Trials volume 26, Article number: 238 (2025) Cite this article
The majority of individuals with chronic stroke have residual upper extremity (UE) disability which they cite as their greatest barrier to recovery. Using orthoses, robotic devices, and functional electrical stimulation (FES) represent rehabilitation techniques that have demonstrated the ability to improve arm and hand function in the chronic stroke population, but individuals with more severe impairments are typically not eligible for these studies. The very few studies incorporating these techniques with the severely impaired population do not utilize volitional FES control or modulated loading, which has been shown to promote greater motor recovery and functional improvement. An UE intervention utilizing an advanced orthosis incorporating volitionally controlled FES and robotically modulated shoulder abduction (SABD) loading may provide a pathway to improved coordinated use of the arm and hand for the more severely impaired chronic stroke population.
In a double-blinded, two-baseline, randomized control trial individuals with chronic moderate to severe stroke resulting in UE hemiparesis will participate in a task-based reaching-grasping-retrieving-releasing (GR3) intervention three times a week for 8 weeks. An anticipated 60 individuals will perform a repeated GR3 task with the ReIn-Hand device (ReIn-Hand), a customized forearm/hand orthosis integrating volitionally controlled FES, to assist with paretic hand-opening. Participants will be randomly assigned to a control group (ReIn-Hand only) or experimental group which will also receive modulated SABD loading via the PACT3D robot. Groups will be compared by (1) their change in function primarily measured by the Box and Blocks Test; (2) change in performance measured by kinematic analysis of reaching and hand-opening and (3) changes in neural motor recovery measured using electroencephalography (EEG) and magnetic resonance imaging (MRI).
The present study will evaluate the effectiveness of a novel interventional device, with and without shoulder abduction assistance, as part of a task-specific training protocol with the moderate to severe chronic stroke population. The focus on the more impaired chronic stroke population provides the opportunity to improve the rehabilitation of this overlooked population. Functional and structural measures using advanced imaging techniques offer the possibility to further delineate recovery and compensation at the neuronal level.
ClinicalTrials.gov ID NCT04077073. Registration date: September 04, 2019.
Note: the numbers in curly brackets in this protocol refer to SPIRIT checklist item numbers. The order of the items has been modified to group similar items (see http://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/).
Title {1} | Effects of device-assisted practice of activities of daily living in a close-to-normal pattern on upper extremity motor recovery in individuals with moderate to severe stroke |
Trial registration {2a and 2b}. | Clinicaltrials.gov ID NCT04077073. |
Protocol version {3} | Version 12; May 3, 2023 |
Funding {4} | U.S. NIH Grant: 5R01HD095187 Department of Physical Therapy and Human Movement Sciences Feinberg School of Medicine, Northwestern University |
Author details {5a} | Justin Drogos*, Carolina Carmona*, Riegele Arceo, Jun Yao |
Name and contact information for the trial sponsor {5b} | Northwestern University Dept of Physical Therapy and Human Movement Sciences 645 N Michigan Ave, suite 1100 Chicago, IL 60611 |
Role of sponsor {5c} | The sponsor has no role in study design; research questions; data collection, management, or analysis; and no authority over report submissions or dissemination. |
Nearly 800,000 individuals experience a stroke in the USA every year, [1] and most of these individuals will be left with upper extremity (UE) impairments [2]. In fact, stroke is a leading cause of disability in the USA with the direct costs of stroke estimated to be over $130 billion by 2035 [3]. Stroke survivors state the greatest barriers to recovery are using the arm in everyday tasks and not having enough upper extremity (UE) movement [4]. The lack of movement is more profound in wrist and finger extensor muscles, which have decreased activation and weakness following stroke, [5] and a loss of independent joint control due to the abnormal flexion synergy where movement at the proximal arm results in involuntary flexion in the distal arm (wrist and hand) [6, 7]. Individuals with chronic stroke (> 6 months post) have less UE function as compared to their peers, [2] and those with moderate to severe impairment (our target cohort) are even more limited, resulting in their exclusion from UE rehabilitation and research. As a result, rehabilitation researchers are looking for new techniques to engage individuals with stroke in task-based arm and hand exercise. Hand and wrist orthotic devices have the potential to address these limitations and allow the severely impaired chronic stroke population to participate in, and receive the benefits of, rehabilitative UE extremity exercise.
It is vital to research rehabilitation and orthotic devices that promote functional recovery in our target cohort because of what device use makes possible—exercise. Exercise is a primary component of post-stroke care, is recommended as part of post-stroke treatment, and is critical for recovery [8]. Exercise prescription is based on evidence showing that movement of the affected limb(s) and the plasticity of the nervous system will allow for changes and subsequent improvement in function [8,9,10]. Among those recommended exercise principles for the post-stroke population are task-specific practice and high dosage practice [10]. Exercise that is task-specific focuses on training a limb to perform a motor task, usually a meaningful activity of daily living, to improve performance of that task [11]. Task-specific training has been shown to be effective in improving performance and cortical activation patters in individuals post stroke [2]. Practice of higher frequency and longer duration has been shown to result in structural cortical changes and improved performance within the chronic stroke population [9, 12, 13]. Despite the increased UE disability in the chronic stroke population [2, 14] and the known benefits of exercise on stroke recovery, individuals with moderate to severe stroke, are largely ignored in the arm and hand intervention literature, and currently there is no effective intervention to restore basic hand function for individuals with chronic moderate to severe stroke. Functional electrical stimulation (FES) of the UE offers an additional means to engage individuals with moderate to severe chronic stroke in interventional studies. Orthotic devices incorporating FES offer an avenue for increased participation in task-specific exercise, but their efficacy is not yet known.
Orthotic devices incorporating FES as a means of improving arm/hand function for individuals with moderate to severe stroke have been studied, but results are inconclusive. Singer et al. [15] reported statistically significant, but clinically insignificant, improvement in UE function and impairment in their study. One study has demonstrated clinically significant improvement in UE function and impairment scores within a chronic stroke population using FES as part of a task-specific intervention, [16] but did not include the most severe stroke survivors and utilized an FES device where stimulation is not volitionally activated. When FES is initiated by volitional muscle activation, it means an individual is asked to voluntarily contract their muscles, and when their muscles’ activity reaches a certain level, the FES is triggered. Research shows FES triggered by volitional muscle activation is superior to non-volitionally, or externally, triggered FES in improving motor control of the upper extremities in the stroke population [17]. A device that utilizes FES and is triggered by volitional muscle activation, like the ReIn-Hand (ReIn-Hand Technologies Inc., DE), may yield better results than devices currently available. If volitionally triggered FES of weakened muscles can achieve superior recovery than an externally triggered FES, it is reasonable to assume incorporating intense, task-specific practice will be even more successful in improving reach and grasp function. Our device, the ReIn-Hand, utilizes both FES and volitional control and has been shown to improve both hand function and cortical activation patterns in the target population—but only with a small number of participants [12, 13].
In addition to using the ReIn-Hand, we will combine it with the PACT3D robot which can modulate the shoulder abduction load. We have evidence demonstrating that robotic modulation of shoulder abduction (SABD) loading during active reaching can reduce the UE synergy both acutely and long term [18,19,20,21]. By combining the ReIn-Hand with a robot, we propose a reaching-grasping-retrieving-releasing (GR3) intervention in individuals with moderate to severe chronic stroke. This design aims to practice activities of daily living (ADLs) in an “anti-synergy” pattern, via augmenting hand opening by the ReIn-Hand and minimizing the effects of the UE synergy by the PACT3D, to maximize potential motor recovery. We will measure not only the intervention-induced changes in clinical outcomes, but also in UE kinematics and functional and morphologic neuroplasticity to disentangle motor compensation versus recovery. Restoration of more typical cortical activation is an important component to consider when evaluating interventions in the stroke population as this can help to overcome the maladaptive synergies mentioned previously. As demonstrated in animal models, allowing compensatory movements, and thus compensatory neural activities, negatively impact neuroplasticity and motor recovery [22,23,24,25,26]. Conversely, trainings that restrict compensation heightened ipsilesional plasticity and enhanced motor recovery, [27,28,29] which is defined as the restoration of a back to pre-injured state at the levels of function, performance, and neural activities [24, 30]. This has prompted the opinion that interventions should focus on maximizing motor recovery versus task accomplishment via compensation [30].
The overarching objective of our research program is to help improve the function of people suffering from UE disability due to stroke, decreasing their disability and increasing their participation, allowing them to lead more purposeful and productive lives. With this intervention, our specific hypothesis is that a device-assisted (ReIn-Hand +/− SABD modulation) intervention that targets performing ADLs in an anti-synergy pattern with individuals with moderate to severe chronic stroke will result in improvements in hand and arm function, reaching performance, and motor recovery at the neural level. Additionally, we hypothesize that all improvements will be greater for the individuals in the experimental group (ReIn-Hand + SABD modulation) as compared to the control group (ReIn-Hand alone). Improved arm and hand function will be measured primarily via the change in score on the Box and Blocks Test. Improved reaching performance will be measured via the change in maximal hand opening area (with and without SABD load) along with the change in compensatory forces at the shoulder and elbow during hand opening.
Improved motor recovery will be measured by measured by functional and structural changes in gray matter density (GMD) and white matter integrity (WMI). We hypothesize that after the intervention both groups will have: (1) hand-opening related cortical activity shift from the contralesional to the ipsilesional hemisphere, (2) increased GMD in ipsilesional and decreased GMD in contralesional sensorimotor cortices; and (3) increased WMI in ipsilesional cortico-fugal tracks and decreased WMI in the contralesional cortico-fugal tracks, with larger changes in the experimental group compared to that in the control group.
For this initial trial of the ReIn-Hand device, we choose the chronic phase of stroke that guarantees a “worst” case situation. Answers to these questions, if positive, are expected to push future related research to the sub-acute or acute phase of recovery and open a door for this large population back to intervention-targeted hand function recovery. Our study population is also moderately to severely impaired, which may make participation difficult. We hypothesize this intervention will be feasible for study participants as evidenced by at least 90% of eligible participants completing their intervention schedule.
This is an interventional randomized controlled trial with parallel assignment.
Study activities will be conducted in Chicago, Illinois, in labs at the Feinberg Medical School campus, an academic hospital setting, both in the Department of Physical Therapy and Human Movement Sciences Department and the Northwestern Memorial Hospital Imaging Center Arkes Pavilion.
Inclusion criteria are as follows: (1) age between 21 and 80; (2) paresis confined to one side, with substantial motor impairment of the upper limb and some residual voluntary movement (Upper Extremity Fugl-Meyer Assessment Score (UEFMA) in the range of 10–40/66, Chedoke McMaster Stroke Assessment Hand Subscale (CMSA_H) ≤ 4); (3) capacity to provide informed consent; (4) ability to elevate their limb against gravity up to at least 75° of shoulder flexion and to generate some active elbow extension; (5) ability to achieve ReIn-Hand assisted hand-open at the level of thumb-to-index finger distance ≥ 4 cm; (6) magnetic resonance imaging (MRI) compatible; (7) discharged from all forms of physical rehabilitation, (8) Intact skin on the hemiparetic arm, (9) Ability to tolerate sitting for no less than one hour, and (10) Montreal Cognitive Assessment (MoCA) score ≥ 23.
Exclusion criteria are as follows: (1) motor or sensory impairment in the non-affected limb; (2) any brainstem and/or cerebellar lesion; (3) severe concurrent medical problems (e.g., cardiorespiratory impairment, uncontrolled hypertension, inflammatory joint disease); (4) History of neurologic disorder other than stroke (Parkinson’s disease, amyotrophic lateral sclerosis, multiple sclerosis, traumatic brain injury, peripheral neuropathy); (5) any acute or chronic painful condition in the upper extremities or spine, indicated by a score ≥ 5 on a 10-point visual analog scale; (6) using cardiac pacemaker, implanted cardioverter defibrillator, neurostimulation system inside brain or spinal cord, bone growth box fusion stimulation; (7) seizures in last 6 months; (8) severe upper extremity sensory impairment indicated by absent sensation on the tactile sensation subscale (light touch and pressure items) of the Revised Nottingham Assessment of Somato-Sensations [31, 32] (score < 4); (9) chemodenervation: botulinum toxin, Myobloc, phenol block, or dysport injection to any portion of the paretic UE within the last 6 months, or phenol/alcohol injections < 12 months before participation; (10) unable to passively attain 90° of shoulder flexion and abduction, measured using a goniometer based on adapted methods; (11) Flexion contractures larger than 45° in the elbow, wrist, metacarpophalangeal joints (MCP) and interphalangeal joints (IP); (12) pregnant or planning to become pregnant; and (13) participating in any experimental rehabilitation or drug studies; (14) inability to attend intervention sessions 3 times a week during 8 weeks, as well as assessments/evaluations and follow-up; (15) UE musculoskeletal impairment limiting function prior to stroke, (16) currently using oxygen, and (17) upper limb amputation.
The informed consent process will be handled by authorized study personnel who are thoroughly familiar with the study and trained in ethical and legal aspects of obtaining consent, including the principal investigator (PI) and co-investigators, evaluator clinicians (physical therapists), and the study coordinator.
Following this trial, there are no planned ancillary studies that may use data collected as part of this trial. However, the informed consent document does include a provision that de-identified data from this study may be shared for future unspecified research studies.
For individuals with moderate to severe stroke, volitional shoulder abduction modulates abnormal UE synergy expression resulting in changes in independent joint control [6, 18, 33, 34] as well as reaching ability [20, 21, 35]. Research shows that exercise interventions targeting shoulder abduction loading during reaching can modify these patterns resulting in improvements in reaching distance [19, 35,36,37]. Our research group has previously demonstrated the ability for the ReIn-Hand to improve hand opening and hand function as part of a task-based GR3 intervention, [12, 13] but those intervention protocols did not account for the effect of SABD loading and the potential benefits of augmenting SABD loading. For this study, the control group will participate in a task-based GR3 intervention with no augmentation of SABD loading, mimicking a real-world scenario and in line with our previous work. The experimental group will participate in the same intervention, but with the addition of SABD augmentation. This group will be able to participate in device-assisted practice of ADLs in an anti-synergic pattern, and thus a close-to-normal movement pattern. This will be the first investigation of this close to normal practice on UE motor recovery in individuals with moderate to severe stroke by evaluating clinical outcomes, kinematics, and neuroplasticity.
Two devices will be used during intervention.
Research participants will participate in a 24-session intervention, ~ 2 h per session, 3 sessions per week, for 8 weeks in total. The individuals involved with the administration of the intervention are two training technicians. For all sessions and both groups, research participants will be seated in a seating system with straps across the chest and waist to prevent unwanted trunk movement. The training technicians will stretch the paretic UE for up to 15 min. The ReIn-Hand will be attached to the paretic upper extremity (UE) and then positioned at a home position in 75° shoulder abduction (SABD), 30° shoulder flexion, and 60° elbow flexion.
The first session will be a “parameter adjusting session,” during which the training Physical Therapist (PT) will determine training parameters, including (1) SABD load, (2) target(jar) distance, (3) jar width, (4) jar weight, (5) jar height, and (6) jar orientation. These training parameters will be established both on the table condition and using a robot. The robot modulates the supporting force in Z-direction applied to the arm while participants are required to lift the arm, thus changing the shoulder abduction (SABD) load. The SABD load will be set as the maximum load that allows the participant to actively reach the target distance, and achieve a ReIn-Hand mediated hand opening no less than 4 cm between the tips of the thumb and index fingers. After establishing SABD load, all the additional parameters (#2–6) will be set, first with the established SABD load as follows: (2) Target distance is at least 70% of the distance of the max reach of the paretic UE when fully supported on a frictionless table created by the robot; (3) Jar width will be increased in 0.5-cm increments, by adding padding around the jar, to the max width the participant can achieve with the ReIn-Hand; (4) Jar height (i.e., distance from the lowest part of the jar to the surface of the table) will be set as 2-cm increments to the max height the participant can successfully (and painlessly) reach the jar; (5) Jar orientation (i.e., the relation of the long axis of the jar to the table surface) will be set as 2° increments to the maximum amount that allows the participant to grasp the jar successfully; and (6) Jar weight will be increased in 100-g increments, stopping if the participant experiences pain or cannot lift the jar. The Training PT will then repeat steps 2–6 to determine these parameters (#2–6) under table condition, i.e., this time without robot support and thus using a height-adjustable table.
Once the intervention parameters are set, the training technician will guide the participant to perform the GR3 activities using these parameters. Participants in the experimental group will be trained using the robot. Their forearm of a participant will be attached to small arm, which will be firmly attached to the robot. Participants in the control group will be trained on a regular height-adjustable table. All training sessions will consist of 40 trials (about 1 h) of “reaching-grasping-retrieving-and- releasing” (GR3) activities, which include (1) reaching towards a plastic jar (diameter = 3 cm, weight = 30 g when empty); (2) activating finger/wrist extensor muscles to trigger the ReIn-Hand, which in turn assists the opening of the paretic hand while reaching; (3) grasping the jar; (4) retrieving the jar to the home position and placing it on the table; and (5) Releasing the jar. In order to avoid fatigue, a resting time of 20–30 s will be provided between trials.
Participants in the control group will be encouraged to perform GR3 activities with the arm above the table. The experimental group will get the necessary SABD support via the PACT3D and thus be required to reach above the table. Both groups will be provided with the same verbal cues. A successful trial requires the completion of all five tasks required during one trial: reaching towards a plastic jar, triggering the ReIn-Hand, grasping the jar, retrieving the jar to the starting position, and releasing the jar. An unsuccessful trial is defined as the failure to complete one of the five tasks during one trial. The results of each of the 40 trials will be recorded by the training technician.
The training technician will consult the Training Physical Therapist (PT) to review the training performance after each session to determine if adjustment of parameters is necessary for the next session. If a participant successfully completes 30/40 trials in 2 successive sessions, the training PT will re-adjust all the parameters at the following session in order to progressively challenge the participant. New parameters then will be implemented by the training technician during that session.
Possible reasons for removal include changes in participant health conditions, changes in experimental inclusion/exclusion criteria, or other unpredictable conditions. Participants are free to choose to stop participating in the study at any time. However, data already collected may not be removed from the study database. Individuals who complete 50% of the intervention will have their data included in analyses. Individuals who do not complete at least 50% of the interventions will not have their data included in the analyses.
Study retention will be promoted by phone calls, emails, and text reminders to participants about upcoming study appointments. We will closely monitor all data and conversations with participants for any reporting of challenges limiting participation. Participants will be given reimbursement for public transportation expenses, parking fees in Northwestern Medical School parking lots, or private Lyft service if needed.
Participants should not begin new forms of physical rehabilitation or UE research while participating in this study.
If participants get ill or get injured as a result of this study (devices or procedures), they should seek medical treatment through their doctor or treatment center of choice and inform the research team. The researchers will not pay for medical care required because of a bad outcome resulting from study participation; however, individuals are not precluded from seeking compensation.
Primary outcome measure:
Secondary Outcome Measures:
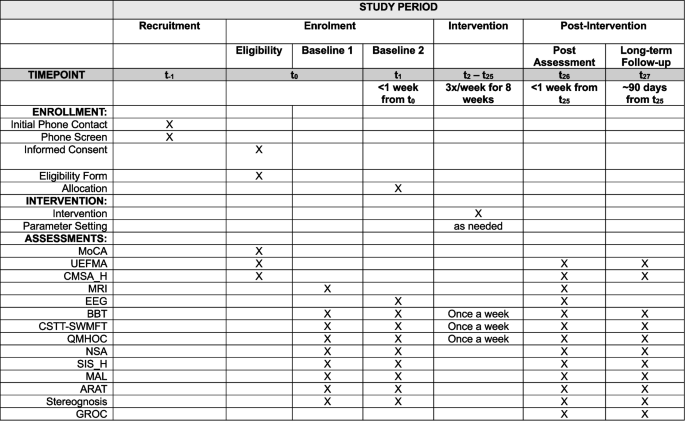
Figure 1 shows the study timeline (see attached).
Sample size calculation is based on a change in the primary outcome from baseline to post-intervention using preliminary results from participants using the ReIn-Hand device only [12, 13] and unpublished results of participants using the ReIn-Hand device with robotic SABD augmentation, the mean difference in BBT for both groups was 2 (SD 2.5). Based on these findings, we conducted a power analysis (G*Power, version 3.1.9.4) to determine how many participants would be needed to detect (1) a significant increase in BBT scores following the intervention within each group, using a one-tailed t-test, and (2) a significantly greater improvement in the experimental group compared to the control group, using a two-sided, two-sample equal-variance t-test. Both tests were set at a significance level of 0.05. The results demonstrated we would need 28 participants per group with a power of 90%. The sample size may change. We will conduct similar power analyses at midterm (N = 30, reaching the post-intervention timepoint) using the primary outcome to assess whether the sample size need to change. Considering a dropout rate of 7% based on a robotic intervention study in a similar population, [37] we will recruit 60 (with 56 valid data in the end) adults (age: 21–81 years) with chronic (> 1 year since stroke) UE hemiparesis resulting from unilateral stroke to participate in this study.
Potential participants will be recruited form the Chicagoland area. Individuals who have had a stroke will be selected from the Clinical Neuroscience Research Registry, maintained by the Physical Therapy and Human Movement Sciences department at Northwestern University and Shirley Ryan AbilityLab (formerly the Rehabilitation Institute of Chicago), containing more than 1000 members. Stroke survivors satisfying our inclusion/exclusion criteria will be contacted by phone or email for recruitment.
In addition to the Clinical Neuroscience Research Registry, the study will be listed on The New Normal Match and ResearchMatch. Both are web-based recruitment portals offered by the Center for Clinical Research, a center in the Northwestern University Clinical and Translational Sciences Institute that allow interested study participants to contact study personnel. We will also contract to BuildClinical, LLC (New York, New York) to enhance participant recruitment. BuildClinical is a data-driven platform that helps academic researchers recruit participants for research studies more efficiently using social media, software, and machine learning. On the study landing page, potential participants complete an online pre-screen questionnaire that gets routed into BuildClinical’s platform and shared with study personnel. In addition to these recruitment techniques, flyers will be distributed in area rehabilitation centers and support groups. We will also accept word-of-mouth recruitment where new participants are referred by previous participants.
Participants who satisfy study criteria will undergo baseline assessments 2 times prior to randomization/allocation (see Fig. 1). Study data is entered into a secured database, REDCap (Research Electronic Data Capture), where it will be maintained. A randomization module will be developed within REDCap to assign participants to one of the training arms using stratified randomization, ensuring balance based on FMA scores. The research coordinator, who will not be blinded, will assign participants, using this randomization module.
REDCap supports role-based access control, with each user assigned a specific role that determines their access to various modules within the database. The research coordinator, the study statistician, and the training technicians are the only users with access to the randomization allocation. Clinical assessors will not have access to the randomization allocations.
To maintain blinding, study personnel and other evaluator therapists in-charge of performing all the assessments and will not have access to group allocation or intervention data. The research coordinator will inform the training technicians that are carrying out the intervention which intervention participants will receive.
Research participants and clinical evaluators will be blind to group assignment. Research coordinator will inform the training technicians about the participant’s group assignment so they can initiate the intervention. All training sessions are conducted privately to prevent participants from observing the interventions received by others. While all participants will have the opportunity to interact with the PACT-3D robot, only those in the experimental group use it during their training sessions. Participants in the control group interact with the robot only during sessions for resetting their training parameters. This may lead some participants to assume in which arm of the trial they belong, but all study members and participants are trained and reminded not to discuss group allocation. Additionally, during assessment sessions, conducted by blinded clinical assessors, participants and training technicians are reminded not to discuss participant treatments or group allocation. Data analysts will be unblinded, but, crucially, analyses will only be performed after data collection is complete. Moreover, analysts will remain uninvolved in recruitment, allocation, intervention, and data collection processes.
Not applicable, no unblinding will be permissible during the study.
Clinical assessments will be completed by the blinded assessor at baseline (2 times), within 1 week of the end of treatment, and 3 months after completion of treatment. Some assessments will be performed weekly during the intervention portion of the study (see Fig. 1). Blinded assessors were trained together to ensure reliability. All outcome measure data is recorded electronically, not on paper, and entered directly into REDCap at the time of collection.
Primary outcome measures
Secondary outcome measures
Study retention will be promoted by phone calls, emails and text reminders to participants about upcoming study appointments. Additionally, participants are compensated $20/h for their participation. We will closely monitor all data and conversations with participants for any reporting of challenges limiting participation. Participants will be given reimbursement for public transportation expenses, parking fees in Northwestern Medical School parking lots, or private Lyft service if needed. If a participant decides to leave, or is removed from the study, an effort will be made to complete all necessary assessments as soon as possible.
All participant demographic information, clinical assessments, intervention behavior, and reported adverse events (AEs) will be recorded and saved in a REDCap database that was specifically designed for this study. As a means of ensuring data completeness, the REDCap platform automatically provides an alert of missing data when a data form is submitted. In addition, data quality will be checked and then backed up on Northwestern University’s Research Database Storage Service (RDSS) platform by the study coordinator on a weekly basis. The RDSS is a secure platform only accessible to the study coordinator and primary investigator. Research data will be retained electronically for a minimum of 3 years after the project’s completion or the funding period, whichever is later.
The name and demographic information of the research participants will be saved in the REDCap database, which only IRB (institutional review board)-trained and approved team members can access. Name and contact information will be saved in a different and locked document, which only recruiters of this study can access. All data output from the REDCap will be deidentified and encoded, which will be used in all the team communication.
Not applicable, no biological specimens will be collected for this study.
The effect of intervention and treatment methods (i.e., two training arms) on all the primary and secondary outcomes will be tested using a linear mixed model or a general linear mixed model (when data distribution shows certain distributions, like Poisson distribution). In this model, we will use the time (pre, post, 3-m follow-up), the groups (experimental and control groups), and their interactions as the main factors, the initial impairment level measured by UEFMA, sex, and years-to-stroke as other adjusted measures, and participant as the random effect.
An interim analysis will be conducted when half of the data sample has competed the intervention (i.e., N = 30 has reached timepoint post-intervention). Only the primary outcome measure will be analyzed. The analysis will be performed by an independent statistician, who will be unblinded to group allocation. Results will be shared with the PI, DSMC, and the NIH (National Institutes of Health), all of which can recommend continuing or terminating the trial.
This study may be stopped prior to its completion if (1) the intervention is associated with adverse effects that call into question the safety of the intervention; (2) difficulty in study recruitment or retention will significantly impact the ability to evaluate the study endpoints; (3) any new information becomes available during the trial that necessitates stopping the trial; or (4) other situations occur that might warrant stopping the trial. The PI will include an assessment of futility in the annual progress report to NIH which may decide to terminate funding of the study.
We will also check whether there are any certain patterns of the participants’ features that their combination or by a single factor can predict the treatment effect of the two treatments conducted in this study.
The primary analysis population will include all participants as initially randomized (intention-to-treat principle). Participants will be analyzed according to the group to which they were originally assigned, regardless of their adherence to the study protocol. This includes individuals who may not have fully complied with the assigned intervention, missed follow-up visits, or deviated from the study procedures. The intention-to-treat approach is employed to maintain the benefits of randomization and provides an unbiased estimate of the treatment effect.
In this study, we will use a linear mixed model (LMM) to handle missing data. The LMM is well-suited for handling incomplete data as it makes use of all available data points, even when some participants have missed sessions or specific measures. All participants with at least one available outcome measurement will be included in the analysis. This approach allows for the inclusion of participants who may have incomplete data due to missed sessions or measures. The analysis will assume that the data are missing at random (MAR), meaning that the probability of missing data is related to observed data but not to the missing data itself. This assumption allows the LMM to provide valid estimates of the treatment effects despite the missingness. The LMM will be implemented using restricted maximum likelihood (REML) estimation. This method provides unbiased estimates of variance components and accommodates the presence of missing data by using all available information. Sensitivity analyses will be conducted to assess the robustness of the results in handling missing data. These may include alternative methods such as multiple imputation or complete case analysis, depending on the extent and pattern of missingness.
To promote transparency and facilitate reproducibility, we plan to grant public access to the study materials as follows:
• The full study protocol will be made available on ClinicalTrials.gov within 12 months of study completion. A redacted version will be provided to protect participant confidentiality and proprietary information, if applicable.
• The de-identified participant-level dataset will be shared in accordance with ethical guidelines and data protection regulations. The dataset will be available upon reasonable request to qualified researchers through a data-sharing platform (e.g., NU SharePoint) after a data use agreement is signed to ensure proper data use. Access will be granted 3 months after the publication of the primary study results.
• The statistical code used for data analysis, including scripts for data cleaning, variable creation, and model estimation, will be provided through an open-access repository such as GitHub. The code
will be uploaded 1 month after the publication of the primary results to allow for independent verification and reproducibility of the findings.
These materials will be shared with the aim of promoting open science and supporting secondary analyses and meta-analyses by other researchers. We encourage the responsible use of these resources in accordance with the original research questions and data use agreements.
The day-to-day management of the trial is overseen by the study coordination team, which includes the PI, study coordinator, training technicians, and clinical assessors. The PI meets weekly with the study coordinator to review recruitment progress, retention rates, protocol deviations, and human resources issues. The study coordinator holds weekly meetings with the training technicians, and, when needed, the clinical assessors to coordinate operational aspects of the trial. The PI attends these team meetings bi-weekly to provide additional oversight and strategic guidance.
To ensure the safety of participants, the data safety monitoring committee (DSMC) will be hosted annually. The DSMC will be composed of the PI (Principal Investigator) of this study, the coordinator of this study, and three independent monitors externally, including one person with a study coordination background, one person with a similar post-stroke clinical trial background, and one person with a post-stroke treatment medical background. The DSMC will review safety data, such as AEs reported by telephone calls or written case reports via letter or email, either by participants or the experimenters. The DSMC will also review the recruitment progress and the quality of the data, such as the data adherence rate and the interim results.
Adverse events (AEs), including any complications potentially related to the intervention or arising in this population, will be monitored and documented throughout the duration of the trial. Harms will be assessed both systematically and non-systematically to ensure comprehensive reporting.
Systematically, participants will be asked at the beginning of each training session about the occurrence of any new or worsening symptoms. In addition, study staff will directly observe participants during sessions for signs of discomfort or physical strain that could indicate an adverse response to the intervention. Non-systematically, participants will also be encouraged to report any issues or concerns spontaneously at any point during the study via phone, text, and/or email notifications.
Potential complications known in the literature for post-stroke populations and upper-limb robotic interventions include musculoskeletal pain, joint stiffness, skin irritation from repeated electrode or device contact, overexertion, fatigue, emotional frustration, and transient increases in muscle tone or spasticity. Though these risks are generally low, they will be actively monitored throughout the trial.
All reported AEs, whether solicited or spontaneous, will be reviewed by the clinical team and recorded in the REDCap database and in accordance with institutional and regulatory guidelines. Serious adverse events (SAEs) will be promptly reported to the PI, IRB officer, and NIH, and reviewed by the DSMC, which is tasked with evaluating their severity, relatedness to the study, and any necessary protocol modifications. Any and all AEs will be reported in future publications and will be described by patient symptoms, and not a specific, standardized vocabulary.
Study progress and safety will be reviewed monthly. Progress reports including patient recruitment, retention/attrition, and AEs will be provided to the independent monitor(s) (DSMC) following each of the monthly reviews. An annual report will be compiled and will include a list and summary of AEs. In addition, the annual report will address (1) whether AE rates are consistent with pre-study assumptions; (2) reason for dropouts from the study; (3) whether all participants met entry criteria; (4) whether continuation of the study is justified on the basis that additional data are needed to accomplish the aims of the study; and (5) conditions whereby the study might be terminated prematurely. The annual report will be sent to the Independent Monitor(s) and will be forwarded to the IRB and National Center for Complementary and Integrative Health (NCCIH) and if applicable, the NIH’s General Clinical Research Center (GCRC)/Clinical and Translational Science Awards (CTSA). The IRB and other applicable recipients will review progress of this study on an annual basis. The PI will also send copies of signed recommendations and comments from the independent monitor(s) to the NCCIH Program Officers within 1 month of each monitoring review.
Modifications to the study protocol that have a potential impact on the following aspects are submitted for review to Northwestern University’s Institutional Review Board Office: study design, eligibility criteria, study procedures, study conduct, and study objectives. Any modifications that have an effect on potential risks or benefits to the participants are also submitted for review and approval prior to implementation. Changes in study team are submitted for modification to the Institutional Review Board and approved by the PI. Trial participants are informed of any protocol amendments either verbally or through re-consenting when appropriate.
The results of this study may also be used for local and regional scientific and healthcare conference presentations, as well as peer-reviewed scientific and medical journal papers. If individual results are discussed, participant identities will be protected by using a study code number rather than a name or other identifying information.
The data collected during the study will be analyzed and reported at the close of the study. Data that may potentially unblind the blinded clinical assessors will not be reported prior to the end of the study. All papers, abstracts, or posters that contain study results will be submitted to the PI for review. The PI will recommend any changes that are deemed appropriate before submitting for publication. It is expected that it may take approximately 4 to 6 months to analyze the final results for journal submission.
The combined use of ReIn-Hand and robotic devices has the possibility to facilitate hand opening and reduce abnormal SABD-induced synergy when practicing ADL-related activities. This EMG-driven device provides synchronized proprioceptive and somatosensory feedback with motor tasks, which is preferred since it increases Hebbian learning by strengthening the involved synapses [65], and acts as a signal for axonal sprouting after cortical lesions [66]. Currently available devices commonly use EMG amplitude to control repeated hand opening and/or closing at a fixed sequence, usually with the arm resting on a table. Due to UE synergies, when an individual with stroke activates proximal muscles during reaching and lifting, EMG-amplitude from both finger/wrist flexors and extensors could significantly increase [34]. Therefore, amplitude-based approaches cannot reliably control the hand in the context of non-tabletop activities in individuals with abnormal UE synergies [67]. Innovatively, we have designed algorithms to reduce the impact of synergic muscle activation, [68] thus guaranteeing a < 1% error rate in the detection of a hand close instead of an open, while keeping a > 90% accuracy rate of detecting hand opening during a functional arm movement. The ReIn-Hand feeds multichannel EMGs into a smart detection algorithm to automatically detect the hand open intention during the arm reaching and retrieving, then automatically stimulate wrist and finger extensor, thus allowing for the engagement of functional, volitionally guided and controlled practice for the first time. Its quick application and user-friendly design have the potential to fill the gap in providing intensive task-specific practice in the more impaired chronic stroke population in an innovative way.
Although the ReIn-Hand can detect hand opening in the context of synergic muscle activities, the resulting hand opening area is usually reduced with an actively abducted arm [69]. Furthermore, it cannot reduce the expression of SABD-induced synergy at the shoulder or elbow either. Due to these issues, interventions without appropriate SABD-support will either be restricted to reaching on a tabletop, thus becoming less like common ADL tasks, or be performed with the abnormal movement patterns caused by SABD-induced synergy. We therefore innovatively propose to combine the ReIn-Hand and PACT-3D robot during training to allow individuals with moderate to severe stroke to move with an anti-synergy movement pattern. By providing the dose-matched control group, who will only get assistance from ReIn-Hand for hand opening, we will be able to investigate the importance of practicing in “close-to-normal” pattern vs. non-controlled way.
The use of robotic-controlled kinematic measures at multiple joints will provide high resolution and accurate data to disentangle motor recovery versus compensation. Using ACT3D robotic modulation of shoulder load, we have developed and validated a set of methods to reliably quantify elbow and hand control abilities under various conditions with high resolution and accuracy [18]. In order to disentangle motor recovery versus compensation for hand control, we propose to use these validated methods to quantify intervention-induced changes simultaneously at multiple joints when maximally opening the paretic hand with or without lifting the arm in individuals with moderate to severe stroke. As suggested, reduced synergy is one of the important signs of motor recovery [70]. In the proposed study, we will measure both hand-opening-induced UE synergies as quantified by coupling torques generated at shoulder and elbow, and the impact of SABD-induced synergy on hand opening ability. A better maintaining of maximal hand opening area while lifting the arm compared to without lifting will indicate motor recovery. Otherwise, a significantly reduced hand opening when lifting will indicate motor compensation, since it reflects that when restricting the compensation from shoulder by requiring an arm lifting, an individual cannot open the hand anymore.
The use of multi-modality imaging methods will provide quantitative measures of intervention-induced neuroplasticity. Motor recovery is not only defined at functional and motor performance levels, but also at the neural level. Up to now, cortical activities related to hand opening with and without lifting the arm following a hand-related intervention have not been widely investigated. We have established validated methods in using high-density EEG approach together with participant-specific MRI-based brain model in estimating the cortical activities with a time resolution of 1 ms and a spatial resolution of 3–5 mm [60,61,62,63,64]. Using this method, we have previously demonstrated abnormal cortical activities that were associated with abnormal synergies [63]. We expect that a post-intervention shift of cortical activity back to ipsilesional sensorimotor cortices will indicate motor recovery since it mimics “normal” hand-related cortical activity. Additionally, we expect motor recovery to be evident in other structural changes. Previous work has shown changes in gray matter (GM) density in ipsilesional sensorimotor cortices [71], along with increases in fractional anisotropy (FA) in the ipsilesional corticospinal tract (CST) [72] in acute and chronic stroke individuals with mild impairments. However, since individuals with moderate to severe stroke are largely ignored in current arm/hand interventions, it is still unknown whether an arm/hand intervention for these more severely impaired post-stroke individuals will result in structural changes. Using advanced anatomical and structural magnetic resonance imaging (MRI) methods, we will measure morphological changes in GM density and descending white matter (WM) integrity. Different from previous work that focused on the ipsilesional side in mildly impaired participants, we will also innovatively quantify these structural changes in the contralesional side. This is based on our recent results that separately evaluated reticulospinal and rubrospinal tract microstructure in chronic stroke individuals with UE motor impairment for the first time [73]. Our results demonstrated that individuals with the greatest UE synergy severity and hand impairments post-stroke have the highest FA in the contralesional reticulospinal tract, a pattern consistent with increased myelination and suggestive of neuroplastic reorganization following stroke-induced compensation. Using a multi-modal MRI approach, the proposed study will provide evidence for morphological neuroplasticity in this more severely impaired large population. We anticipate simultaneous structural changes in the form of a decrease in the contralesional GM density and FA, and an increase in these structural measures in the ipsilesional side. This will agree with “synaptic competition” theory and support the motor recovery.
This study is unique in its study population, interventional devices, and mutli-dimensional measures of function and recovery. We hope to demonstrate whether individuals with moderate to severe chronic stroke are capable of functional improvement, and further understanding of the structural and functional characteristics that define recovery and compensation.
Protocol version number: 12
Protocol date: May 3, 2023
Date recruitment began: September 23, 2019
Approximate date when recruitment will be completed: September 1, 2024
All of the collected data are for research purposes only, and data will be kept in strict confidence. With the exception of the form needed to collect participant contact information required for the success of the study, all remaining data collection forms are designed so that only the study identification number appears as an identifier. Data collection paper forms will be kept in a locked, secure file cabinet at the PI’s office at NU. Electronic data collection forms will be secured in an encrypted study folder stored on the server provided by the Feinberg School of Medicine at NU. Access to the server is regulated and monitored by the PI and only qualified study personnel authorized by IRB. The PI, with support from the project manager, will monitor compliance with IRB and HIPAA regulations. No identifiable information will be given to any unauthorized person without permission from the participant. The consent form will include statements required by the NU IRB regarding information disclosure requirements (e.g., NIH, FDA -Food and Drug Administration- audits).
All data will be merged and stored on the server provided by the Medical School of NU. The database will be secured with password protection. The informatics manager will receive only coded information that is entered into the database under those identification numbers. Electronic communication with outside collaborators will involve only unidentifiable information. All information published on papers or presented at conferences will be identified as a number without identification of participant personal information.The link between the study identification numbers and identifying information (e.g., name, address, age, telephone numbers, and email addresses) will be kept separate from the dataset. Only the research study staff has need (and necessary access) to cross-reference files such as when it is necessary to contact a participant for monthly check-ins, scheduling follow-up appointments, and/or clarification of survey answers (e.g., interpreting hand-written comments). All MRI data will be uploaded to the Northwestern University Research Image Processing System (NURIPS), an online collaborative research environment for securely storing, managing, analyzing and sharing de-identified medical imaging, associated data (e.g., behavioral), and results from advanced customizable processing pipelines. NURIPS is supported by both NUIT (Northwestern University Information Technology) and FSM-IT (Feinberg Information Technology) and takes advantage of the NU high-performance computing cluster, Quest. NURIPS is a secure environment that supports the latest NU policy and procedures for encryption of data during transit and rest, provides granular project level access controls with varying permissions based on user groups, and allows non-NU collaborators access once they obtain an affiliate NetID (Northwestern credentials). All data are backed up and have restore points that go back for 30 days. Users have access to common data analysis pipelines and the opportunity to create and share their own pipelines.
- Rein-Hand:
-
Rein-Hand device
- UE:
-
Upper extremity
- FES:
-
Functional electrical stimulation
- SABD:
-
Shoulder abduction
- GR3:
-
Reaching-grasping-retrieving-releasing
- ADLs:
-
Activities of daily living
- EMG:
-
Electromyography
- PT:
-
Physical therapist
- BBT:
-
Box and Blocks Test
- CSTT-SWMFT:
-
Cutaneous Sensory Touch Threshold using Semmes–Weinstein Monofilaments
- UEFMA:
-
Upper Extremity Fugl-Meyer Assessment Score
- ARAT:
-
Action Research Arm Test
- SIS_H:
-
Stroke Impact Scale Hand function domain’
- MAL:
-
Motor Activity Log
- CMSA_H:
-
Chedoke McMaster Stroke Assessment Hand Subscale
- NSA:
-
Revised Nottingham Sensory Assessment- Kinaesthesia Subscale
- GROC:
-
Global Rating of Change
- HPA:
-
Hand pentagon area
- LI:
-
Laterality Index for Cortical Activity
- CAR:
-
Cortical activity ratio
- GM:
-
Gray matter
- WM:
-
White matter
- FA:
-
Fractional anisotropy
- REDCap:
-
Research Electronic Data Capture
- DTI:
-
Diffusion Tensor Imaging
- RDSS:
-
Research Data Storage Service
- EEG:
-
Electroencephalography
- MRI:
-
Magnetic resonance imaging
- AE:
-
Adverse events
- IRB:
-
Institutional review board
- NIH:
-
National Institutes of Health
- LMM:
-
Linear mixed model
- MAR:
-
Missing at Random
- REML:
-
Restricted maximum likelihood
- DSMC:
-
Data Safety Monitoring Committee
- PI:
-
Principal Investigator
- SAE:
-
Serious adverse events
- NCCIH :
-
National Center for Complementary and Integrative Health
- GCRC:
-
NIH’s General Clinical Research Center
- CTSA:
-
Clinical and Translational Science Awards
- NU:
-
Northwestern University
- NU PTHMS:
-
Physical Therapy and Human Movement Sciences Department of Northwestern University
- NMH:
-
Northwestern Memorial Hospital
- FDA:
-
Food and Drug Administration
- RIPS:
-
Northwestern University Research Image Processing System
- NUIT:
-
Northwestern University Information Technology
- FSM-IT:
-
Feinberg Information Technology
- NetIDs:
-
Northwestern credentials
Gaubert CS, Mockett SP. Inter-rater reliability of the Nottingham method of stereognosis assessment. Clin Rehabil. 2000;14(2):153–9. Available from: https://pubmed.ncbi.nlm.nih.gov/10763792/
Carod-Artal FJ, Coral LF, Trizotto DS, Moreira CM. Self- and proxy-report agreement on the Stroke Impact Scale. Stroke. 2009;40(10):3308–14. Available from: https://pubmed.ncbi.nlm.nih.gov/19661469/
Lin KC, Fu T, Wu CY, Hsieh YW, Chen CL, Lee PC. Psychometric comparisons of the Stroke Impact Scale 3.0 and Stroke-Specific Quality of Life Scale. Qual Life Res. 2010;19(3):435–43. Available from: https://pubmed.ncbi.nlm.nih.gov/20127418/
van der Lee JH, Beckerman H, Knol DL, De Vet HCW, Bouter LM. Clinimetric properties of the Motor Activity Log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35(6):1410–4. Available from: https://pubmed.ncbi.nlm.nih.gov/15087552/
Chen P, Liu TW, Tse MMY, Lai CKY, Tsoh J, Ng SSM. The predictive role of hand section of Fugl-Meyer Assessment and Motor Activity Log in Action Research Arm Test in people with stroke. Front Neurol. 2022;13. Available from: https://pubmed.ncbi.nlm.nih.gov/35873769/
van der Lee JH, De Groot V, Beckerman H, Wagenaar RC, Lankhorst GJ, Bouter LM. The intra- and interrater reliability of the Action Research Arm Test: A practical test of upper extremity function in patients with stroke. Arch Phys Med Rehabil. 2001;82(1):14–9. Available from: https://pubmed.ncbi.nlm.nih.gov/11239280/
Barreca SR, Stratford PW, Lambert CL, Masters LM, Streiner DL. Test-retest reliability, validity, and sensitivity of the Chedoke Arm and Hand Activity Inventory: A new measure of upper-limb function for survivors of stroke. Arch Phys Med Rehabil. 2005;86(8):1616–22. Available from: https://pubmed.ncbi.nlm.nih.gov/16084816/
Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24(1):58–63. Available from: https://pubmed.ncbi.nlm.nih.gov/8418551/
Wu C yi, Chuang I ching, Ma H ing, Lin K chung, Chen C ling. Validity and responsiveness of the Revised Nottingham Sensation Assessment for outcome evaluation in stroke rehabilitation. Am J Occup Ther. 2016;70(2):1–8. Available from: https://doi.org/10.5014/ajot.2016.018390.
Bradley A, Yao J, Dewald J, Richter CP. Evaluation of electroencephalography source localization algorithms with multiple cortical sources. PLoS One. 2016;11(1). Available from: https://doi.org/10.1371/journal.pone.0147266.
Chen A, Yao J, Kuiken T, Dewald JPA. Cortical motor activity and reorganization following upper-limb amputation and subsequent targeted reinnervation. Neuroimage Clin. 2013;3:498–506. Available from: https://pubmed.ncbi.nlm.nih.gov/24273732/
Yao J, Chen A, Kuiken T, Carmona C, Dewald J. Sensory cortical re-mapping following upper-limb amputation and subsequent targeted reinnervation: A case report. Neuroimage Clin. 2015;8:329–36. Available from: https://pubmed.ncbi.nlm.nih.gov/26106558/
Yao J, Chen A, Carmona C, Dewald JPA. Cortical overlap of joint representations contributes to the loss of independent joint control following stroke. Neuroimage. 2009;45(2):490–9. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1053811908012639
Yao J, Dewald JPA. Evaluation of different cortical source localization methods using simulated and experimental EEG data. Neuroimage. 2005;25(2):369–82. Available from: https://pubmed.ncbi.nlm.nih.gov/15784415/
Iftime-Nielsen SD, Christensen MS, Vingborg RJ, Sinkjær T, Roepstorff A, Grey MJ. Interaction of electrical stimulation and voluntary hand movement in SII and the cerebellum during simulated therapeutic functional electrical stimulation in healthy adults. Hum Brain Mapp. 2012;33(1):40–9. Available from: https://pubmed.ncbi.nlm.nih.gov/21591025/
Fugl Meyer AR, Jaasko L, Leyman I. The post stroke hemiplegic patient. I. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7(1):13–31. Available from: https://pubmed.ncbi.nlm.nih.gov/1135616/
Yao J, Sheaff C, Carmona C, Dewald JPA. Impact of shoulder abduction loading on brain-machine interface in predicting hand opening and closing in individuals with chronic stroke. Neurorehabil Neural Repair. 2016;30(4):363–72. Available from: https://pubmed.ncbi.nlm.nih.gov/26216789/
Lan Y, Yao J, Dewald JPA. The impact of shoulder abduction loading on EMG-based intention detection of hand opening and closing after stroke. Annu Int Conf IEEE Eng Med Biol Soc. 2011;2011:4136–9. Available from: https://pubmed.ncbi.nlm.nih.gov/22255250/
Yao J, MaagDenberg F, Lan Y, Sullivan J, Dewald Jules P. Effects of wide pulse neuromuscular electrical stimulation on hand Opening in individuals with moderate to severe stroke. In: 36th Annual International IEEE EMBS Conference. Chicago; 2014.
van Kordelaar J, Van Wegen EEH, Nijland RHM, Daffertshofer A, Kwakkel G. Understanding adaptive motor control of the paretic upper limb early poststroke: The EXPLICIT-stroke program. Neurorehabil Neural Repair. 2013;27(9):854–63. Available from: https://pubmed.ncbi.nlm.nih.gov/23884015/
Gauthier L V., Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: Plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39(5):1520–5. Available from: https://pubmed.ncbi.nlm.nih.gov/18323492/
Fan YT, Lin KC, Liu HL, Chen YL, Wu CY. Changes in structural integrity are correlated with motor and functional recovery after post-stroke rehabilitation. Restor Neurol Neurosci. 2015;33(6):835–44. Available from: https://pubmed.ncbi.nlm.nih.gov/26484696/
Owen M, Ingo C, Dewald JPA. Upper extremity motor impairments and microstructural changes in bulbospinal pathways in chronic hemiparetic stroke. Front Neurol [Internet]. 2017;8. Available from: https://pubmed.ncbi.nlm.nih.gov/28659855/
The authors would like to acknowledge Jasmine Benitez Albert Chang, Kailee Kozicki, and Jennifer Shao, Anna Li, and Caroline Graff for their assistance with recruitment, administrative duties, and data collection.
Funding for this protocol comes from the National Institute of Health and Departmental Fund of the Physical Therapy and Human Movement Sciences Department of Northwestern University (NU PTHMS). Non-financial support from the NU PTHMS Department include the following: The facilities and other resources available at NU include everything needed to undertake and complete the proposed research successfully. The appropriate personnel, laboratories, and existing equipment are in place.
Laboratory: The Department of Physical Therapy and Human Movement Sciences (PTHMS) includes eight laboratories, each approximately 500 sq. ft. in size. We will be using three of these laboratories to perform our research. One of the laboratories contains height-adjustable tables, comfortable chairs and cabinets to conduct the proposed tests. The other 2 labs, which are shielded rooms, contain the latest in robotic technology, 3 surface EMG recording systems, 1 active portable EEG/EMG system, and 2 wireless EEG/EMG systems.
Clinical: The NUPTHMS is affiliated with the Shirley Ryan AbilityLab and Northwestern Memorial Hospital (NMH). Both hospitals have stroke, spinal cord injury, and head trauma wards as well as a large outpatient facility. Participants chosen for the research component of this proposal belong to our 700+ outpatient participant database and the Clinical Research Registry (CRR) maintained through Shirley Ryan AbilityLab. The proximity to the Shirley Ryan AbilityLab and NMH, as well as access to the CRR, contributes substantially to the recruitment of research participants and, thus, the potential success of the proposed study.
Computer: The NUPTHMS maintains a large computer simulation laboratory including the latest PCs, a 100 core PSSC labs cluster computer, as well as installed analysis software (Matlab, CURRY, Analyzor, Cortech, FMRIB Software Library (FSL)) for EEG/EMG/MRI/DTI data processing, source reconstruction and musculoskeletal modeling purposes. Furthermore, Northwestern University, Feinberg IT department provides a minimum 1 TB of desktop mountable storage for research purpose to guarantee the safety of the research data. The combination of these information technologies contributes to the potential for success by assuring both efficient data handling and optimal communication among members of the research team.
Office: The NUPTHMS investigators working on this project have their own offices at NU. Furthermore, the NUPTHMS department will provide administrative support. These facilities assure that the PI and the immediate research team will have the necessary space in which to formulate experiments, analyze results, and prepare manuscripts for publication.
Other resources:
Machine Shop: The NUPTHMS also maintains a machine and electronics shop (800 sq. ft.) that is available to construct and build custom equipment under the direction of the department engineer. The machine shop has been expanded to include the latest in computer-controlled milling devices, a large electronic bench, aluminum welding equipment, and a brand-new lathe. This equipment allows us to manufacture new parts for ReIn-Hand as needed and repair/upgrade broken parts or electronics. The availability of this machine shop provides a valuable resource for on-demand fabrication, alteration, and repair of hardware used in our experiments.
HD Video-conferencing facilities: NUPTHMS has 3 HD videoconference rooms (400 sq. ft.) that house brand new HD conference systems with an 80-in. touch screen TVs, which allows for effective communication with external collaborators.
The study protocol, the informed consent forms, participant recruitment materials, and additional study-related documents will be reviewed and approved by the IRB Office of NU, which is responsible for protecting the rights and welfare of human research participants. In addition to yearly reviews that include progress and safety reports, the IRB Office also reviews any modifications to the study protocol, the informed consent forms, participant recruitment materials, and additional study-related documents made throughout the year.
All study participants are asked to review the informed consent document in the presence of a research team member that can address their questions and concerns before being asked to sign and provide written, informed consent for their participation in the study.
Written informed consent will be obtained from each participant at entry into the study. Informed consent is obtained by the following process:
1. The participant will be asked to review the study consent form.
2. A trained member of the study team will meet with the participant to review the form, to confirm the participant’s understanding of the study, and to answer any questions that the participant might have.
3. Once the participant demonstrates understanding of the study and agrees to participate in the study, the consent will be signed in the presence of study team member, who will also witness the document.
See Appendix A.
Dr. Jun Yao, co-author and primary investigator, developed the investigational device for this study. Three authors (Carmona, Drogos, and Yao) have ownership stakes in ReIn-Hand Technologies, Inc., the corporation that currently holds the exclusive license for this investigational device. Importantly, the corporation was founded after applying for, and receiving, NIH funding; and the corporation has not received any funding as part of this project. Northwestern University owns the patent for this device.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Drogos, J.M., Carmona, C., Arceo, R. et al. Effects of device-assisted practice of activities of daily living in a close-to-normal pattern on upper extremity motor recovery in individuals with moderate to severe stroke: study protocol of a randomized control trial. Trials 26, 238 (2025). https://doi.org/10.1186/s13063-025-08930-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13063-025-08930-7