BMC Oral Health volume 25, Article number: 791 (2025) Cite this article
Dental plaque accumulation plays a key role in caries development. This study explored the relationships between dental caries experience, the bacterial composition of dental plaque, and oral health behaviors in children with mixed dentition and fair-to-good oral hygiene, as assessed by the Silness and Löe Plaque Index (PlI).
This study included 54 children (6–12 years, mean ± SD = 8 ± 2) from Greater Poland Province. The participants’ parents completed a questionnaire on diet, oral hygiene, and dental care. Two calibrated pediatric dentists performed the dental examinations. Supragingival plaque samples were collected from intact enamel, and bacterial detection was conducted via polymerase chain reaction (PCR).
Among the 54 children, 39 had a history of dental caries (International Caries Detection and Assessment System – Decayed, Missing, and Filled teeth index for permanent and primary dentition, ICDAS-DMF/dmf > 0), 15 were caries-free, and 37 had at least one cavitated lesion, restoration, or extraction (DMF/dmf > 0). Streptococcus mutans was significantly more prevalent in children with ICDAS-DMF/dmf > 0 (p = 0.026) and DMF/dmf > 0 (p = 0.038). Parascardovia denticolens was significantly more prevalent in children with DMF/dmf > 0 (p = 0.027). Children harboring S. mutans and P. denticolens had significantly higher PlI scores (median: 0.5) than did those without these bacteria (median: 0.2) (p = 0.028, p = 0.014). The median intake of cariogenic beverages was greater in children with ICDAS-DMF/dmf > 0 (p = 0.038). A lack of dental visits in the past 12 months was associated with detectable S. mutans (p = 0.021) and Actinomyces viscosus (p = 0.041). Bottle feeding beyond 18 months correlated with the presence of Scardovia wiggsiae (p = 0.028). Nighttime meals or drinks were associated with dental caries experience (p = 0.046) and S. mutans detection (p = 0.016).
The strong association of S. mutans with caries, along with the frequent detection of P. denticolens in children with cavitated lesions, highlights the importance of these species as potential targets for preventive strategies. The association of S. wiggsiae with prolonged bottle-feeding might suggest that early feeding behaviors influence microbial colonization. In children with fair-to-good oral hygiene, parental education on nighttime feeding, limiting cariogenic beverages, and promoting regular dental visits remain crucial for caries prevention.
Dental caries is the most common oral disease and is characterized by the degradation of tooth tissues caused by acids produced during bacterial fermentation of dietary carbohydrates. The etiology of this disease is multifactorial and involves both environmental and genetic factors [1]. Caries development is largely dependent on the presence of a harmful biofilm—dental plaque—formed by complex microbial interactions occurring on the tooth surface. These interactions involve oral microaggregates, bacterial metabolites, saliva components, and dietary carbohydrates [1, 2]. Dental plaque is a highly organized structure in which microbial cells are embedded in an extracellular matrix that provides stability and protection. Over 1000 different bacterial species [3] have been identified within dental biofilms, with their attachment, growth, and survival shaped by physical, metabolic, and molecular interactions [4].
A key risk factor for caries development is poor oral hygiene, which leads to excessive plaque accumulation [1]. However, some children who brush regularly still develop caries, raising important questions about what additional factors shape their microbiome and disease susceptibility.
A group of cariogenic bacteria, particularly Streptococcus mutans and Lactobacillus species, are most commonly linked to tooth decay. Significant efforts have been made to understand the virulence factors of these bacteria. S. mutans is especially harmful because of its aciduric and acidogenic properties, as well as the ability to produce polysaccharides that aid bacterial attachment to the tooth surface [2, 5]. It has been particularly well studied for its role in initiating caries through enamel demineralization. Lactobacillus species have also been studied for their association with the progression of deeper lesions in dentin, as they thrive in more acidic environments created during advanced stages of caries [6].
However, more recent research has expanded the focus to include other bacterial species that contribute to caries. Studies have shown that dental caries is a polymicrobial disease involving a diverse range of bacteria, including members of the phylum Actinomycetota. Within this phylum, several genera relevant to dental caries have been identified, including Actinomyces, Bifidobacterium, Parascardovia, and Scardovia [7]. S. wiggsiae has emerged as a potential indicator of severe early childhood caries [8]. Scardovia inopinata, Bifidobacterium dentium, and P. denticolens have been frequently isolated from caries lesions [9, 10], while Actinomyces species are linked to the etiology of root caries [11].
These bacteria participate in the complex biofilm environment, interacting with S. mutans and other microorganisms while contributing to the acidic conditions necessary for caries development. Despite their significant role in the oral ecosystem, members of Actinomycetota have been studied less extensively than S. mutans, even though they influence both oral health and disease.
This study explored the relationships between dental caries experience, the bacterial composition of dental plaque, and oral health behaviors in children with mixed dentition and fair-to-good oral hygiene. Our goal is to identify additional factors that shape the oral microbiome and contribute to caries development, despite the absence of poor brushing habits. Specifically, we examined the presence of S. mutans and selected species from the genera Actinomyces, Bifidobacterium, Parascardovia, and Scardovia to assess bacterial patterns associated with caries risk.
We hypothesized that, in children with fair-to-good oral hygiene, the presence of specific plaque bacteria and certain behavioral patterns would be significantly associated with caries experience.
This was a cross-sectional observational study conducted in a university dental clinic setting. The study protocol was approved by the University Bioethics Committee (Resolution No. 115/21), and written informed consent was provided by all participants’ parents. Because no medical intervention was administered, registration clinical trial number was not applicable to this study.
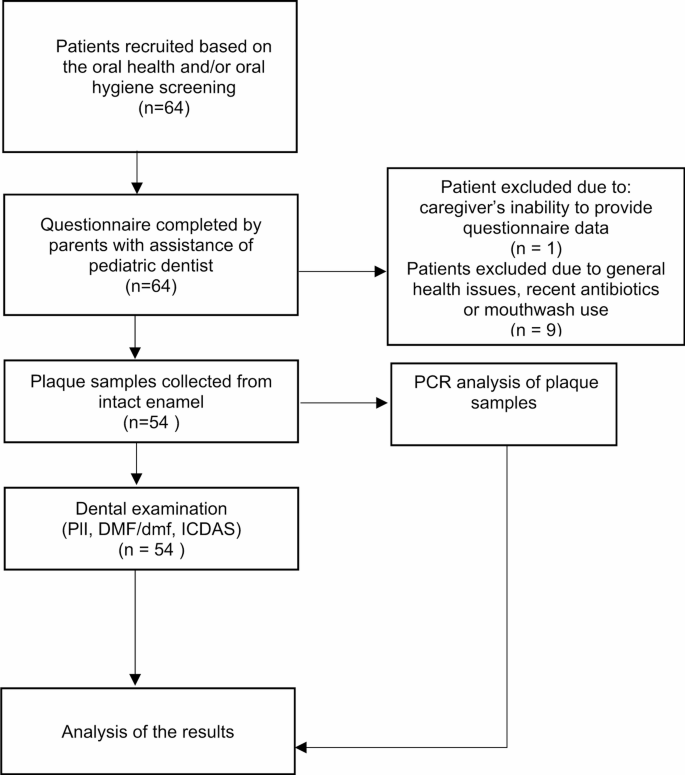
A flowchart of the study methodology is shown in Fig. 1. The study group consisted of 54 children (24 females and 30 males) aged 6–12 years (mean ± SD = 8 ± 2) who were residents of Poznań and its vicinity (Greater Poland Province). Participants were recruited from patients attending check-up visits at the Pediatric Dentistry Clinic of the University Center of Stomatology and Specialist Medicine in Poznań.
Children were excluded from the study if they had any systemic diseases, were using orthodontic appliances or antibacterial mouthwashes, had complications of dental caries (such as clinical signs of periapical inflammation), had received antibiotics in the past six months, or exhibited poor oral hygiene (Silness–Löe Plaque Index [PlI] ≥ 1.9) [12].Therefore, all participants in the final sample had PlI scores below 1.9, which we refer to as fair-to-good oral hygiene.
Sample size calculation was carried out via G*Power (version 3.1.9.4) on the basis of the study by Henne et al. [9], which detected S. mutans in 62% of children with caries and 10% of caries-free subjects. The calculation was performed for a one-tailed test, with a significance level (α) of 0.05 and statistical power (1-β) of 0.80, indicating that a minimum of 12 subjects per group (24 in total) would be required to detect a significant difference.
We included a larger study group: 17 caries-free children and 37 children with dental caries experience. The decision to oversample children with caries ensured a more representative distribution of caries status in the population from Greater Poland, where the prevalence of caries reached 77% in 6-year-olds and 55% in 12-year-olds [13].
The questionnaire was developed by the authors specifically for this study (see supplementary material). Its content was reviewed by a panel of pediatric dentists for clinical relevance and clarity, and it was piloted on five parents to evaluate comprehensibility. No major difficulties in understanding were reported. During the main study, pediatric dentists were available to assist parents while completing the questionnaire to ensure clarity and completeness of responses.
The questionnaire gathered information on feeding behaviors (including breast and bottle-feeding duration and nighttime feeding), as well as dietary intake. Nighttime meals or drinks were defined as the consumption of any food or beverage (excluding water) after the child had brushed their teeth for bed or during nighttime awakenings. The number of daily meals, including snacks, was also reported.
Parents were asked to assess the frequency of their child’s consumption of various food and drink items. Responses were recorded using a six-point scale with the following categories:
The points assigned to each response were designed to approximate the daily frequency of consumption for each food or drink item. The total points for items within specific food and drink groups were then summed to calculate the average daily intake for each category:
These calculations provided an estimate of the daily intake frequency for each subgroup with different caries statuses and microbial profiles.
Oral hygiene behaviors were assessed by asking parents about the frequency of brushing, the type of toothpaste used, and the age at which brushing began. Dental care was evaluated through questions regarding the age at the first dental visit, the reason for the visit, and the timing of the most recent dental appointment.
Oral Hygiene Examination.
Dental examinations were conducted by two calibrated pediatric dentists (N.T.-W. and R.S.) using visual and tactile methods. Prior to data collection, the examiners underwent calibration against each other, achieving inter-examiner and intra-examiner agreement with Cohen’s kappa values 0.85 and 0.90, respectively.
Children were examined in the early afternoon, at least three hours after their last meal and a minimum of six hours after morning toothbrushing, to standardize the conditions for plaque assessment and microbial sampling.
The assessment of the Silness–Löe Plaque Index (PlI) [12] was based on recording both soft debris and mineralized deposits on the six index teeth: 16, 12, 24, 36, 32, and 44. In the absence of teeth, primary teeth 55, 52, 64, 75, 72, or 84 were examined. Each of the four tooth surfaces (buccal, lingual, mesial, and distal) was assigned a score from 0 to 3, where: 0 indicated no plaque; 1 indicated a thin film of plaque adhering to the free gingival margin and adjacent area of the tooth, detectable by a probe; 2 indicated a moderate accumulation of soft deposits within the gingival pocket or on the tooth and gingival margin, visible to the naked eye; and 3 indicated an abundance of soft deposits within the gingival pocket and/or on the tooth and gingival margin. The PlI of the subject was calculated by summing scores from four surfaces of each index tooth and averaging across all scored surfaces. The subject’s plaque status was assigned as follows: good (0–0.6), fair (0.7–1.8), and poor (1.9–3.0). Patients with poor oral hygiene were excluded from the study.
Collection of dental plaque samples.
A sterile plastic disposable excavating spoon was used to collect a pooled supragingival plaque sample from intact enamel. Sampling included eight buccal and accessible proximal surfaces, avoiding any sites with visible caries, restorations, or enamel defects. Plaque was pooled into one sample per participant to obtain a representative profile of the supragingival microbiota.
The collected plaque was transferred into a tube containing 1.0 mL of DNA stabilization buffer (Canvax). Each sample was processed for DNA isolation using the HigherPurity™ Stool DNA Isolation Kit (Canvax) at the laboratory of the Poznań University of Life Sciences. Briefly, the dental plaque lysates in the stabilization buffer were transferred to bead tubes and homogenized at 30 Hz for 1 min in a Mixer Mill M300 (Retsch), followed by incubation at 95 °C for 20 min. The lysate was centrifuged at full speed, and 600 µl of the supernatant was transferred to a new tube. From this point, the isolation was performed according to the kit manual. The selected bacteria were detected via polymerase chain reaction (PCR).
Since we used oligonucleotide primers previously published in scientific literature, we did not conduct additional laboratory verification of their specificity. However, before selecting the primers, we conducted a literature search to identify various primer sequences used for detecting the target species. We then verified these candidate sequences in silico using the NCBI Primer-BLAST tool to ensure specificity to the target species and avoid cross-reactivity with non-target genomes. As positive controls, we used genomic DNA from the respective bacterial strains obtained from DSMZ – German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). In some instances, PCR products were purified and sequenced to confirm amplification specificity. As negative controls, No Template Controls (NTC) were used to monitor for potential contamination.
The PCR reaction mixture (20 µl) was composed of 2 µl of 10×PCR buffer for RUN DNA polymerase (containing 15 mM MgCl2), 200 µM of each dNTP, 0.4 µM of each species-specific primer required [11,12,13,14,15], 0.4 U of RUN DNA polymerase (RUN DNA polymerase, A&A Biotechnology, Gdańsk, Poland), and 5 µl of template DNA. Amplification was carried out in a Veriti thermal cycler (Life Technologies, Warsaw, Poland). The amplification consisted of one cycle at 95 °C for 5 min; 40 cycles at 94 °C for 30 s, Ta according to Table 1 for 30 s, and 72 °C for 1 min; and a final cycle at 72 °C for 5 min. Amplified products were electrophoresed on a 1.5% agarose gel, stained with ethidium bromide, and visualized under UV transillumination.
After professional teeth cleaning, examiners assessed dental caries experience by recording the number of decayed teeth (D and d for permanent and primary teeth, respectively), teeth extracted due to dental caries (M and m for permanent and primary teeth, respectively), and teeth restored due to caries (F and f for permanent and primary teeth, respectively). The teeth were gently dried, and a dental mirror was used to detect discolouration, cavities, and restorations. Caries lesions were identified using the WHO criteria [19]. In addition, incipient caries lesions were marked on the diagram according to the International Caries Detection and Assessment System [20].
Each participant was assigned to one of two groups on the basis of dental caries experience: the group with ICDAS-dmf and/or ICDAS-DMF > 0 and the group without a history of caries. Additionally, participants were categorized into one of two further groups on the basis of the presence or absence of at least one tooth with a history of cavitated caries lesions (DMF and/or dmf > 0).
All the statistical analyses were performed via MedCalc® Statistical Software version 20.115 (MedCalc Software Ltd., Ostend, Belgium 2022). The Shapiro‒Wilk test was used to assess the normality of the data distribution. Since all variables demonstrated a nonnormal distribution, nonparametric tests were applied for further analysis. The Mann‒Whitney U test was used to compare differences between groups for continuous variables. Chi-square test was used to compare proportions when all expected cell counts were ≥ 5. When this assumption was violated Fisher’s exact test was applied. A p value < 0.05 was considered statistically significant.
Table 2 summarizes descriptive statistics for caries-related parameters in the study population. The PlI ranged from 0 to 1.8, with a median value of 0.4 (Q1: 0.2; Q3: 0.8).
The DMF index ranged from 0 to 4, with a median of 0.0 (Q1: 0.0; Q3: 0.0). The dmf index ranged from 0 to 11, with a median of 1.5 (Q1: 0.0; Q3: 6.0). The ICDAS-DMF scores for permanent teeth ranged from 0 to 6, with a median of 0.0 (Q1: 0.0; Q3: 3.0). The ICDAS-dmf scores for primary teeth ranged from 0 to 11, with a median of 2.0 (Q1: 0.0; Q3: 6.0). Among the 54 children, 39 had an ICDAS-DMF/dmf > 0, 15 were caries-free, and 37 had a DMF/dmf > 0.
Table 3 presents the results of the analysis of the presence of selected bacteria in relation to dental caries status.
S. mutans was significantly more prevalent in children with ICDAS-DMF/dmf > 0 than in the caries-free group (p = 0.026) and in children with DMF/dmf > 0 than in the group without a history of cavitated caries lesions (p = 0.038). P. denticolens was significantly more prevalent in children with DMF/dmf > 0 than in those without a history of cavitated caries lesions (p = 0.027). Other bacteria were not significantly associated with the dental caries indices. S. inopinata was not detected in any sample.
Table 4 presents dental caries experience, microbial profiles, and associated numerical variables: age and dietary and oral hygiene behaviors.
The median ages were similar across subgroups with different caries statuses and microbial profiles, with no statistically significant differences, except for children who harbored A. naeslundii, who were significantly older than those without this bacterium (p = 0.026).
The median PlI values varied among the subgroups. Statistically significant differences were observed between subgroups with different microbial profiles (median PlI: 0.5 for S. mutans or P. denticolens carriers vs. 0.2 for children without these bacteria, p = 0.028 and p = 0.014, respectively).
All the children used fluoridated toothpaste (data not listed in the tables).
Most children reported brushing their teeth three times daily, with no significant differences across subgroups on the basis of caries experience or microbial profiles (p > 0.05). The median age at first toothbrushing and dental visit, as well as the median number of daily meals (5–6), did not significantly differ among the subgroups (p > 0.05). Similarly, the median daily intake of tooth-friendly, cariogenic solid foods, and neutral/low-cariogenic foods did not differ among the subgroups (p > 0.05).
The median intake of cariogenic beverages varied across subgroups and was significantly greater in children with ICDAS DMF/dmf > 0 (p = 0.038).
Table 5 presents the relationships between dental caries experience, microbial profiles, and nominal variables such as sex, early-life feeding habits, and dental visit patterns.
The distributions of females and males were not significantly different across the subgroups (p > 0.05).
Children whose first dental visit was due to oral health problems were significantly more likely to have experienced dental caries (ICDAS-DMF/dmf > 0; p = 0.021) and to harbor S.mutans or P. denticolens in their plaque samples (p = 0.044 and p = 0.003, respectively).
Children who had not attended a dental appointment within the last 12 months were significantly more likely to have S. mutans or A. viscosus detected in their plaque (p = 0.021 and p = 0.041, respectively).
Prolonged bottle-feeding beyond 18 months of age was significantly associated with colonization by S. wiggsiae (p = 0.028), while breastfeeding beyond 18 months showed no statistically significant associations with caries or specific bacterial species.
Nighttime consumption of food or drinks was significantly associated with caries experience (p = 0.046) and the presence of S. mutans in supragingival plaque (p = 0.016).
This study highlights key associations between dental caries, oral hygiene practices, dietary behaviors, and microbial profiles in children aged 6–12 years. We excluded children with poor oral hygiene to isolate the influence of bacterial and behavioral factors independent of heavy plaque accumulation, which is a dominant caries risk factor. Our findings confirm the well-established role of S. mutans in cariogenesis, with a significant association between its presence and increased caries experience. Children with a history of dental caries were more likely to harbor S. mutans, reinforcing its pathogenic role as a primary cariogenic bacterium. Its acidogenic and aciduric properties, along with its ability to synthesize glucans that increase biofilm formation, make it a crucial target for preventive strategies [2]. Zheng et al. [21] reported significant differences in S. mutans abundance between groups with a high caries index (dmft ≥ 10) and those without caries, reinforcing its role as a “core microbe” in caries development. Recent observations suggest that S. mutans forms “rotund” clusters over white-spot lesions, further supporting its role as a primary driver of enamel demineralization [22].
On the other hand, Qudeimat et al. [23] reported that S. mutans was not consistently present in all individuals with active caries, even when plaque samples from multiple tooth surfaces were pooled. Similarly, our study did not detect S. mutans in 46% of individuals with dental caries.
In contrast to Aas et al. [24], who detected S. mutans only in dental plaque from dentin caries lesions but not in dental plaque from healthy enamel or white-spot lesions, our study identified S. mutans on the intact enamel of 54% of children with ICDAS DMF/dmf > 0 and 20% of children without caries lesions. Becker et al. [25] reported a strong relationship between S. mutans and caries at all lesion depths, with significantly lower levels found on intact enamel in caries-active and caries-free individuals.
Mitrakul et al. [6] detected S. mutans, Lactobacillus, and Bifidobacterium in both initial and mature plaques of children with severe early childhood caries and in caries-free groups. While Lactobacillus and Bifidobacterium levels were significantly correlated with the dmf score and plaque index, S. mutans levels were correlated with only the dmf score. In our study, S. mutans was associated with both dental caries and the dental plaque index.
Notably, in this study, we excluded children with poor oral hygiene, although their dental plaque is likely to contain higher levels of cariogenic microbiota. This selection was based on findings from previous studies, which consistently demonstrated that oral hygiene is the most significant risk factor for dental caries in children with primary and permanent dentition from the Greater Poland Province [26, 27]. By focusing on children without heavy plaque accumulation, we aimed to investigate additional risk factors beyond oral hygiene that may contribute to the development of caries.
The interplay between S. mutans and other bacteria is crucial in caries development. De Matos et al. [28] demonstrated that S. mutans produces more acid when cocultured with Bifidobacterium species such as P. denticolens, S. inopinata, and B. dentium, contributing to a more acidic environment that promotes enamel demineralization. Additionally, Kressier et al. reported that S. wiggsiae lacks arginine deaminase activity, preventing it from neutralizing acidic conditions and further contributing to caries development [29]. Laboratory findings suggest that, compared with S. mutans, Bifidobacterium species exhibit superior acid resistance, allowing them to thrive in low-pH environments and evade fluoride-induced metabolic inhibition [30].
Anaerobic culture methods confirmed strong associations between severe early childhood caries and both S. mutans and S. wiggsiae. Notably, S. wiggsiae was detected in some caries-affected children who did not harbor S. mutans, suggesting that it may serve as an alternative pathogen in caries development. Computational analysis of the microbiota, dietary patterns, and clinical data revealed that children with both S. mutans and S. wiggsiae experienced more rapid caries progression, indicating that these bacteria are potential indicators of severe disease [8].
Boisen et al. [31] detected S. mutans in caries-free individuals, but the prevalence and relative abundance were significantly lower than those in caries-active individuals. S. wiggsiae was more abundant in caries-active children, although the difference was not statistically significant. In our study, S. wiggsiae was detected more often than S. mutans was, and it was not significantly associated with dental caries.
Modesto et al. [10] examined the presence of S. inopinata, B. dentium, and P. denticolens in both dental plaque and caries lesions and reported that S. inopinata was the most frequently isolated species in carious lesions, B. dentium was more common in plaques, and P. denticolens was present in both habitats. Our study, which analyzed plaque exclusively from intact enamel, revealed no presence of S. inopinata. However, P. denticolens was frequently detected, especially in children in the cavitated stage of dental caries (DMF/dmf > 0), which suggests that its presence can be an indicator of more advanced stages of caries. Among the saliva samples collected from healthy adult volunteers by Beighton et al. [32], the predominant isolate was B. dentium, which was isolated from 82.2% of the subjects, whereas S. inopinata and P. denticolens were detected in 4.7% and 3.2% of the individuals, respectively. In contrast to the results of the abovementioned studies, we managed to isolate B. dentium from only one individual affected by caries. According to Henne et al., B. dentium does not colonize hard surfaces, so its numbers are very low or under the detection limit on caries-free tooth surfaces. Dental caries initiation by other species facilitates the attachment of B. dentium and its proliferation, so it can be found mainly in deep lesions [9].
Interestingly, we detected B. catenulatum in the supragingival plaque of 5 individuals with dental caries. In the study by Moore et al., B. catenulatum, which is typically isolated from human feces, was found to constitute 0.01% of the microflora in periodontitis [33]. The role of this species in the development of dental caries requires further study.
The variability in research findings may stem from differences in sampling methods, microbial detection techniques, population characteristics, and environmental factors.
The role of Actinomyces species, such as A. naeslundii and A. viscosus, in root surface caries is well documented [11, 34–35]. Chokshi et al. [36] reported a positive correlation between dental caries indices and Actinomyces abundance in children with mixed and permanent dentition. These bacteria are early colonizers of dental plaque [37], and their ability to produce ammonia from urea can increase plaque pH and potentially inhibit caries progression [38]. Richards et al. [7] reported that A. naeslundii was significantly more abundant in the dental plaque of caries-free individuals than in the plaque on intact and carious enamel and dentin of individuals with caries. In the study by Tang et al., A. naeslundii predominated in children’s supragingival plaque in terms of both its prevalence and proportion. It was detected in 80% of caries-free children and 69% of caries-active individuals [38]. Similarly, in our study, the presence of A. naeslundii was not significantly associated with dental caries. Moreover, A. naeslundii was the most frequently detected species, which suggests that it plays an important role in the microenvironment of the supragingival dental plaque of children.
With respect to behavioral factors related to caries, the increase in dental caries over the centuries aligns with increased sugar availability and consumption [39, 40]. Our dietary analysis revealed that liquid sugars in beverages were a strong determinant of dental caries, whereas solid sugars were not, despite children with caries consuming far fewer sugary drinks (1 serving/day) than solid sugary foods (4 servings/day). This aligns with studies by Evans et al. [41] and Laniado et al. [42], which highlight the strong association between sugar-sweetened beverage consumption and increased caries experience. The experience of dental caries in 12-year-old adolescents from Greater Poland depends on only one significant nutritional factor—the daily consumption of sweet carbonated drinks [24]. In contrast, Kumar et al. [43] reported a stronger link between solid sugar consumption and caries risk, underscoring the variability in dietary influences.
This discrepancy may be explained by reporting biases or differences in consumption patterns. It is possible that parents do not accurately recall or quantify their children’s beverage intake, particularly if sugary drinks are consumed in small sips throughout the day rather than in distinct servings. Sugary drinks may be consumed between meals when salivary flow is lower, increasing cariogenic potential, whereas solid foods may be eaten during meals when protective saliva flow is greater.
According to a systematic review by Angarita-Díaz et al., a sugar-rich diet causes a decrease in the microbial diversity of the oral microbiome and the predominance of cariogenic bacterial species [44]. A study by Jurczak et al. revealed that children with an elevated sucrose taste threshold were more than 10 times more likely to harbor S. mutans [45].
Emerging evidence also emphasizes the role of early-life nutrition in shaping the oral microbiota and risk of dental caries. Eshriqui et al. highlighted how infant feeding practices can influence long-term microbial colonization [46]. Cordova-Carrillo et al. reported that exclusively breastfed infants exhibited lower levels of S. mutans compared to those receiving formula or mixed feeding [47]. Furthermore, several studies have demonstrated that prolonged breastfeeding or bottle-feeding is linked to an increased risk of early childhood caries. In a systematic review, Tham et al. concluded that breastfeeding up to 12 months may protect against caries when compared to formula feeding, but extended breastfeeding beyond 12 months was associated with greater caries risk [48]. Similarly, Sritangsirikul et al. observed a protective effect of breastfeeding between 6 and 17 months, whereas feeding beyond 18 months was linked to higher caries prevalence [49].
Our results did not reveal any significant association between exposure to cariogenic foods and the microbial profile, nor between early feeding patterns and dental caries. However, prolonged bottle-feeding (> 18 months) was associated with the presence of S. wiggsiae (p = 0.018), supporting findings that feeding habits influence microbial colonization. Notably, Bifidobacterium efficiently ferments lactose, which may explain its frequent detection in children consuming lactose-rich diets [28]. Moreover, our study confirms that nighttime eating and drinking habits significantly increase the risk of caries and the likelihood of S. mutans colonization. Saliva plays a crucial role in neutralizing acids and remineralizing enamel, but its flow rate decreases during sleep, prolonging acid exposure from fermentable carbohydrates [50]. Interventions such as discouraging late-night snacking, promoting fluoride use, and ensuring effective brushing before bedtime could mitigate caries risk and reduce S. mutans colonization.
Finally, children’s dental visit patterns revealed important preventive care gaps. Many children with caries lesions seek dental care only because of pain or visible decay, emphasizing the need for earlier routine checkups. The higher prevalence of S. mutans and A. viscosus in children who had not visited a dentist for more than 12 months can be attributed to prolonged plaque accumulation, lack of professional cleaning, and fluoride prophylaxis, all of which create a more favorable environment for cariogenic and plaque-associated bacteria to thrive [51].
This study has several limitations. The sample size was relatively small and calculated based on S. mutans detection. The lower prevalence of some other species may have limited the strength of conclusions for these taxa. Additionally, self-reported behaviors may be prone to recall bias. Finally, the exclusion of children with poor oral hygiene limits the generalizability of findings to the broader pediatric population. Despite these limitations, the study provides valuable insights into the microbial and behavioral factors associated with dental caries in children.
This study underscores the multifactorial nature of dental caries. Our findings support the hypothesis that, in children without heavy plaque accumulation, caries development is associated with the presence of specific bacterial species and behavioral factors.
The strong association of S. mutans with caries, along with the frequent detection of P. denticolens in children with cavitated lesions, highlights the importance of these species as potential targets for preventive strategies. The association of S. wiggsiae with prolonged bottle-feeding might suggest that early feeding behaviors influence microbial colonization. In children with fair-to-good oral hygiene, parental education on nighttime feeding and limiting cariogenic beverages remain crucial for effective caries prevention.
The data that support the findings of this study are not openly available due to reasons of sensitivity and are available from the corresponding author upon reasonable request. Data are located in controlled access data storage at the Department of Pediatric Dentistry of Poznan University of Medical Sciences.
- PlI:
-
Silness–Löe Plaque Index
- DMF:
-
number of permanent teeth with caries lesions (excluding incipient caries), extracted, or restored due to caries
- dmf:
-
number of primary teeth with caries lesions (excluding incipient caries), extracted, or restored due to caries
- ICDAS-DMF:
-
number of permanent teeth with caries lesions (including incipient caries), extracted, or restored due to caries
- ICDAS-dmf:
-
number of primary teeth with caries lesions (including incipient caries), extracted, or restored due to caries
- PCR:
-
polymerase chain reaction
The authors would like to thank the patients and their parents, who agreed to participate in this study.
The research was supported by the Poznan University of Medical Sciences statutory funds. The funding body had no role in the design of the study; the collection, analysis, and interpretation of the data; or the preparation of the manuscript.
All procedures performed in this study were conducted in accordance with the 1964 Declaration of Helsinki and its subsequent amendments. The study protocol was approved by the University Bioethics Committee (Resolution No. 115/21), and written informed consent was obtained from the parents of all participants.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Opydo-Szymaczek, J., Torlińska-Walkowiak, N., Maćkowiak, K. et al. Supragingival plaque microbiota and caries risk factors among children with mixed dentition. BMC Oral Health 25, 791 (2025). https://doi.org/10.1186/s12903-025-06123-x