BMC Geriatrics volume 25, Article number: 443 (2025) Cite this article
Sarcopenic obesity (SO), the coexistence of low muscle mass and excess fat, is increasingly recognized in older adults. Its definition and clinical implications remain debated. This study evaluated SO using the European Society for Clinical Nutrition and Metabolism–European Association for the Study of Obesity (ESPEN–EASO) criteria and examined its associations with geriatric syndromes and metabolic risk factors.
We conducted a retrospective cross-sectional study of 364 hospitalized patients aged ≥ 65 years who underwent bioelectrical impedance analysis. Geriatric assessments included Activities of Daily Living (ADL), Instrumental ADL (IADL), Mini Nutritional Assessment–Short Form (MNA-SF), Mini-Mental State Examination (MMSE), Geriatric Depression Scale (GDS), Clinical Frailty Scale (CFS), and handgrip strength. Mortality data were collected. Associations with SO were analyzed using univariate and multivariate logistic regression.
SO prevalence was 24.5%. Patients with SO had worse ADL, IADL, MNA-SF scores, and lower handgrip strength (all p < 0.01). Independent predictors of SO included older age (OR = 1.064, p = 0.002), female gender (OR = 3.574, p < 0.001), hypertension (OR = 2.094, p = 0.007), and diabetes mellitus (OR = 3.342, p < 0.001). The SO group had higher mortality (15.1% vs. 9.3%, p = 0.03). A geriatric model including frailty, MNA-SF (OR = 0.472, 95% CI: 0.306–0.727), and handgrip strength (OR = 0.741, 95% CI: 0.641–0.856) showed better model fit (Nagelkerke R² = 0.474) than the metabolic model (R² = 0.223).
SO is a distinct clinical condition linked to frailty, functional decline, and mortality. Geriatric factors show stronger associations than metabolic indicators, underscoring the importance of comprehensive geriatric screening in older adults.
Not applicable.
Sarcopenia, characterized by the age-related decline in muscle mass and strength, and obesity, marked by the excessive accumulation of body fat, are two distinct medical conditions that often manifest concurrently, leading to the emergence of a novel entity known as sarcopenic obesity (SO) [1, 2]. The relationship between sarcopenia and obesity is particularly concerning, as the two conditions can exacerbate each other and lead to even more severe functional impairments. Sarcopenic obesity (SO) has emerged as a critical entity in geriatrics, particularly due to its underestimated impact on older adults. Many older individuals who appear well-nourished due to obesity may be sarcopenic, a condition often overlooked. This phenomenon has significant implications, as sarcopenia is already well known for its detrimental effects in older adults, including increased frailty, higher fall risk, reduced functional capacity, and greater hospitalization rates [1, 2]. Moreover, previous studies suggest that sarcopenic obesity exacerbates these negative outcomes even further. While sarcopenia and obesity have been extensively studied separately, the interplay between these conditions and their cumulative effects on geriatric health remains insufficiently explored [3,4,5]. Studies suggest that SO affects functionality more than sarcopenia or obesity alone.
Many definitions of SO have been proposed in the literature, leading to significant differences in reported prevalence and associations with functional problems. The European Society for Clinical Nutrition and Metabolism (ESPEN) and the European Association for the Study of Obesity (EASO) introduced a consensus definition in 2022 to standardize SO classification, improving comparability across studies. The ESPEN-EASO criteria are particularly relevant as they integrate muscle quality and body composition, addressing the inconsistencies in prior definitions. Using these standardized thresholds, our study ensures a more precise evaluation of SO’s clinical impact and its differentiation from isolated sarcopenia or obesity. However, despite introducing these criteria, studies focusing on their application in real-world clinical settings and their implications for frailty and mortality remain scarce [6].
SO is a complex condition that requires tailored interventions to address the dual challenges of preserving muscle mass while reducing the health risks associated with excess body fat. Targeted interventions, including the incorporation of functional foods and sustainable physical activity, have been identified as potential strategies to mitigate the burden of sarcopenia and obesity. As the global population ages and the prevalence of these conditions rises, a comprehensive understanding of their multifactorial causes and the development of effective, multidisciplinary approaches to prevention and management will be crucial in addressing this growing public health challenge.
The causes of these complex conditions are multifactorial, involving a combination of lifestyle, genetic, and environmental factors. Factors such as disuse, changing endocrine function, chronic diseases, inflammation, insulin resistance, and nutritional deficiencies have all been identified as potential contributors to the development of sarcopenia. Similarly, poor diet, sedentary lifestyle, and aging have been linked to the rising prevalence of obesity, with the coexistence of sarcopenia and obesity, known as SO, presenting a particularly concerning challenge [3, 4, 7, 8].
Previous studies have demonstrated that sarcopenic obesity (SO) negatively impacts activities of daily living, frailty, and mortality in older adults. However, there is a lack of research comparing SO with sarcopenia and obesity alone using the standardized 2022 ESPEN-EASO criteria. Our study aims to address this gap by evaluating whether sarcopenic obesity (SO) represents a mere coexistence of sarcopenia and obesity or constitutes a distinct clinical entity with unique health implications. We also examine how SO is associated with geriatric syndromes, particularly frailty and mortality, in comparison to sarcopenia and obesity individually. Through the use of comprehensive geriatric assessment and comparative clinical modeling, this study offers critical insights into the clinical relevance of SO in aging populations, contributing to improved strategies for screening, classification, and management.
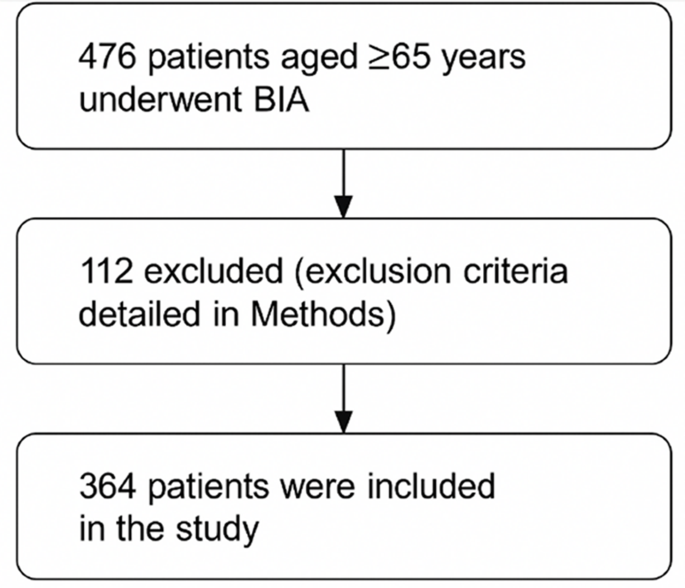
In this cross-sectional retrospective study, 364 patients with appropriate files and data were included among 476 patients aged 65 and over who underwent Bioelectrical Impedance Analysis (BIA) in two different hospitals between July 2015 and September 2023 (Fig. 1). Those who gave informed consent for the tests underwent a comprehensive geriatric assessment, and individuals with full data and measurements of mental and other functional abilities were included in the study. Patients whose data were incomplete in the file (those not suitable for BIA, laboratory values were missing, comprehensive geriatric assessment tests could not be performed or were missing) were excluded from the study. Patient Selection and Exclusion Criteria: A total of 476 patients aged ≥ 65 years who underwent bioelectrical impedance analysis (BIA) were initially screened. Of these, 112 patients were excluded due to the following reasons: presence of metallic implants (e.g., joint prostheses, defibrillators), active malignancy, end-stage organ failure, severe infection, medications affecting body composition (e.g., corticosteroids, immunosuppressants), unexplained weight loss > 10% in the last 6 months, severe cognitive impairment or inability to provide informed consent, conditions leading to secondary sarcopenia (e.g., endocrine disorders, recent major surgery), and incomplete geriatric assessment data due to physical or neurological disabilities. Finally, 364 patients were included in the analysis.
For the necessary analyses, patients were first categorized into two groups: sarcopenic obese and non-sarcopenic obese. Then, to conduct a more detailed evaluation of body composition, they were further stratified into four subgroups based on the definition of sarcopenic obesity (SO): Group 1– Normal; Group 2– Obese only (O); Group 3– Sarcopenic only (S); Group 4– Sarcopenic Obesity (SO).
Geriatric assessments included the Katz Activities of Daily Living Index (ADL), Lawton Instrumental Activities of Daily Living Scale (IADL), Mini-Nutritional Assessment Short-Form (MNA-SF), Geriatric Depression Scale (GDS), and Mini-Mental Status Examination (MMSE). We used the Clinical Frailty Score to define and grade frailty. Frailty was evaluated using the Clinical Frailty Scale (CFS), which rates patients from 1 (very fit) to 9 (terminally ill) based on physical and functional status.See details in Table 1.
We defined sarcopenic obesity (SO) and its components according to the diagnostic criteria provided by the European Society for Clinical Nutrition and Metabolism (ESPEN) and the European Association for the Study of Obesity (EASO) [6]. The 2022 ESPEN–EASO criteria were chosen because they provide a standardized and widely accepted framework for identifying SO, enabling comparability across studies. These criteria encompass both muscle function and obesity metrics, allowing for a comprehensive evaluation of body composition. Unlike earlier definitions, the ESPEN–EASO classification reduces discrepancies in prevalence and outcome estimates by providing clear diagnostic thresholds for both sarcopenia and obesity. We used body mass index (BMI) and waist circumference as screening tools for obesity. Handgrip strength was used as part of the sarcopenia diagnosis, in accordance with ESPEN–EASO recommendations. For details on body composition measurements using bioelectrical impedance analysis (BIA), please refer to Table 1. The diagnosis of SO was made using a two-step process: initial screening followed by confirmation, as outlined in the ESPEN–EASO algorithm.1.
Individuals were initially screened based on obesity indicators, including body mass index (BMI) or waist circumference (WC), using ethnicity-based cutoffs. Sarcopenia Risk Indicators: Clinical suspicion of sarcopenia was evaluated using validated screening tools such as the SARC-F questionnaire.
Following a positive screening result, the SO diagnosis was confirmed by a Muscle Function Assessment, which included handgrip strength and the chair stand test to evaluate muscle function. Body Composition Analysis: Bioelectrical Impedance Analysis (BIA) measured lean and fat mass. Individuals with excessive fat and low skeletal muscle mass were classified as having SO.
This standardized approach allowed for consistent identification and classification of SO within our study population [6]. In our study, we followed the diagnostic phase of these recommendations by confirming obesity and sarcopenia based on fat percentage and muscle mass measured using Bioelectrical Impedance Analysis (BIA). Additionally, we assessed muscle functionality through gait speed and handgrip strength measurements.
Mortality data were obtained from the Death Notification System managed by the Public Health Agency of the Turkish Ministry of Health, ensuring standardized and government-verified mortality tracking [9]. We identified those who died at least one year after their application as mortality. We determined the mortality status as a percentage on a case-by-case basis.
Biochemical laboratory results presented and compared in Table 2 were utilized. Biochemical parameters were assessed using venous blood samples analyzed in the hospital’s central laboratory under standard operating procedures. The following variables and their respective measurement techniques were recorded: Fasting blood glucose (mg/dL; reference range: 74–100): Measured using enzymatic methods. Glomerular filtration rate (eGFR): Calculated based on serum creatinine levels. Calcium (mg/dL; 8.8–10.6): Measured using the spectrophotometric method, which quantifies light absorption at specific wavelengths. Total protein (g/L; 66–83): Determined via spectrophotometry. Albumin (g/L; 35–52): Determined via spectrophotometry. Leukocyte count (×10⁹/L; 4.5–11): Counted using automated hematology analyzers. Hemoglobin (g/dL; 11.7–16.1): Measured by the cyanmethemoglobin method. Vitamin B12 (pg/mL; 126.5–505): Assessed using the spectrophotometric method. Thyroid-stimulating hormone [TSH] (µIU/mL; 0.38–5.33): Evaluated via the Electrochemiluminescence Immunoassay (ECLIA), which is widely used to measure hormones and proteins with high sensitivity. C-reactive protein [CRP] (mg/L; 0.0–5.0): Measured by the turbidimetric method, which detects the concentration of suspended particles through light scattering. 25-hydroxy vitamin D (µg/L; 10–60): Analyzed using High-Performance Liquid Chromatography (HPLC), an advanced technique for separating and quantifying biological compounds.
The sample size was calculated based on an SO prevalence of approximately 34%, requiring at least 102 patients for a power of 95% and an alpha of 0.05. Statistical analysis was performed using SPSS 26. The normality of variables was assessed using both visual (histograms and probability plots) and analytical methods (Kolmogorov-Smirnov and Shapiro-Wilk tests). Descriptive statistics included mean and standard deviation for normally distributed variables and median with maximum-minimum values for non-normally distributed variables. Frequencies of categorical variables were expressed as percentages. Chi-square and ANOVA tests were used for group comparisons, followed by Bonferroni post hoc tests. For comparisons between two (SO-non-SO) independent groups, the Independent Samples t-test was used for normally distributed variables, while the Mann-Whitney U test was applied for non-normally distributed variables.
Variables found significant in the ANOVA analysis were further analyzed using regression analyses. Multicollinearity among independent variables was checked using Pearson, Spearman, or Kendall’s tau-b correlation analyses, and variables with multicollinearity were not included in the same regression models. Logistic regression results for SO were reported as odds ratios (OR) with a 95% confidence interval (CI). To further examine the association of different factors with the development of sarcopenic obesity (SO), two separate logistic regression models were constructed: (1) a metabolic model including demographic and metabolic risk factors (age, hypertension, and diabetes mellitus), and (2) a comprehensive geriatric assessment model including frailty, malnutrition, and functional parameters. Model performances were compared using Nagelkerke R² values, Akaike Information Criterion (AIC), and Bayesian Information Criterion (BIC) scores. A p-value of less than 0.05 was considered significant.
The study included 364 patients with a mean age of 77.11 ± 6.97 years, of whom 64.6% were female. To evaluate the impact of sarcopenic obesity (SO), patients were categorized into two broad groups: SO and non-SO. Further stratification yielded four subgroups: normal (39.6%), obese only (16.5%), sarcopenic only (19.5%), and sarcopenic obese (24.5%).
SO patients exhibited significantly worse functional and cognitive performance compared to other groups. Specifically, activities of Daily Living (Katz ADL) and Instrumental Activities of Daily Living (Lawton-Brody IADL) scores were significantly lower in the SO group (p < 0.01), indicating greater dependency. Mini Nutritional Assessment (MNA-SF) scores were the weakest in SO patients (p < 0.001), suggesting a strong link between SO and nutritional deficits. Mini-Mental State Examination (MMSE) scores were significantly lower in the SO group (p < 0.01), indicating a strong association with cognitive impairment. Handgrip strength was markedly reduced in SO patients (p < 0.001), confirming impaired muscle function. Logistic regression analysis showed that female gender, advanced age, hypertension, diabetes mellitüs, low Mini-Nutritional Assessment (MNA-SF) scores, and reduced handgrip strength were significantly associated with SO.
Sarcopenic obesity (SO) was significantly associated with increased frailty, as SO patients exhibited the highest Clinical Frailty Scale (CFS) scores compared to other body composition groups (p < 0.05). In logistic regression analysis, frailty demonstrated a trend-level association with SO (OR = 1.835, 95% CI: 0.986–3.416, p = 0.057). Subgroup analysis further indicated that both sarcopenia and SO were independently associated with higher frailty scores, whereas obesity alone was not significantly linked. The comprehensive geriatric model incorporating frailty, nutritional status, and handgrip strength yielded a better model fit and higher explanatory power for SO than the traditional metabolic model (Nagelkerke R² = 0.474 vs. 0.223; AIC = 112.385 vs. 353.847), emphasizing the central role of geriatric factors. Moreover, the mortality rate in the SO group was significantly higher than in the non-SO group (15.1% vs. 9.3%, p = 0.03), suggesting that SO may independently contribute to adverse outcomes, including mortality, beyond the additive effects of sarcopenia and obesity alone.
Older age was significantly associated with SO in our model (OR = 1.064, p = 0.002), supporting its role as a contributing factor. Female gender was significantly associated with SO (OR = 3.574, p < 0.001), highlighting a gender-based vulnerability. Hypertension (OR = 2.094, p = 0.007) and diabetes mellitus (OR = 3.342, p < 0.001) significantly increased the risk of SO. The presence of both metabolic conditions further compounded SO risk, necessitating targeted interventions.
Tables 2 and 3, and 4 provide a detailed comparison of functional, cognitive, and metabolic parameters across study groups.
Logistic regression analysis identified female gender (OR = 3.574, p < 0.001), advanced age (OR = 1.064, p = 0.002), hypertension (OR = 2.094, p = 0.007), and diabetes mellitus (OR = 3.342, p < 0.001) as the strongest independent risk factors for sarcopenic obesity (SO) in the metabolic model. In the geriatric model, low Mini Nutritional Assessment (MNA-SF) scores (OR = 0.472, p = 0.001) and reduced handgrip strength (OR = 0.741, p < 0.001) emerged as significant determinants of SO. Depression scores were inversely associated with SO (OR = 0.759, p = 0.002), while cognitive function (MMSE) and walking speed did not show a significant independent association with SO.
Our study demonstrated that sarcopenic obesity (SO) is associated with significant impairments in functional measures, as well as increased frailty and mortality. Logistic regression analysis showed that female gender, advanced age, hypertension, diabetes mellitus, low Mini-Nutritional Assessment (MNA-SF) scores, and reduced handgrip strength were significantly associated with SO. Importantly, our findings suggest that geriatric factors, particularly malnutrition and muscle weakness, are more strongly associated with SO than metabolic risk factors alone.
SO was associated with poorer ADL and IADL scores, reflecting greater physical dependence. However, logistic regression analysis did not significantly associate ADL and IADL with SO. This may be due to the more decisive influence of other geriatric factors, such as muscle weakness (handgrip strength) and nutritional deficits (MNA-SF), which have been significantly associated with SO. Previous studies have reported a strong association between SO and functional decline, as SO exacerbates mobility limitations due to combined muscle weakness and excess fat mass [5, 7, 17]. However, our findings suggest that ADL and IADL impairments may be mediated by other factors such as frailty, underlying comorbidities, and hospitalization status rather than being directly associated with SO. Additionally, ADL and IADL are subjective measures, and psychological, environmental, and social factors can influence their assessment. Hospitalized individuals, such as those included in our study, may already exhibit lower functional scores due to acute illness or overall frailty, which might have attenuated the strength of association between these variables and the presence of SO. Furthermore, IADL impairment has also been linked to cognitive dysfunction, which was not significantly associated with SO in our model, possibly contributing to the lack of an independent relationship between SO and IADL [18,19,20]. These findings suggest that while SO negatively impacts physical function, its effects may be more strongly reflected through muscle strength and nutritional status rather than self-reported functional scores. Future studies should explore whether objective functional measures, such as gait speed or balance testing, provide a stronger link between SO and functional decline.
Although cognitive impairment has been linked to SO in some studies [18, 21, 22], our logistic regression analysis did not identify Mini-Mental State Examination (MMSE) scores as independently associated with SO. This discrepancy may stem from multiple factors. First, cognitive impairment is a multifactorial condition influenced by vascular, metabolic, and neurodegenerative mechanisms. It SO alone may not be sufficient to drive cognitive decline without additional risk factors such as neuroinflammation. Second, MMSE may not be sensitive enough to detect subtle cognitive impairments, particularly those related to executive function and processing speed, which have been suggested as key domains affected in SO populations. Third, the relatively homogeneous nature of our hospitalized older adult cohort, with many patients already exhibiting baseline cognitive decline, may have resulted in an underestimation of SO’s independent contribution to cognitive dysfunction. Future studies using more detailed neuropsychological assessments and including community-dwelling older adults may provide a clearer understanding of the relationship between SO and cognitive impairment.
Frailty is a key concern in SO, with affected individuals showing significantly higher frailty scores than those with sarcopenia or obesity alone [23, 24]. The increased mortality risk observed in the SO group further underscores the compounded burden of these two conditions [3, 4, 25, 26]. The mechanisms underlying this association may include chronic inflammation, hormonal dysregulation, and increased oxidative stress, all contributing to both frailty and mortality [27,28,29]. Given these risks, targeted interventions such as resistance exercise, nutritional optimization, cognitive monitoring, and metabolic management are essential to delay or mitigate frailty progression in SO patients. Implementing structured exercise programs, ensuring adequate protein intake, and controlling metabolic risk factors may help maintain functional independence and reduce adverse outcomes.
Our study found that geriatric factors, particularly low Mini Nutritional Assessment (MNA-SF) scores and reduced handgrip strength, are more strongly associated with sarcopenic obesity (SO) than metabolic risk factors such as hypertension and diabetes mellitüs. This finding suggests that geriatric syndromes play a more significant role in SO development than metabolic syndromes, highlighting the importance of addressing malnutrition and muscle weakness in preventive and therapeutic strategies. Previous studies have shown that SO is associated with both metabolic and geriatric factors; for instance, a study reported that the coexistence of obesity and sarcopenia increases frailty and disability risk, while another study linked sarcopenia and obesity to cardiovascular diseases and mortality [30]. However, these studies did not differentiate whether metabolic or geriatric factors had a stronger association with SO. Our study adds to the literature by demonstrating that nutritional status and muscle function are more direct determinants of SO than metabolic disturbances alone. Malnutrition contributes to muscle wasting and functional decline, accelerating SO progression, while decreased muscle strength directly impairs physical function and increases dependency. Although metabolic factors such as hypertension and diabetes mellitüs may not be the primary drivers of SO, they could contribute indirectly by exacerbating inflammation, insulin resistance, and energy metabolism imbalances, which may accelerate sarcopenia and malnutrition [8]. This interaction suggests that metabolic and geriatric factors are not isolated but rather interconnected in the pathogenesis of SO. Given these findings, a comprehensive approach addressing metabolic and geriatric factors is crucial for effective SO prevention and management, focusing on early nutritional support, resistance training, and metabolic control to mitigate the adverse outcomes associated with SO.
Due to SO’s multifactorial nature, a multidisciplinary approach is required for effective management. To identify at-risk individuals early, routine screening using MNA-SF, MMSE, and handgrip strength should be incorporated into clinical practice [6, 31]. These tools comprehensively evaluate nutritional deficits, cognitive impairment, and muscle strength, crucial components in SO management. Once SO is identified, an individualized treatment plan should be developed to address each patient’s needs. Management strategies should include structured resistance exercise programs to enhance muscle strength, functional capacity, and mobility [23, 24]. Exercise interventions, particularly progressive resistance training, have mitigated muscle loss and improved overall functional performance in SO patients. In addition to exercise, nutritional optimization is fundamental, emphasizing adequate protein intake and caloric balance to prevent further muscle wasting and promote anabolic processes [8]. The recommended dietary approach should focus on high-quality protein sources, leucine-enriched supplementation, and adequate caloric intake to counteract the catabolic effects of SO. Nutritional interventions should be individualized, considering comorbid conditions such as renal function and metabolic disorders. Furthermore, cognitive and functional monitoring should be regularly conducted to detect and manage cognitive decline and physical impairment at early stages [18, 19].
Lastly, comprehensive metabolic control is essential, particularly for individuals with diabetes mellitüs and hypertension, as these conditions can contribute to the inflammatory and metabolic dysfunction underlying SO progression [31, 32]. Optimizing glycemic control, blood pressure regulation, and lipid profile management through pharmacological and lifestyle interventions may help mitigate the systemic effects contributing to SO. A multidisciplinary team approach, including geriatricians, dietitians, physiotherapists, and endocrinologists, is recommended to implement a holistic, patient-centered management plan that effectively addresses SO and its complications.
Prospective cohort studies are needed to establish causality between SO and adverse health outcomes. Additionally, randomized controlled trials should investigate the efficacy of exercise and nutrition-based interventions in reducing SO prevalence and mitigating its complications. Future research should also explore the biological mechanisms linking SO with frailty, cognitive decline, and mortality, particularly the role of inflammatory and hormonal pathways [32,33,34].
This study has several limitations that should be acknowledged to provide a comprehensive understanding of the findings. The retrospective cross-sectional design of our study limits the ability to infer causal relationships between sarcopenic obesity (SO) and the clinical outcomes observed. While associations are evident, the lack of temporal data prevents establishing whether SO is a direct cause or a consequence of these outcomes. Future longitudinal studies are necessary to confirm causality and explore the progression of SO over time.
Although multivariate analyses were conducted to adjust for some variables, such as hypertension and diabetes mellitüs, this study did not account for other potential confounding factors. Systemic syndromes, other comorbidities (e.g., chronic kidney disease, cardiovascular diseases), and lifestyle factors (e.g., physical activity levels, dietary habits) could have influenced the results. The inability to fully control for these factors is a limitation that may have introduced bias into our findings.
Another potential limitation of this study is the extended duration of data collection for a cross-sectional design. We performed a time-segmented analysis to address potential sampling and measurement bias concerns, dividing the dataset into different collection periods. Although the study spans multiple years, we did not observe any major institutional or procedural changes that would be expected to introduce systematic bias over time. Therefore, we assume the data to be temporally consistent. Furthermore, to minimize variability, all data collection procedures followed standardized protocols across the study duration, ensuring uniformity in measurements and assessments. While this approach mitigates the impact of long-term data collection on study findings, we acknowledge that residual biases may still exist.
Our sample was from hospitalized patients, which may not represent the general geriatric population. Hospitalized individuals often exhibit more severe health conditions and frailty compared to community-dwelling older adults. This selection bias limits the external validity of our findings and may overestimate the prevalence and impact of SO in the broader population. However, it is also important to note that sarcopenic obesity and its associated negative health consequences may be more prominently observed in hospitalized older adults, making this population particularly relevant for studying the condition’s clinical significance. Future research involving community-based samples could provide more generalizable insights. Furthermore, while detailed nutritional intake records were unavailable, we ensured all participants underwent the Mini Nutritional Assessment (MNA-SF), a validated and reliable tool widely used in geriatric populations. This provides an objective measure of nutritional status, helping to account for variations in dietary intake.
We acknowledge that specific inflammatory biomarkers were not comprehensively assessed regarding inflammatory status. However, key laboratory parameters such as C-reactive protein (CRP), leukocyte count, erythrocyte sedimentation rate (ESR), and albumin levels were available and analyzed. This study has several strengths that contribute significantly to understanding sarcopenic obesity (SO) in older adults. First, the comprehensive use of geriatric assessment tools, including ADL, IADL, MMSE, MNA-SF, GDS, and handgrip strength, allows for a multidimensional evaluation of physical, cognitive, and psychosocial health. Additionally, the study provides a detailed analysis of SO, highlighting its distinct impacts compared to obesity or sarcopenia alone. By utilizing logistic regression, the study identifies independent risk factors for SO, such as diabetes mellitüs, hypertension, and poor nutritional and cognitive status, offering valuable insights for targeted interventions. Furthermore, the association of SO with frailty and mortality underscores its clinical importance and emphasizes the need for early detection and management. The hospital-based real-world data strengthen the study’s relevance while including diverse functional, cognitive, and biochemical parameters to enrich its findings. Overall, the study addresses an underexplored area in the literature, providing a robust foundation for future research and clinical applications. Importantly, this study is among the first to apply the ESPEN–EASO consensus criteria for diagnosing SO in a hospitalized older adult population. The use of these standardized criteria enhances the comparability of findings across studies and supports a unified diagnostic framework. Notably, our analysis demonstrated that geriatric parameters—particularly handgrip strength, nutritional status, and frailty—offered greater discriminatory power for SO than traditional metabolic indicators. These findings underscore the importance of integrating geriatric assessments into SO screening and highlight the clinical value of ESPEN–EASO-based diagnostic approaches in routine geriatric care.
Sarcopenic obesity (SO) is a clinically significant condition in older adults, associated with increased frailty, functional decline, and mortality. Our findings indicate that geriatric factors—particularly malnutrition and muscle weakness—are more strongly associated with SO than traditional metabolic risk factors. These results highlight the importance of incorporating routine geriatric assessments, including handgrip strength and nutritional screening, into clinical practice. A multidisciplinary approach targeting both metabolic and geriatric domains is essential for the early detection and management of SO.
No datasets were generated or analysed during the current study.
Not applicable.
This research received no external funding.
The study was conducted in accordance with the principles of the Declaration of Helsinki. Ethical approval was obtained from the Clinical Research Ethics Committee No. 1 of Ankara Bilkent City Hospital (Approval No: E1-22-2391, Date: 29/06/2022). Written informed consent was waived by the ethics committee due to the retrospective nature of the study.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Silay, K., Selvi Oztorun, H. Sarcopenic obesity is linked to worse clinical outcomes than sarcopenia or obesity alone in hospitalized older adults. BMC Geriatr 25, 443 (2025). https://doi.org/10.1186/s12877-025-06105-2