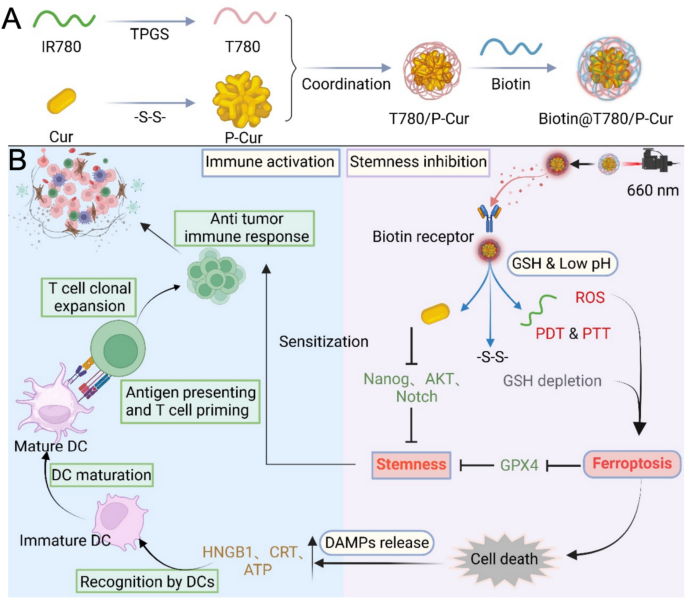
Journal of Nanobiotechnology volume 23, Article number: 500 (2025) Cite this article
Immunotherapy represents a transformative advance in cancer treatment; however, its efficacy in head and neck cancer (HNC) remains constrained by tumor cell stemness and profound immunosuppression within the tumor microenvironment (TME). Overcoming these barriers necessitates innovative strategies to simultaneously eradicate stem-like populations and reprogram the TME.
We engineered a tumor-targeted polymer prodrug nanoplatform, Biotin@P-Cur/T780 NPs, integrating disulfide-linked polycurcumin (P-Cur) and the photothermal agent T780. DSPE-PEG-Biotin surface functionalization enables active tumor targeting. The nanoplatform exploits high intratumoral glutathione (GSH) to trigger disassembly, releasing curcumin monomers and T780. This elicits dual GSH depletion and reactive oxygen species (ROS) amplification, inducing ROS-mediated apoptosis and ferroptosis. Concurrently, localized near-infrared irradiation activates T780, synergizing photothermal (PTT) and photodynamic (PDT) therapies to intensify immunogenic cell death (ICD).
The Biotin@P-Cur/T780 NPs potently suppressed tumor cell stemness in vitro and in vivo. ROS/ferroptosis-driven ICD, amplified by PTT/PDT, reversed TME immunosuppression, enhancing dendritic cell maturation and cytotoxic T lymphocyte infiltration. This multimodal mechanism significantly inhibited primary tumor growth and metastasis in HNC models, while extending survival.
Our prodrug nanoplatform overcomes key resistance mechanisms in HNC by coordinately targeting stemness, inducing dual apoptosis/ferroptosis, and potentiating ICD through PDT/PTT-enhanced immunomodulation. This strategy provides a potent combinatorial approach to augment immunotherapy efficacy in refractory tumors.
Immunotherapy offers considerable promise in revolutionizing cancer treatment by improving the effectiveness of chemotherapy and mitigating its associated side effects [1]. However, despite these promising advancements, substantial challenges have emerged, frequently leading to diminished effectiveness of current therapies and complicating the achievement of sustained treatment responses. Tumor cell stemness and immune suppression within the TME are major contributors to treatment resistance and cancer progression [2]. Consequently, there is an urgent need for innovative strategies to address and overcome these challenges.
Growing evidence indicates that nanoparticles, particularly those functionalized with bioactive compounds, hold significant potential for enhancing antitumor efficacy and overcoming resistance mechanisms [3]. Curcumin (Cur), a natural polyphenol with strong anticancer potential, stands out for its capacity to regulate key signaling pathways associated with cancer cell stemness, growth, survival, and metastasis. Nonetheless, its clinical use is limited by low bioavailability and rapid systemic clearance [2, 4, 5]. To address these limitations, we have designed and synthesized P-Cur, a curcumin derivative linked by disulfide bonds, to improve curcumin’s stability, solubility, and therapeutic efficacy [6]. Additionally, P-Cur is endowed with the capability to induce ferroptosis by depleting glutathione (GSH) [7, 8]. To optimize the sensitizing effects of Cur on tumor immunotherapy, integrating phototherapy into the current experimental design is a strategic approach.
For solid tumors, the combination of phototherapy with immunomodulators shows great promise as a therapeutic strategy [9]. Photodynamic therapy (PDT) under near-infrared (NIR) laser irradiation induces oxidative stress-mediated apoptosis, promoting the release of tumor-associated antigens and damage-associated molecular patterns (DAMPs), including adenosine triphosphate (ATP), calreticulin (CRT), and high-mobility group box 1 (HMGB1). This process effectively triggers a strong immunogenic cell death (ICD) response [9, 10]. The incorporation of PTT further enhances the immunogenicity of the TME and augments oxidative stress-related cell death pathways (including apoptosis and ferroptosis), thereby significantly amplifying the effectiveness of therapeutic interventions.
Herein, we developed a novel Biotin@P-Cur/T780 nanoparticle system designed to address the limitations of immunotherapy in head and neck cancer, particularly targeting the challenges posed by tumor cell stemness. Specifically, the synthesis of the photothermal agent T780 by introducing TPGS (D-α-tocopheryl polyethylene glycol 1000 succinate) into IR780 (a near-infrared (NIR) dye) leverages the amphiphilic properties of TPGS to enhance IR780’s water dispersibility and stability [11]. Curcumin molecules were linked and polymerized via disulfide bonds to form P-Cur. The T780 was then co-assembled with P-Cur to generate P-Cur/T780 nanoparticles (P-Cur/T780 NPs). To further minimize off-target effects, these nanoparticles were coated with tumor-targeting biotin, culminating in the development of the final Biotin@P-Cur/T780 nanoparticle platform (Biotin@P-Cur/T780 NPs, Scheme 1A) [12]. In this nanoparticle platform, biotin enables targeted delivery of P-Cur and T780 to tumor cells through binding to biotin receptors. In the high-GSH environment of tumor cells, disulfide bonds are specifically hydrolyzed, leading to the disassembly of P-Cur/T780 NPs. This process releases curcumin monomers and T780, which each perform distinct roles. The accumulation of ROS and the depletion of GSH induced by these components activate oxidative stress-related apoptosis and ferroptosis, thereby triggering ICD. Furthermore, this strategy reduces tumor cell stemness and enhances the sensitivity of head and neck cancer cells to immunotherapy, resulting in potent immunological destruction of the tumor (Scheme 1B). Through comprehensive in vitro and in vivo experiments, we have shown that this nanoplatform effectively suppresses tumor growth while reducing the stemness of head and neck cancer cells, leading to an enhanced immune response. This innovative approach holds great potential for addressing the current challenges in head and neck cancer treatment.
(A) Preparation of Biotin@T780/P-cur NPs. (B) Illustration of the potential mechanism by which Biotin@T780/P-cur NPs enhance and amplify the immunotherapy sensitivity of head and neck cancer
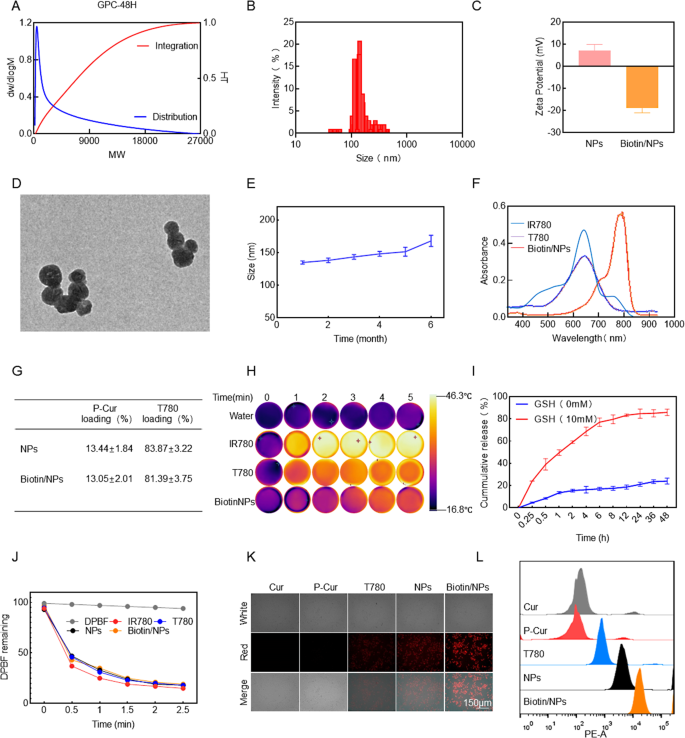
The biocompatible phototherapy agent T780 was synthesized through a two-step reaction process. Initially, IR780 was reacted with aminocaproic acid under the catalysis of triethylamine (TEA) to yield the intermediate IR780-COOH. Subsequent esterification of IR780-COOH with polyethylene glycol succinate (TPGS) afforded the final product T780 (Figure S1A, Supporting Information). The structure of T780 was confirmed by proton nuclear magnetic resonance (¹H NMR) spectroscopy, which revealed the characteristic absorption peak of the ester bond proton at 3.8 ppm (Figure S1B, Supporting Information), thereby verifying the successful synthesis of T780. For the synthesis of polymer curcumin (P-Cur), dithiopropionic acid was first activated via its carboxyl group using DMAP, NHS, and EDC as catalysts (Figure S1C, Supporting Information). Curcumin monomers were then reacted with the activated dithiopropionic acid at room temperature for 48 h, resulting in the formation of P-Cur with disulfide bonds. This modification endowed the curcumin with GSH-depleting capability (Figure S1D, Supporting Information). The structures of both curcumin and P-Cur were characterized by 1H NMR spectroscopy, and the presence of characteristic peaks confirmed the successful synthesis of the desired products (Figure S1E-F, Supporting Information). The polymerization kinetics of curcumin were systematically investigated using gel permeation chromatography (GPC) at reaction intervals of 24 and 48 h. Quantitative analysis demonstrated that curcumin underwent successful radical-mediated polymerization to form P-Cur. The number-average molecular weight (Mn) of the product progressively increased from 800 to 1 500 as the reaction time extended from 24 to 48 h. Notably, the absence of molecular weight reduction or oligomer peaks in the GPC chromatograms at 48 h confirmed the exceptional structural integrity of P-Cur under prolonged reaction conditions (Fig. 1A, S2A, Supporting Information). Additionally, the shift observed in the UV absorption spectrum provided further confirmation of the successful synthesis of P-Cur (Figure S2C, Supporting Information).
To fabricate Biotin/T780/P-Cur nanoparticles (NPs), T780 was mixed with P-Cur at a 1:1 ratio. The mixture was ultrasonically dispersed and then rotary evaporated to form T780/P-Cur NPs. Subsequent DSPE-PEG-biotin modification refined the particle size distribution, yielding Biotin/T780/P-Cur NPs with a hydrodynamic diameter of 142.6 ± 12.3 nm (polydispersity index: 0.18 ± 0.07) (Fig. 1B and S2B, Supporting Information). The Zeta potential was determined to be -17.7 ± 1.8 mV (Fig. 1C). Transmission electron microscopy (TEM) imaging revealed uniform spherical morphology of the NPs in aqueous solution (Fig. 1D). Notably, the NPs exhibited excellent stability, maintaining a consistent particle size for up to 6 months (Fig. 1E). UV-Vis spectroscopy analysis indicated that both T780 and Biotin/T780/P-Cur NPs have a maximum absorption wavelength at 660 nm, in contrast to free IR780, which absorbs maximally at 808 nm (Fig. 1F). This blue shift in the absorption peak confirms the successful synthesis of the NPs and provides critical data for subsequent laser irradiation experiments. The pronounced absorption in the near-infrared (NIR) region highlights their potential for phototherapy applications. Given this spectral characteristic, using a 660 nm laser for irradiating Biotin/T780/P-Cur NPs is more practical. The drug loading efficiencies are depicted in Fig. 1G. The drug loading efficiencies of the NPs are presented in Fig. 1G. The loading efficiency of P-Cur in Biotin/T780/P-Cur NPs was determined to be 13.05 ± 2.01%, while the loading efficiency of T780 was significantly higher at 81.39 ± 3.75% (Fig. 1G). Infrared thermal imaging of water, IR780, T780, and Biotin/T780/P-Cur NPs after NIR laser exposure revealed that Biotin/T780/P-Cur NPs demonstrated superior photothermal conversion efficiency compared to free IR780 and T780. Specifically, Biotin/T780/P-Cur NPs and T780 were irradiated with a 660 nm laser, while IR780 was exposed to an 808 nm laser, both at a power density of 1.0 W/cm2 for 5 min (Fig. 1H). The enhanced heating properties of Biotin/T780/P-Cur NPs were both dose- and time-dependent, and functional modifications did not significantly affect the photothermal performance of the NPs (Figure S2D-F, Supporting Information).
To assess the tumor-specific release efficiency of the nanoparticles in vivo, we simulated the high reductive GSH environment typically found within tumors to evaluate the nanoparticles’ drug release performance [13]. The results indicate that in a high-GSH environment, the nanoparticles exhibit a significant ability to release and disassemble, driven by the specific dissociation of disulfide bonds by GSH (Fig. 1I) Subsequently, the photodynamic efficacy of Biotin/NPs was evaluated using the 1,3-diphenylisobenzofuran (DPBF) probe. After 2.5 min of NIR laser irradiation, the remaining DPBF absorbance levels for Biotin/NPs, NPs, free IR780, and T780 were comparable, indicating that the prepared nanoparticles possess a strong capability to generate and release reactive oxygen species (ROS). (Fig. 1J and S2 I-K, Supporting Information). Given the critical role of effective nanoparticle internalization in drug efficacy, we first assessed the cellular uptake capabilities of several components. The results demonstrated that biotin-modified nanoparticles exhibited a significant advantage in penetrating the head and neck cancer cell line AMC-HN-8 compared to other control groups, as confirmed by flow cytometry analysis (Fig. 1K-J). This finding suggests that rational modification of nanoparticles indeed enhances drug penetration efficiency, providing strong support for subsequent anti-tumor studies.
Preparation and Characterization of Biotin@T780/P-cur. (A) Molecular weight distribution change diagram of the P-cur within 48 h. (B) Size distribution analysis and (C) zeta potential of Biotin@T780/P-cur NPs. (D) TEM image of Biotin@T780/P-cur NPs. Scale bar: 200 nm. (E) Size distribution of Biotin@T780/P-cur NPs over 6 months. (F) UV-vis absorption spectra of IR780, T780, and Biotin@T780/P-cur NPs. (G) Drug loading efficiency of Biotin@T780/P-cur NPs. (H) Infrared thermal images and temperature variations of IR780, T780, and Biotin@T780/P-cur NPs in water after laser irradiation (λ = 808 nm for IR780 and 660 nm for T780 and Biotin@T780/P-cur NPs, P = 1.0 W/cm2; irradiation time = 2 min). (I) The in vitro release of IR780 from Biotin@T780/P-cur NPs in PBS was evaluated under conditions of 0 mM and 10 mM GSH, respectively. (J) Residual levels of DPBF after NIR laser irradiation of IR780, T780, T780/P-cur NPs, and Biotin@T780/P-cur NPs (with IR780 at 808 nm and T780, T780/P-cur NPs, and Biotin@T780/P-cur NPs at 660 nm). (K) Red fluorescence microscopy images of AMC-HN-8 cells incubated with Cur, P-Cur, T780/P-cur NPs, and Biotin@T780/P-cur NPs at 4 h. Scale bar: 150 nm. (L) Flow cytometry analysis was conducted to assess the time-dependent cellular uptake of T780. For panels b, d, and i, the results are presented as mean ± SD, with n = 3
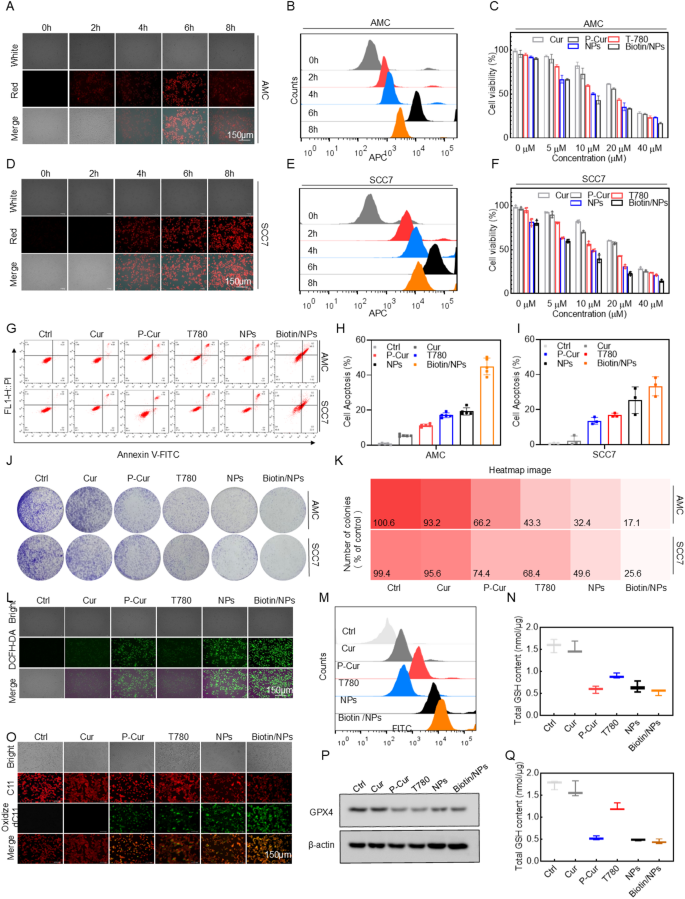
Following the validation of the nanoparticles’ fundamental properties, we performed in vitro studies to evaluate the cytotoxicity and underlying mechanisms of the nano-drugs. The optimal treatment duration was first established and validated in the head and neck cancer cell lines AMC-HN-8 and SCC7. The results showed that cellular uptake was maximized at 6 h of drug exposure (Figs. 2A-B, D-E). The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay results confirmed that the nanoparticles exert significant cytotoxic effects on tumor cell lines, highlighting their potential for effective in vitro tumor cell eradication (Figs. 2C-F). Apoptotic cell death was further assessed by flow cytometry using dual staining with propidium iodide (PI) and Annexin-V FITC. (Figs. 2G-I). The results indicated that the Biotin/NPs group exhibited a significantly higher percentage of apoptotic cells compared to the other control groups. The nanoparticles demonstrated enhanced synergy in inducing apoptosis in both cell lines in vitro, outperforming the effects of monotherapy. Following this, the antiproliferative potential of Biotin/NPs was evaluated using colony formation assays. As depicted in Figs. 2J and K, the results were consistent with those of other antiproliferative experiments, confirming the pronounced antitumor potential of this nanoplatform.
Building on the prior nanoparticle (NP) design, we investigated whether the cytotoxicity of Biotin/NPs is linked to cellular ROS accumulation or ferroptosis [14]. To explore this, we measured cellular oxidative stress using the ROS-sensitive fluorescent probe 2’,7’-dichlorofluorescein diacetate (DCFH-DA). The results showed that, under optimal laser irradiation conditions, fluorescence intensity was notably higher in all cell lines treated with Biotin/NPs compared to those treated with individual drugs or NPs alone (Figs. 2L and S3A). These findings were further corroborated by flow cytometry (Figs. 2M and S3B). Considering that Ferroptosis is marked by the build-up of lipid peroxidation products, we employed C11-BODIPY (581/591) as a sensor for lipid peroxidation [15–16]. The data indicated that, at the maximum excitation wavelength, lipid peroxidation levels were markedly elevated in head and neck cancer cells treated with Biotin/NPs (Figs. 2O and S3C, Supporting Information). Flow cytometry results also demonstrated a similar synergistic effect (Figures S3D-E, Supporting Information). The expression levels of GPX4 in cell samples from various treatment groups further corroborated the potent ability of nanoparticles to induce ferroptosis in tumor cells (Fig. 2P). The results of the experiment assessing the impact of nanoparticles on intracellular GSH levels revealed a significant reduction (Figs. 2N, Q). Treatment with the nanoparticles caused a pronounced decrease in cellular GSH levels compared to the untreated controls. This notable depletion highlights the nanoparticles’ capacity to compromise cellular antioxidant defenses, thereby enhancing their potential to induce oxidative stress.
In vitro cellular uptake, toxicity, and oxidative stress induction potential of Biotin@T780/P-cur NPs. (A-B, D-E) Fluorescence microscopy images and flow cytometry data of AMC-HN-8 and SCC7 cells incubated with Biotin@T780/P-cur NPs for 2, 4, 6, and 8 h. Scale bar: 150 nm. (C, F) Cell viability of AMC-HN-8 and SCC7 cells after treatment with the specified formulations. (G-I) Flow cytometry results and bar graphs of apoptosis in AMC-HN-8 and SCC7 cells following treatment with different formulations. (J-K) Colony formation assay and heatmap analysis of AMC-HN-8 and SCC7 cells. (L-M) ROS levels in AMC-HN-8 following different treatments, measured by fluorescence imaging (L) and flow cytometry (M). Scale bar: 150 μm. (O) Lipid peroxidation levels in AMC-HN-8 after different treatments, measured by fluorescence imaging. Scale bar: 150 μm. (P) Western blot experiments were conducted to validate the expression levels of GPX4 within cells. (N, Q) Total intracellular GSH levels in AMC-HN-8 and SCC7 cells following treatment with the specified formulations
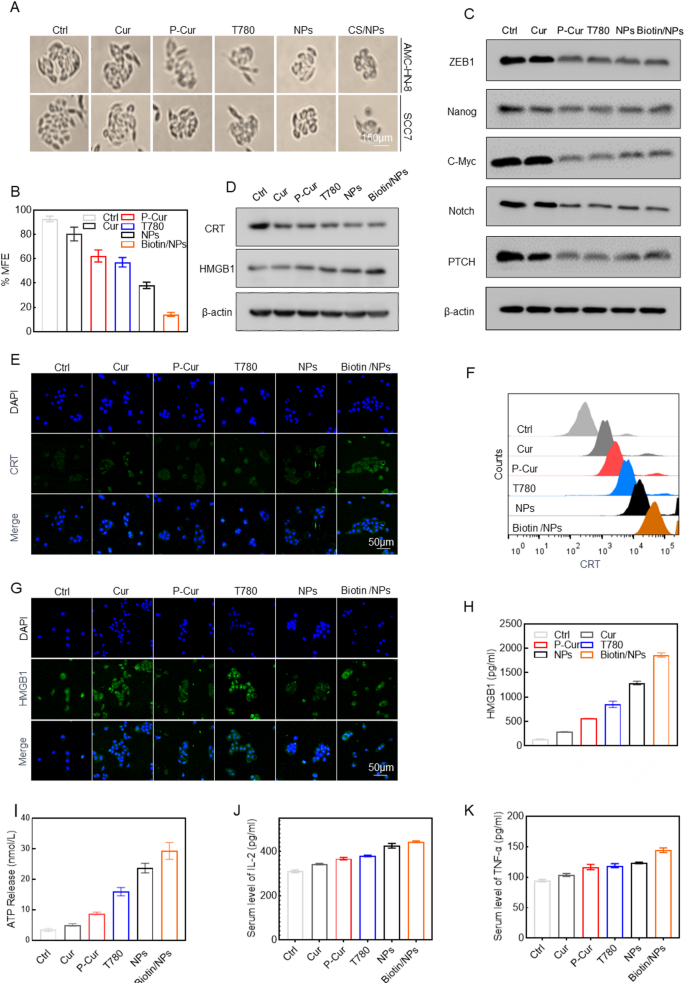
Extensive research has highlighted the anticancer potential of curcumin, with several studies confirming its ability to reduce tumor cell stemness—a critical factor in the efficacy of cancer immunotherapy. In this section of our study, we explored the effects of Biotin@T780/P-cur NPs on tumor stemness and the immune response. Initially, head and neck cancer cells (SCC7) were incubated under various treatment conditions and cultured as cell spheres for 7 days in 96-well ultra-low attachment plates. The results demonstrated that the Biotin@T780/P-cur NPs treatment group significantly impaired the sphere-forming ability of the tumor cells (Fig. 3A-B). Additionally, treatment with Biotin@T780/P-cur NPs significantly decreased the expression of stem cell biomarkers ZEB1, Nanog, the oncogene c-Myc, Notch, PTCH, and EMT markers in head and neck cancer cells. These findings suggest that curcumin-based antitumor nanoparticles effectively reduce tumor cell stemness (Fig. 3C).
Given the pronounced induction of oxidative stress and associated ferroptosis in tumor cells, as well as the significant reduction in cell stemness induced by nanoparticles, we subsequently conducted a detailed evaluation of the effects of nanoparticles on the tumor cell immune response. The expression levels of CRT, HMGB1, HSP70, and ATP secretion were measured after treating head and neck cancer cells with PBS, Cur, P-Cur, T780, P-Cur/T780 NPs, and Biotin@P-Cur/T780 NPs. Notably, Western blot analyses revealed an upregulation of HSP70 and CRT in AMC-HN-8 tumor cells to varying degrees (Fig. 3D). Since CRT externalization and HMGB1 release are crucial immunological markers of ICD, aiding the immune system in recognizing and clearing dead cells while triggering and amplifying immune responses, we conducted further investigation into these phenomena. CRT and HMGB1 distributions were examined using laser confocal scanning microscopy, and flow cytometry was utilized to quantify CRT exposure on the cell surface and HMGB1 intracellular release. The results demonstrated that Biotin@P-Cur/T780 NPs significantly enhanced CRT externalization and HMGB1 release compared to other drug treatment groups or the control group (Fig. 3E-H, Figure SG-J, Supplementary Information), indicating that Biotin@P-Cur/T780 NPs have a substantial capacity to induce ICD.Consistently, ELISA detection of the supernatants showed a significant increase in the release of HMGB1, ATP and pro-inflammatory cytokines (IL-2 and TNF-α) (Fig. 3I-K). The observed changes in CRT, HMGB1, HSP70, ATP, and pro-inflammatory cytokine levels following treatment with Biotin@P-Cur/T780 NPs directly demonstrate the nanoparticles’ capacity to induce ICD in tumor cells, which, based on prior insights, provides crucial evidence for tumor immunosensitization. These findings support our hypothesis that treating head and neck cancer cells with Biotin@P-Cur/T780 NPs can substantially enhance their immunogenicity through the release of large amounts of DAMPs, potentially contributing to the subsequent activation of tumor-specific CD4+ and CD8+ T cells, thereby enhancing the antitumor immune response.
The in vitro mechanisms by which Biotin@T780/P-cur NPs attenuate tumor cell stemness and augment immunotherapy sensitivity. (A-B) Head and neck cancer cells (SCC7) were incubated under different treatments and grown as cell spheres for 7 days in 96-well ultra-low attachment plates. (C) Immunoblot analysis of ZEB1, Nanog, c-Myc, Notch, and PTCH in SCC7 cells with indicated treatments. (D) Western blot showed the effects of different treatments on the expression levels of CRT and HSP70. (E-F) Immunofluorescence staining and flow cytometry analysis of CRT expression on the surface of SCC7 cells after different treatments. Scale bar: 50 μm. (G-H) SCC7 cells were subjected to HMGB1 immunofluorescence labeling and flow cytometry analysis after various treatments. (I-K) Quantification of ATP and pro-inflammatory factors IL-2 and TNF-α in SCC7 cells following the specified treatments
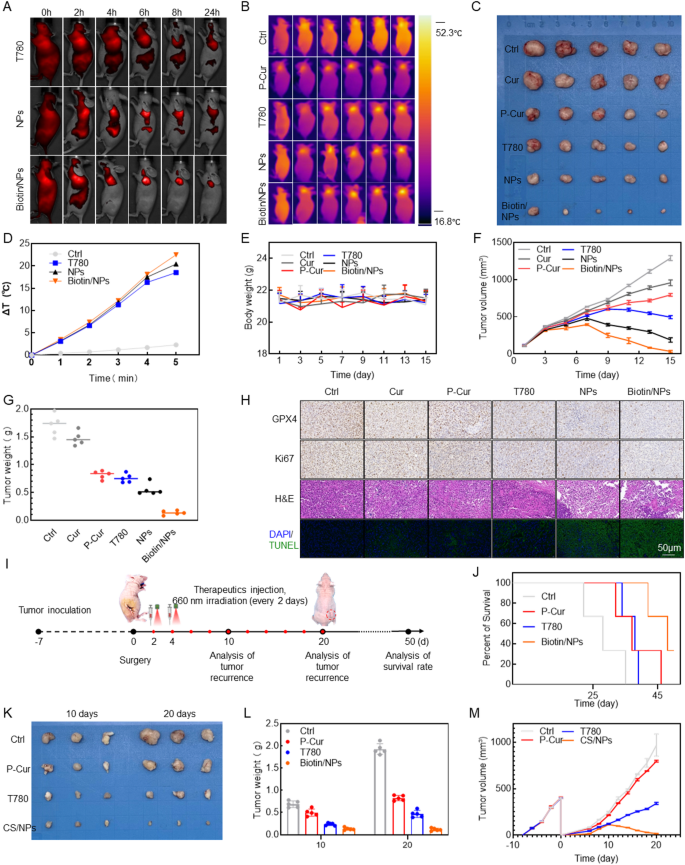
In light of the promising results of nanoparticles in in vitro experiments, it is crucial to further validate their efficacy by assessing their ability to promote tumor regression and inhibit stemness-driven recurrence in vivo. By employing a NIR fluorescence imaging system, Biotin@P-Cur/T780 NPs were used as fluorescent probes to monitor their in vivo biodistribution and tumor accumulation in real time, ensuring adequate therapeutic efficacy (Fig. 4A). The results showed that T780, P-Cur/T780 NPs, and Biotin@P-Cur/T780 NPs reached peak fluorescence at 6 h, which led to the selection of laser irradiation 6 h post-intravenous administration for subsequent PDT and PTT. Thermal images and heating curves were analyzed at 6 h post-injection. Tumors were irradiated in situ using 660 nm NIR light at 1 W/cm2 power for 3 min. As shown in Fig. 4B, D, there was minimal temperature change in the tumor region for the saline group following NIR laser irradiation. In contrast, the tumor surface temperature in the Biotin@P-Cur/T780 NPs group increased by approximately 22 °C within 3 min post-irradiation, which was higher than that observed in any of the control groups. These results demonstrate that Biotin significantly enhances active targeting, improving both intratumoral drug uptake and the efficacy of photothermal therapy. Drugs were administered every 2 days at a dose of 2 mg/kg body weight, and tumor specimens were collected after 2 weeks. The results of drug efficacy and changes in mouse body weight are presented in Fig. 4C, G. The data show that, without significantly affecting mouse body weight, the nanoparticles demonstrated a marked ability to promote regression of the subcutaneous head and neck cancer model, with the Biotin-coated actively targeted nanoparticle formulation showing particularly pronounced effects. The remarkable antitumor efficacy of Biotin@P-Cur/T780 NPs was further confirmed through TUNEL assays and H&E staining of tumor tissues. Notably, H&E staining of excised organs from mice in different treatment groups showed no significant nanomedicine-induced damage (Figure S4A). Serological analyses in mice confirmed the favorable biosafety profile of the drug (Figure S4B-E). Moreover, these analyses revealed a significant reduction in the expression of the ferroptosis-related protein GPX4 (Fig. 4H). These findings indicate that Biotin@P-Cur/T780 NPs can significantly inhibit tumor progression in vivo, primarily by inducing ferroptosis in tumor cells. Additionally, the analyses demonstrated a marked decrease in the expression of both GPX4 and the tumor proliferation marker Ki67 (Fig. 4H). These results indicate that Biotin@P-Cur/T780 NPs effectively promote tumor cell death and inhibit proliferation through the induction of ferroptosis, all while maintaining a strong biosafety profile.
Based on our earlier insights into the mechanisms by which nanoparticles inhibit tumors, we developed a tumor recurrence model in subsequent in vivo experiments to evaluate the ability of nanoparticles to reduce tumor cell stemness and mitigate postoperative recurrence (Fig. 4I). Compared to the control group, treatment with Biotin@P-Cur/T780 NPs significantly reduced tumor recurrence, demonstrating similar cytotoxic potential against both localized and systemic tumor cells. This treatment effectively decreased tumor cell stemness and inhibited postoperative tumor recurrence (Fig. 4J-M). Overall, the Biotin@P-Cur/T780 NPs developed in this study show promising potential for tumor therapy and stemness reduction, making them an excellent candidate for multifunctional drug delivery and cancer treatment.
Evaluation of in vivo antitumor effects and tumor recurrence inhibition. (A) Biodistribution of T780, T780/P-cur NPs, and Biotin@T780/P-cur NPs at various time points in an AMC-HN-8 tumor-bearing BALB/c nude mouse model. (B, D) Infrared thermography and corresponding temperature change graphs of AMC-HN-8 tumor-bearing nude mice subjected to different treatments after exposure to 808 nm or 660 nm laser (1 W/cm²) for 1, 2, 3, 4, and 5 min. (C) Representative image of isolated tumor from nude mice. Scale bar: 1 cm. (E) Line graph of mouse body weight changes. (F) Tumor volume change graph. (G) Scatter plot of final tumor weight changes. (H) GPX4, Ki67, H&E staining, and TUNEL assays of tumors at the end of the antitumor study. Scale bar: 150 μm. (I) Schematic illustration of tumor recurrence model establishment and subsequent treatment. (J) Kaplan-Meyer survival function of mice in different treatment groups. (K-L) Ex vivo images and bar graph analysis of tumors from the recurrence model 10 and 20 days post-establishment. (M) Time-dependent tumor volume changes in the recurrence model across different treatment groups
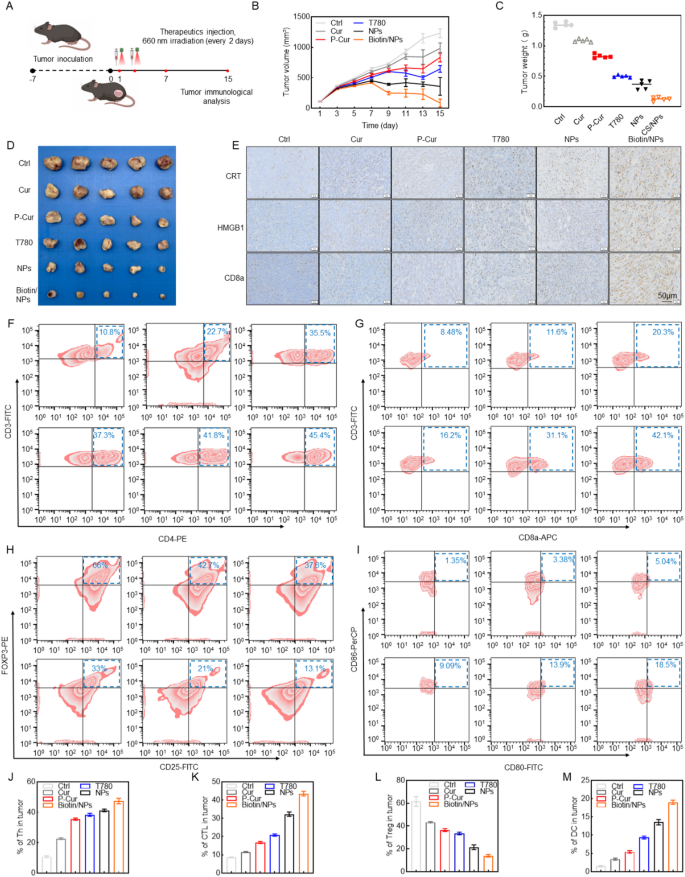
To evaluate the immunostimulatory effects and mechanisms of Biotin@P-Cur/T780 NPs in vivo, we established an SCC7 head and neck carcinoma model in C57BL/6 mice (Fig. 5A). Biotin@P-Cur/T780 NPs exhibited significant tumor suppression efficacy comparable to other treatments in this SCC7 tumor-bearing mouse model (Fig. 5B-D). Immunohistochemical analysis revealed elevated levels of CRT exposure and HMGB1 release in tumors from the Biotin@P-Cur/T780 NPs group relative to the Ctrl and other treatment groups (Fig. 5E), consistent with the in vitro findings.
Additionally, we analyzed tumor-infiltrating CD4⁺ and CD8⁺ T cell populations in the SCC7/C57BL/6 mouse model. Treatment with Biotin@P-Cur/T780 NPs increased infiltration of CD4⁺ (Fig. 5F, J) and CD8⁺ (Fig. 5G, K) T cells into tumor tissue compared to control groups. Given that regulatory T cells (Tregs), a CD4⁺ T cell subset with immunosuppressive activity, promote tumor progression [17, 18], we assessed their intratumoral frequency. Treatment with Biotin@P-Cur/T780 NPs significantly increased the proportion of Treg cells (Fig. 5H, L). This observation suggests the nanoparticles may modulate the tumor immune microenvironment, potentially influencing immunotherapeutic responses.
Dendritic cells (DCs) are pivotal in antigen presentation and the activation of adaptive antitumor immune responses [19–20]. We further assessed the proportion of mature DCs (Fig. 5I, M). The percentage of mature DCs (CD80+CD86+) in the Biotin@P-Cur/T780 NPs group was 18.5%, which was significantly higher compared to the control groups. These results suggest that Biotin@P-Cur/T780 NPs can effectively stimulate a systemic immune response. Additionally, effective polarization of macrophages from the M2 to M1 phenotype has been shown to significantly enhance tumor immunotherapy. As shown in Fig. 5N-O, Biotin@P-Cur/T780 NPs treatment successfully induced the polarization of macrophages from the M2 to M1 phenotype. In summary, Biotin@P-Cur/T780 NPs improve immune surveillance by stimulating immune cells and diminishing immunosuppressive cells, thereby effectively triggering a systemic immune response.
The Biotin@T780/P-Cur NPs-mediated in vivo immune activation. (A) Illustration of the Development and Treatment of a Tumor Immune Response Model in Healthy Mice. (B) Representative images of the dissected tumors and (C) tumor volume of different groups (n = 5). (D) The tumor weight of different groups. (E) Immunohistochemical Analysis of CRT, HMGB1, and CD8a in Tumor Tissues Following Different Treatments. (F-O) Flow cytometry and quantitative analysis of the proportions of key immune cells within the tumors of mice across different treatment groups, including CD4+ T cells, CD8+ T cells, Tregs, DCs, M1 macrophages, and M2 macrophages
In this study, we developed a biotin-functionalized curcumin-based polymer prodrug nanoplatform (Biotin@P-Cur/T780 NPs) designed to enhance the efficacy of combined photo-immunotherapy for head and neck cancer. The radical-mediated polymerization of curcumin was optimized over a 48-hour reaction period, resulting in a gradual increase in number-average molecular weight from. This improved polymerization supports sustained release and pH-responsive drug delivery through acid-labile ester linkages, which help preserve curcumin’s bioactivity until release in the tumor microenvironment. The nanoplatform demonstrated tumor-targeting capability, GSH/pH-sensitive release behavior, and the ability to induce moderate ICD in vitro and in murine tumor models. While reduced cancer stemness markers and partial reprogramming of the immunosuppressive tumor microenvironment were observed, these effects were modest and warrant further mechanistic validation.
The therapeutic benefit, though encouraging, remains constrained by the limited penetration depth of phototherapies and the complex immune landscape of head and neck tumors. Despite these limitations, this study provides a proof-of-concept for integrating polymer prodrug chemistry with responsive nanocarrier systems to improve curcumin delivery and enable multi-modal cancer therapy. Further work is needed to refine drug loading efficiency, evaluate long-term immune memory effects, and assess the platform’s efficacy in more clinically relevant models, including patient-derived xenografts and immunocompetent large animals.
No datasets were generated or analysed during the current study.
The authors would like to thank the Pub-lab of West China School of Basic Medical Sciences and Forensic Medicine, Sichuan University, for providing various laboratory instruments.
Ningbo Top Medical and Health Research Program (No. 2023030514), Ningbo Clinical Research Center for Otolaryngology Head and Neck Disease (No. 2022L005), Ningbo Natural Science Foundation (No. 2023J030), Chengdu Science and Technology Program (No. 2024-YF05-00227-SN), Postdoctoral Research Project, West China Hospital, Sichuan University (No. 2024HXBH110), Natural Science Foundation of Sichuan Province (No. 2024NSFSC1905, 2025ZNSFSC1042), and the Zhong Nanshan Youth Science and Technology Innovation Award Fund of the China Youth Entrepreneurship and Employment Foundation (No. P24041587729) supported this work.
All animal experiments conformed to the requirements of the institutional animal use and care system of Sichuan University.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Shen, Z., Jiang, H., Lu, S. et al. Curcumin-based polymer prodrug nanoplatform for high-efficiency immunotherapy by synergistically suppression of head and neck cancer cell stemness. J Nanobiotechnol 23, 500 (2025). https://doi.org/10.1186/s12951-025-03559-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-025-03559-9