a peer-reviewed funding grant that matched unrestricted funds from the Fonds de Recherche du Québec–Santé public agency, the Cancer Research Society, and Merck Canada. F.P., É.R., P.O.R., and J.M.B. are supported by Fonds de recherche du Québec–Santé’s Clinical Research Scholars awards.
This study was conducted in compliance with the declaration of Helsinki and received authorization from the central research ethic board and all participants provided written informed consent prior to their inclusion in the study.
Not applicable.
Senior authors J.M.B. and F.P. have received consultancy honoraria from, and are local principal investigators for, Novartis. J.M.B. is a local principal investigator for Point Biopharma.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Zamanian, A., Rousseau, É., Buteau, FA. et al. The tumour sink effect on 68Ga-PSMA-PET/CT in metastatic castration-resistant prostate cancer and its implications for PSMA-RPT: a sub-analysis of the 3TMPO study. Cancer Imaging 25, 91 (2025). https://doi.org/10.1186/s40644-025-00910-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40644-025-00910-z