Critical Care volume 29, Article number: 237 (2025) Cite this article
The duration of episodes of intracranial hypertension is related to poor outcome, hence the need for prompt diagnosis. Numerous issues can lead to delays in the implementation of invasive intracranial pressure (ICP) monitoring, thereby increasing the dose of intracranial hypertension to which the patient is exposed. The aim of this prospective, observational, multicenter study was to assess the magnitude of this delay, evaluating the time required for initiation of invasive ICP monitoring, from indication (T1) to initiation of the maneuver (T2) when performed by neurosurgeons compared to intensive care physicians.
We evaluated the impact of the operator performing the maneuver (neurosurgeon vs. intensivist) on the T2-T1 time interval, where T1 represents the time at which indication for invasive ICP monitoring is declared, and T2 the time at which the maneuver starts, defined as the skin incision. The effect of the operator performing the maneuver was evaluated through a parametric survival model. Both intraparenchymal catheters (IPCs) and external ventricular drains (EVDs) were considered as invasive ICP monitoring devices. Invasive monitoring could be performed in intensive care unit (ICU) or in operating room (OR).
A total of 112 patients were included into the final analysis; 39 IPCs were placed by intensivists within the ICU, and a total of 73 IPCs and EVDs by neurosurgeons both within the ICU and OR settings. The mean difference in T2-T1 time for IPCs placement in the ICU was 69 min (CI 50.1–94.8) in the intensivist group and 145 min (CI 103.4–202.9) in neurosurgeon group. The mean difference between these groups, 76 min, was found to be statistically significant (p-value = 0.0021). In the group treated by neurosurgeons, no statistically significant differences were found in timing between the ICU and the OR.
Invasive ICP monitoring performed with IPCs in ICU begins earlier when performed by intensivists rather than neurosurgeons. This finding suggests the possibility to obtain a prompt diagnosis of intracranial hypertension when intensivists intervein directly at patient’s bedside. Further studies are needed to confirm these findings and investigate their effect on outcome.
Invasive intracranial pressure (ICP) monitoring is of paramount importance in managing patients with acute brain injury, allowing accurate diagnosis and treatment of intracranial hypertension [1,2,3,4,5].
Intracranial pressure dose, which reflects both the intensity and duration of the intracranial hypertensive episode, has been identified as an independent predictor of mortality and severe disability in several studies [6,7,8,9], highlighting the importance of prompt diagnosis in the effort to improve patient outcomes.
The placement of invasive devices for ICP monitoring has traditionally been a procedure reserved exclusively for neurosurgeons. However, factors such as shortages of neurosurgeons in certain areas and limited resources can delay the placement of these devices, thereby increasing the ICP dose to which patients are exposed [10]. In recent years the placement of devices for ICP monitoring (in particular IPCs), has been increasingly performed by non-neurosurgeons, including intensivists, with a similar low incidence of complications, as described in literature [11,12,13,14,15,16,17]. The ability of trained intensivists to intervene directly at the patient's bedside by safely placing an invasive device as soon as intracranial hypertension is suspected, and its monitoring is indicated, could lead to a reduction in the ICP dose. With these assumptions in mind, the TIMING-ICP study group (Timing of Invasive intracranial pressure MonitorIng placement between NeurosurGeons and Intensive Care Physicians) decided to carefully analyze the timing required to initiate invasive ICP monitoring. The goal was to assess the magnitude of any delays in its implementation and to examine whether there are significant differences when the procedure is performed directly by intensivists versus neurosurgeons called to carry it out.
The primary objective of the study was to compare the amount of time required to initiate invasive ICP monitoring, following its indication, when performed by neurosurgeons versus intensive care physicians. Importantly, this is a consecutive series and therefore randomization was not performed. Given the observational nature of the study, the choice of operator performing the procedure was based solely on the standard clinical practices at the center.
The secondary objective was to compare post-procedural complications between the group in which the device was placed by intensivists and the group treated by neurosurgeons.
This observational, prospective, multicenter national study was promoted and coordinated by the Department of Neuroanesthesia, Neurocritical and Post-operative Care of the ASST Spedali Civili University affiliated Hospital of Brescia and involved the intensive care unit and neurosurgical units of 7 centers throughout Italy.
Data collection started in April 2021, and was extended from the initial ending date of April 2023 to February 2024 due to delays caused by the Covid-19 pandemic. The study protocol was approved by the local Ethics Committee (NP 4628) and subsequently by the Committees of all participating centers. Since the patients were all unconscious, informed consent was obtained retrospectively in cases where mental faculties were restored, as required by Italian law.
Data were initially collected using an electronic data collection sheet, after which they were fully transferred and stored in a database within RedCap.
The study included centers where catheter placement was performed only by neurosurgeons, as well as centers where it was carried out by both neurosurgeons and intensivists. In the latter case, the choice of specialist performing the maneuver was based on an internal logistical decision at each center, as further detailed in the following sections.
The study included all patients aged 18 years or older with acute brain injury in whom urgent indication for invasive ICP monitoring was declared.
Patients were excluded if ICP monitoring was not urgently indicated, if the external ventricular drain (EVD) was primarily placed for reasons other than urgent ICP monitoring, or if a significant coagulation abnormality contraindicated the drill hole procedure.
Epidural catheters used for monitoring after decompressive craniectomy were not considered as urgently placed, as this did not meet the inclusion criteria. Instead, patients who underwent neurosurgery and later required invasive ICP monitoring during their hospital stay were included.
The parameter identified for comparison between the group treated by intensivists and the group treated by neurosurgeons was the time interval (ΔT2-T1) between the moment at which invasive ICP monitoring was deemed necessary, indicated as T1, and the moment at which device placement procedure began, defined as skin incision and denoted as T2.
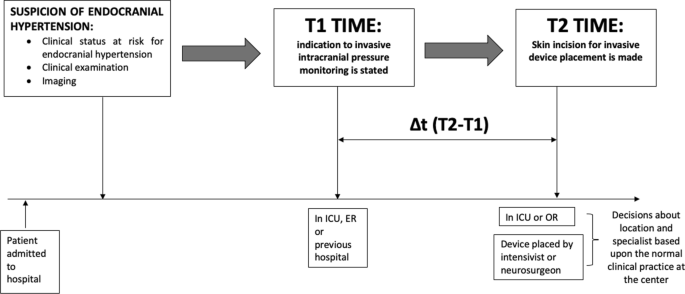
The primary outcome was the comparison of the mean time interval (ΔT2-T1) in patients managed by neurosurgeons versus those managed by intensivists. Subgroups were created based on the type of device used and the location where the procedure was performed (Fig. 1).
Visual representation of the study design. T1 is the moment at which invasive intracranial pressure monitoring is stated; T2 is the moment at which skin incision is performed to allow intracranial device placement. ICU: Intensive Care Unit, ER; Emergency Room, OR: operatory room
We excluded the procedural time from the analysis, as it was performed by trained personnel. Specifically, the neurosurgeons and intensivists were all attending physicians, and the intensivists who performed the procedure had specific training. Therefore, the procedural time was considered consistent. The greater variability in timing was expected due to delays in starting the maneuver itself, which was the focus of the study.
Indication for invasive ICP monitoring could be provided in the emergency department, during ICU stay or in the hospital ward where the patient was first admitted.
The decision to perform invasive ICP monitoring in traumatic brain injury (TBI) was made following consultation between the intensivist and the neurosurgeon, in accordance with the Brain Trauma Foundation guidelines, fourth edition [3, 18].
In cases of spontaneous intraparenchymal hemorrhage, the main criteria for indicating invasive ICP monitoring were a GCS (Glasgow Coma Scale) score ≤ 8, evidence of transtentorial herniation on CT scan, hemorrhagic involvement of the ventricular system or hydrocephalus, and recent guideline recommendations for managing spontaneous intracerebral hemorrhage [19].
In other clinical conditions where intracranial hypertension may occur, specific guidelines [20, 21], along with clinical judgement were used to determine the need for invasive ICP monitoring.
If the neurosurgeon determined the need for an EVD primarily for cerebrospinal fluid (CSF) drainage, the patient was excluded from the study. However, patients were included if EVD placement was the only option for ICP monitoring or if there was an urgent need to measure and monitor ICP and, based on the brain CT, CSF drainage was highly probable to reduce elevated ICP.
In centers where both clinicians placed the catheters, the procedure was typically performed by the intensivist. If an intensivist was unavailable, the consulting neurosurgeon performed the procedure instead. In centers where the neurosurgeons were the only clinicians performing invasive monitoring, they carried out the procedure. While the intensivist managed the patient directly at bedside in the ICU, the location where the neurosurgeon performed the maneuver depended on factors such as internal protocols or agreements, and the availability of the operating room (OR). The setting where the maneuvers were performed, whether in the ICU, OR or emergency department (ED), were appropriately recorded.
The procedure for placing intraparenchymal devices involved positioning the patient supine, with the head raised at a 30° angle. The Kocher point was identified, and the surgical field was prepared by shaving and disinfecting the scalp with an antiseptic solution and placing sterile drapes. Local anesthesia and sedation were administered if necessary. An incision was then made in the skin and subcutaneous tissue with a 2–3 cm antero-posterior cut at the Kocher point. The tissues were retracted, the periosteum scraped off and the drill tip was prepared. Once the drill was ready, a hole was made by positioning the instrument perpendicular to the skull surface, with a slight inclination. The hole was irrigated with sterile saline, and the dura mater was exposed and opened with an appropriate needle (e.g. a 18-G spinal needle). The bolt was then screwed into the skull to provide support, and a stylet was passed through the hole to clear the passage. The bolt was irrigated, and the catheter tip was zeroed before being inserted through the bolt and secured to the scalp to prevent displacement.
The procedure used for EVD placement was similar to that described for the intraparenchymal catheter up to the point of dura mater opening. At this stage, a trocar was advanced subcutaneously from the lower edge of the surgical wound, emerging about 5 cm posteriorly. The catheter, mounted on a stylet, was then passed through the burr hole and advanced toward the contralateral medial canthus, perpendicular to the cranial surface for 6.5 cm, until reaching the anterior horn of the ipsilateral lateral ventricle. The stylet was then removed, and correct placement was confirmed by cerebrospinal fluid outflow. Using the trocar previously placed subcutaneously, the ventricular shunt was tunneled in the subcutaneous tissue, sutured to the skin, and then connected to the drainage system and zeroed. Imaging-guided techniques were not used for device placement. Correct positioning was confirmed post-procedure using a brain CT scan.
The complications included: catheter-related intracranial hemorrhage (with or without the need for neurosurgical intervention), CFS infection (confirmed by microorganism isolation), catheter mispositioning (defined as the catheter penetrating a few millimeters into the brain parenchyma after initial correct placement or the absence of the pressure waveform), and catheter dysfunction (defined as a failure to display a correct pressure waveform within 12 h of catheter placement).
Data were summarized using mean and standard deviation, median and IQR, for quantitative variables, counts and percentages for qualitative variables.
Delta time in the placement of invasive ICP monitoring is assumed as T2-T1, declared in minutes.
Typology operator (neurosurgeon vs intensivist) impact on delta time was evaluated through a parametric survival model with an exponential family for a time-to-event outcome with robust variance estimate to account for intra-center correlation (clustering effect).
The incidence of complications, valued as a binary variable, was evaluated through a logistic model fitted using a GLMM (generalized linear mixed model) with the organization exposed in the dedicated data element. Results were reported as estimates and corresponding 95% confidence interval. The analyses assumed a two-sided 5% significance level and were performed using R (version 4.4.1).
Sample size assessment was performed by Monte Carlo simulation (B = 500) using an exponential parametric model. Assuming a time decrease (T2-T1) of 70 min in the procedure carried out by an intensivist compared to a neurosurgeon, with a mean time of 140 min with 1:1 allocation between the two groups (intensivist:neurosurgeon), a total number of 70 patients (35 treated by intensivists and 35 by neurosurgeons), allows to evaluate the interest effect with a power of at least 85%, and a significance level of 5% (Fig. 1).
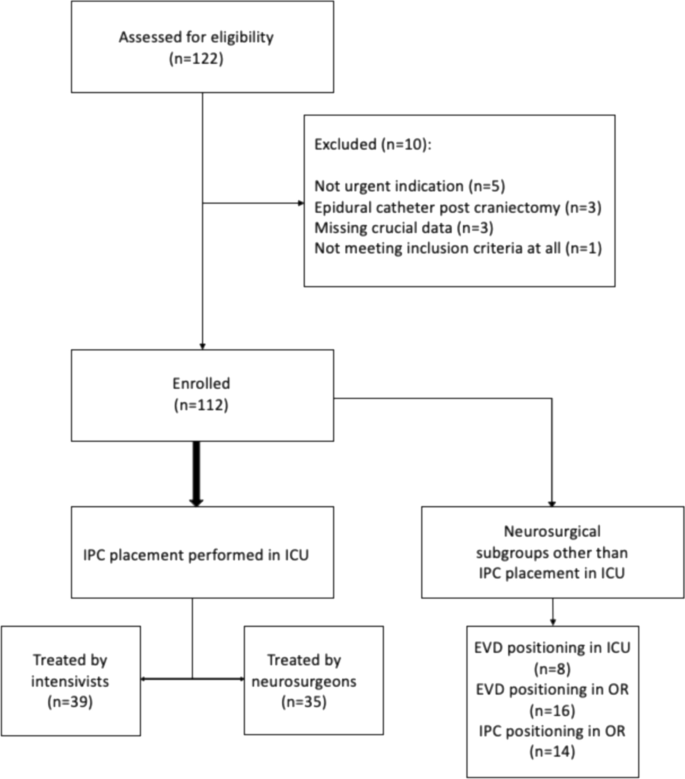
A total of 112 out of 122 patients assessed for eligibility, were included into the study (Fig. 2).
The flow chart illustrates the process of selection of eligible patients and their division into two groups according to the specialist who placed the invasive intracranial pressure monitoring device. The location where the device was placed is also indicated. IPC: intraparenchymal catheter, EVD: external ventricular drain, ICU: intensive care unit, OR: operatory room
Although the initial estimated sample size was 70 patients, a larger number of patients was ultimately included in the study to ensure the comparability of the resulting categories. The placement of IPCs in the OR and EVDs solely for intracranial pressure monitoring was rarely observed during data collection, so it was decided to continue including in the neurosurgical group only patients who received intraparenchymal devices placed in the intensive care unit. This was done mainly to obtain a group directly comparable to the one treated by intensivists. In fact, it is notable that while the group treated by intensivists was homogeneous, in that all patients were treated in the ICU setting, the neurosurgical group was not, as it included patients treated both in the ICU and in the OR. Data from patients who had intraparenchymal catheters placed in the OR and those receiving EVDs, which did not reach the expected sample size, were combined into a separate subgroup, as shown in the Fig. 2.
Demographic data, etiologies, and clinical and radiological severity scores are presented in Table 1. In particular, traumatic brain injury (TBI) was the most common (61%), with a median Marshall score of 4 (IQR 2), followed by subarachnoid hemorrhage (SAH) (21%), with a median Fisher score of 4 (IQR 1) and intraparenchymal hemorrhage (ICH) (11%), with a median ICH score of 3 (IQR 1).
As required by study protocol, neurosurgical consultation was performed in all cases. The median GCS score at T1 was 4 in the group treated by the intensivists and 7 in the neurosurgical group, while the median RASS (Richmond Agitation Sedation Scale) score at T1 was −4 in both groups.
The indication for invasive ICP monitoring was declared in the ICU in 74% of cases, in the ED in 19% of cases, and in the hospital where the patient was previously admitted in 7% of cases. Invasive ICP monitoring was performed in the ICU in 72% of cases and in the OR in 28% of cases (Table 2).
Out of 112 procedures, 64 were performed during the day (08:00–20:00) and 48 at night (20:00–8:00). A chi-square test showed no significant difference in the frequency of procedures performed during the day versus night (χ2 = 2.29; p-value 0,13). Even when comparing the group treated by intensivists to the group treated by neurosurgeons, the chi-square test did not reveal any statistically significant differences regarding the time of day the procedure was performed (χ2 = 0.24; p-value = 0,63).
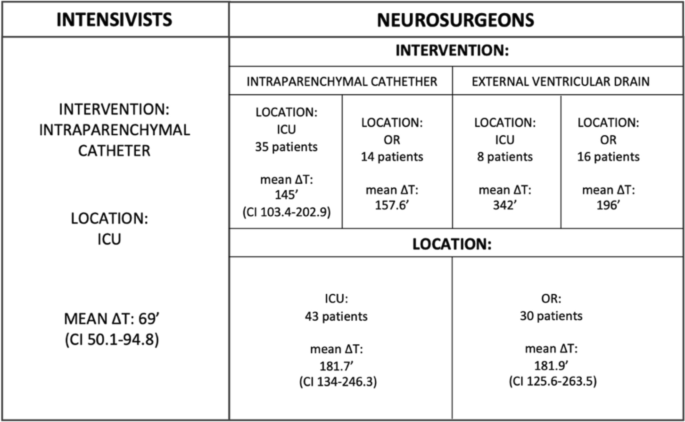
In particular, 39 patients were treated by intensivists with intraparenchymal catheter placement in the ICU, with a mean Δt (T2-T1) of 69 min (CI 50.1—94.8), while 35 patients were treated by neurosurgeons, who placed 35 intraparenchymal catheters in the ICU with a mean Δt (T2-T1) of 145 min (CI 103.4–202.9). This difference in mean time (76 min) was found to be statistically significant (p-value = 0.0021) (Fig. 3).
Results of the study divided according to the intervening specialist. The neurosurgery section shows the results by both the type of device placed (intervention) and the location where the maneuver was performed (location). ICU: intensive care unit, OR: operating room; ΔT: T2-T1
The subgroup treated by neurosurgeons with EVDs (in both the ICU and OR) and IPCs in the operating room included a total of 38 patients. Of these, 14 underwent intraparenchymal catheter placement in the operating room (OR) with a mean Δt (T2-T1) of 157.6 min, 16 patients underwent EVDs placement in the OR with a mean Δt (T2-T1) of 196 min, and 8 underwent EVDs placement in the intensive care unit (ICU) with a mean Δt (T2-T1) of 342 min.
The average intervention time of neurosurgical patients were analyzed according to the location where the procedure was carried out. The average Δt (T2-T1) for patients treated in the ICU (n = 43) was 181.7 min (CI 134.0–246.3), while for those in the OR (n = 30) it was 181.9 min (CI 125.6–263.5). The difference between these averages was found to be non-significant (p-value 1) (Fig. 3).
The average ICP at opening was 14.1 mmHg; 19% of patients with TBI had an ICP > 22 mmHg, while intracranial hypertension (defined as ICP > 20 mmHg) affected 13% of patients with other etiologies.
The chi-square test did not show statistically significant differences between the groups of patients who underwent device placement within a ΔT2-T1 of 60 min and those treated after 60 min (χ2 = 0.96; p-value = 0.33,) regarding the presence of opening pressure greater than 20 mmHg. The same result was obtained when considering a 35 min intervention window (χ2 = 0.39; p-value = 0.53).
Regarding the secondary objective of the study, although no clinically relevant complications were recorded, the complication rate recorded according to the study criteria was 11.8%.
In the group treated by intensivists, the only recorded complication was optic fiber misplacement. In contrast, 15 complications were observed in the neurosurgical group (Table 3). The most frequent was fiber malposition (8 cases), followed by catheter malfunction (4 cases), CSF infection (2 cases), and one case of bleeding related to an intraparenchymal catheter, which did not require neurosurgical intervention. However, statistical analysis revealed that the difference in complications rates between the two groups was not statistically significant. In particular, the probability of the complication occurring was 2.6% (95% CI: 0.4–16.1) in the group treated by intensivists and 8.6% (95% CI: 2.8–23.4) in the neurosurgical group. The odds ratio was 3.56 (95% CI: 0.35–35.94), with a p-value of 0.28.
This study found a statistically significant difference of 76 min in the average time from indication to initiation of ICP monitoring with IPCs between patients treated by intensivists (mean ΔT2-T1: 69 min) and those treated by neurosurgeons (mean ΔT2-T1: 145 min) within the ICU setting.
It is clear that involving the intensivist directly at patient’s bedside in the ICU, as soon as intracranial hypertension is suspected, offers a significant benefit. This early intervention has the potential of reducing the duration of elevated ICP, minimizing the patient’s exposure to intracranial hypertension. Indeed, numerous studies have demonstrated that both the intensity and duration of the episode are pivotal determinants of the patient's ultimate outcome. As highlighted in this analysis, the neurosurgeon may not always be readily available for the placement of an invasive device, which is currently considered crucial for managing intracranial hypertension. This can lead to delays in the targeted treatment, potentially affecting patient outcomes.
Although invasive intracranial pressure monitoring is traditionally performed by neurosurgeons in most parts of the world, to the best of our knowledge, its implementation by other specialists is increasingly common in several European countries, the United States, and some low-income nations [13, 15,16,17, 22,23,24]. Therefore, we believe the results are generalizable. However, conducting a multicenter international study would provide more robust evidence for their generalizability.
The chi-square test did not show statistically significant differences in the presence of opening pressure values indicative of intracranial hypertension (defined as ICP > 20) between the groups treated within a ΔT2-T1 of 60 min or less and those treated beyond this ΔT2-T1 (p-value = 0.33). This result was also confirmed when considering a ΔT2-T1 of 35 min (p-value = 0.53). This may be due to confounding factors, such as emergency interventions performed shortly before based on strong clinical and radiological suspicion of intracranial hypertension. However, the data collection did not include information on therapeutic interventions. Additionally, opening pressure may not be entirely accurate; nevertheless, it was chosen as the endpoint to standardize measurements. Further studies, extending into the post-device placement period with sequential time points, may provide more clarity. Finally, our findings further support the importance of invasive intracranial pressure monitoring, which remains the gold standard for evaluating intracranial pressure.
The analysis of the entire cohort treated by the neurosurgeons revealed no significant differences between the observed OR and ICU intervention times. Therefore, the location of the procedure does not appear to significantly affect the timing of monitoring initiation. What seems to have a greater impact is the availability of an operator who can immediately intervene to place the device. Nevertheless, obtaining a recommendation from a specialist on the need for invasive intracranial pressure monitoring is crucial. This can be achieved remotely through teleconsultation, enabling the initial management of patients with suspected intracranial hypertension, even in facilities without a neurosurgical unit.
Patients treated by intensivists had a median GCS at T1 of 4, whereas those treated by neurosurgeons had a median GCS at T1 of 7. This difference may represent a selection bias, as intensivists might have initiated monitoring earlier, even when not strictly necessary. To account for this potential bias, an analysis was conducted to adjust the results for GCS at T1. After adjusting for GCS at T1, patients treated by neurosurgeons still had a significantly longer T2-T1 time compared to those treated by intensivists (estimates = 2.07, 95% CI [1.34–3.21], p-value = 0.001). However, GCS at T1 was not a significant predictor of T2-T1 time (estimates = 1.03, 95% CI [0.97–1.09], p-value = 0.309), suggesting that the difference in intervention times between physician groups was independent of the initial GCS score. These results indicate that despite the lower baseline GCS in the intensivist-treated group, the treating physician remained a significant predictor of intervention timing and intensivists did not intervene earlier because they cared more critically ill patients.
No significant difference was found in the distribution of procedures performed during the day (n = 64) versus night (n = 48) (p-value = 0.13). Similarly, no statistically significant differences were found between intensivists and neurosurgeons regarding the time of day the procedure was performed (p-value = 0.63). These findings suggest that the timing of the procedure, whether performed during the day or at night, does not significantly differ between the overall cohort or when comparing patients treated by intensivists and neurosurgeons. Other factors, such as clinical urgency or staffing, may have played a larger role in determining the timing of the procedures.
Our analysis found no statistically significant differences in complication rates between patients treated by intensivists and those treated by neurosurgeons. The most common complication was catheter misplacement, which occurred in seven neurosurgical patients: 2 TBI cases (median Marshall: 3), 2 SAH cases (median Fisher: 3), 2 ICH cases (median ICH: 3), and 1 with a cerebral neoplasm. The median presenting GCS was 9 in these cases. Compared to the overall cohort (median Marshall: 4, Fisher: 4, ICH: 3, GCS: 7), clinical and radiological severity does not appear to be associated with a higher risk of misplacement. Additionally, the incidence of this and other complications aligns with rates reported in the literature.
This finding is consistent with previous studies and supports our hypothesis that intensivist-performed procedures are safe and time-efficient, particularly in centers where this practice has been integrated into routine clinical workflows. In fact, when analyzing average Δt (T2-T1), we found that in the two centers where intensivists routinely perform this procedure, the average times were 35 and 57 min (each based on 12 patients). Conversely, in centers where this approach was recently introduced, the average times were 74, 116, and 122 min (each based on 5 patients). However, the time differences between these groups were not statistically analyzed to ascertain their significance, since the study was not powered for such an analysis. Despite this, the multicenter design, which included units with varying levels of experience, allowed us to observe these trends.
This study allowed us to assess the time required to initiate intracranial pressure monitoring, revealing a statistically significant average difference of 76 min between the two groups. However, the average time of 69 min in the intensivist group also warrants improvement. Literature [6, 7] suggests that even short episodes of intracranial hypertension are associated with worse outcomes. We hope these findings encourage physicians managing at-risk patients to implement strategies that minimize delays in initiating invasive monitoring.
To the best of our knowledge, this is the first study to investigate the timing required to initiate invasive intracranial pressure monitoring performed by intensivists and neurosurgeons.
Based on the promising results of this study, we plan to conduct an international multicenter study, possibly a randomized controlled trial, to confirm our findings. This study will also assess how delays in intracranial pressure monitoring impact patient outcomes, including mortality, long-term neurological outcomes, and the need for additional interventions.
Additionally, a further parameter marking the conclusion of the invasive device placement procedure should be included in future studies to assess potential discrepancies in procedural timing between the two groups, as this aspect was not considered in the current study.
One key limitation of this study is that it was not designed with sufficient statistical power to detect significant differences in patient outcomes between those who underwent early ICP monitoring and those who had it placed later. As a result, we are unable to draw definitive conclusions about the effects of delayed monitoring compared to early monitoring on critical outcomes such as mortality, morbidity, and neurological recovery. To better understand the impact of ICP monitoring timing, future studies with larger sample sizes and greater statistical power will be necessary.
Additionally, we recognize that the presence of intracranial hypertension is a crucial factor in evaluating patient outcomes, and accurate assessment of this condition is essential for determining the appropriate interventions. However, due to the scope of the current study, we did not explore the relationship between delayed ICP monitoring and subsequent ICP elevations or the potential need for more aggressive treatments. We acknowledge that this is an important area for future investigation and that further research is needed to address this gap.
Other limitations should be considered when interpreting the results of this study. First, the collection of data regarding therapeutic interventions was not included, as it was beyond the scope of the research. Therapeutic interventions may have influenced management strategies in patients with suspected intracranial hypertension, potentially delaying the indication for the placement of an invasive device. This could have certainly impacted the timing of T1 and possibly also T2. The lack of data on therapeutic interventions prevented us from precisely analyzing the actual presence of intracranial hypertension at the time of device placement, as we cannot determine whether the recorded values were influenced by any treatments administered. While this issue extends beyond the study’s scope, it remains an important factor for future research.
Additionally, more specific information regarding the operators' level of experience was not available for this analysis. We acknowledge that this aspect is important for better understanding the impact of operator experience on managing timing. We believe that this issue warrants further investigation in future studies.
This study revealed that within the ICU setting, intraparenchymal catheter placement for invasive ICP monitoring is initiated quicker when performed by intensivists compared to neurosurgeons, with similar low complication rates. This may reduce the overall burden of intracranial hypertension to which the patient is exposed. Further investigation, preferably through a randomized controlled trial, is warranted to investigate the impact of procedural delays, the duration of the device placement itself, and the effect of early monitoring on patient outcomes.
No datasets were generated or analysed during the current study.
- ICU:
-
Intensive care unit
- OR:
-
Operatory room
- ED:
-
Emergency department
- ICP:
-
Intracranial pressure
- IPCs:
-
Intraparenchymal catheters
- EVDs:
-
External ventricular drains
- CSF:
-
Cerebrospinal fluid
- TBI:
-
Traumatic brain injury
- SAH:
-
Subarachnoid hemorrhage
- ICH:
-
Intraparenchymal hemorrhage
- GCS:
-
Glasgow Coma Scale
- RASS:
-
Richmond Agitation Sedation Scale
- CI:
-
Confidence interval
- IQR:
-
Interquartile range
- ICH score:
-
Intracerebral hemorrhage score
- Marshall TBI:
-
Marshall classification of traumatic brain injury
- ASPECTS:
-
Alberta Stroke Program Early CT score
- CT:
-
Computed tomography
The authors of TIMING-ICP would like to dedicate this paper to Dr. Emanuele Russo, who passed away December 2024 at the age of 47, and to his wonderful family who must deal with this incredible void he leaves behind. Emanuele, who worked at the Bufalini hospital of Cesena and led the TIMING-ICP study for this site, was a dedicate clinician, researcher, and friend.
The present study has not been funded.
The study protocol and its amendments were approved by the ethics committee of Brescia, located at Spedali Civili Hospital, Brescia, (NP 4628) and subsequently by the ethics committees of the centres participating in the study.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Cite this article
Mariani, L., Calza, S., Gritti, P. et al. From indication to initiation of invasive intracranial pressure monitoring time differences between neurosurgeons and intensive care physicians: can intracranial hypertension dose be reduced? TIMING-ICP, a multicenter, observational, prospective study. Crit Care 29, 237 (2025). https://doi.org/10.1186/s13054-025-05384-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-025-05384-w