Archives of Public Health volume 83, Article number: 150 (2025) Cite this article
Our study assesses the association between cumulative handgrip strength and longitudinal changes in cognitive function and daily functioning.
Two comparative cohort studies were used, including the English Longitudinal Study of Ageing (ELSA) and the Survey of Health, Ageing and Retirement in Europe (SHARE). Cumulative handgrip strength was calculated using three repeated measurements of handgrip strength. Linear mixed regression models evaluated the association between cumulative handgrip strength and longitudinal changes in cognitive function and daily functioning. Cox regression models were performed to determine the association between cumulative handgrip strength and the risk of cognitive and functional impairment.
Individuals with lower levels of cumulative handgrip strength had lower global cognition (β: -0.244; 95% CI: -0.317, -0.170 for ELSA and -0.359; -0.406, -0.311 for SHARE) and experienced a faster decline in cognitive function over time (-0.025; -0.037, -0.013 for ELSA, and -0.019; -0.026, -0.013 in SHARE). We found lower levels of cumulative handgrip strength were associated with lower daily functioning (β: 0.267; 95% CI: 0.161, 0.374 for ELSA and 0.153; 0.079, 0.227 for SHARE), and a faster decline in daily functioning over time (0.105; 0.081, 0.129 for ELSA and 0.217; 0.195, 0.238 for SHARE). Furthermore, lower levels of cumulative handgrip strength were related to a higher risk of cognitive and functional impairment.
Our study suggested that lower levels of cumulative handgrip strength was related to an accelerated decline in cognitive function and daily functioning. Persistently strengthening muscle strength should be emphasized in preventing neurodegenerative disorders and disabilities.
Text box 1. Contributions to the literature |
|---|
➢ Evidence on the association between cumulative handgrip strength, cognitive function, and daily functioning remains limited. |
➢ Our study suggested that lower levels of cumulative handgrip strength was related to an accelerated decline in cognitive function and daily functioning. |
➢ Persistently strengthening muscle strength should be emphasized in preventing neurodegenerative disorders and disabilities. |
With the surging growth of the aging population, the number of adults who have dementia and functional disability is rising [1]. Globally, an estimated 50 million and 101 million older adults live with dementia and severe functional disability; these numbers are projected to increase to 150 million and 277 million by 2050, respectively [1, 2], substantially raising the worldwide cost of dementia and disability [3]. Preserving and enhancing cognitive and functional abilities in later life is essential for promoting healthy aging and alleviating the growing social and economic burdens associated with dementia and disability.
Handgrip strength is a convenient and well-established measurement of muscle strength [4]. It serves as a critical indicator of frailty and is associated with cognitive decline (or even dementia) [5] and functional disability [6] in older adults. A meta-analysis of 15 longitudinal cohort studies found that low handgrip strength was associated with an increased risk of cognitive decline and dementia [7]. Furthermore, declines in handgrip strength are sensitive to the integrity of the nervous system centers, particularly those mediating the control of coordinated movement. Thus, deterioration in handgrip strength could lead to functional disability [8], defined as reliance on activities of daily living (ADLs) and/or instrumental activities of daily living (IADLs). A recent meta-analysis showed that low handgrip strength was related to worsened ADLs (odds ratio [OR]: 1.51, 95% CI:1.34, 1.70; 10 studies) and IADLs (OR: 1.59, 95% CI: 1.04, 2.41;4 studies) [6], respectively. Nevertheless, most existing studies examining the relationship between handgrip strength, cognitive function, and daily functioning rely on single time-point measurements (usually baseline level). Given that the handgrip strength is subject to dynamic change due to factors such as aging and physical activity [9, 10], results based on a single measurement might be unreliable. Cumulative exposure of handgrip strength, calculated as the product of the dose level and the exposure time using repeated measurements, accounts for the dynamic change and cumulative exposure effects of muscular strength [11]. Previous studies have explored the association of cumulative handgrip strength and the risk of incident diabetes [11] and cardiovascular disease [12]. However, evidence on the association between cumulative handgrip strength, cognitive function, and daily functioning remains limited.
To address this gap, we utilized data from the English Longitudinal Study of Aging (ELSA) and Survey of Health, Ageing, and Retirement in Europe (SHARE) to investigate 1) the associations between cumulative handgrip strength and longitudinal changes in cognitive function and daily functioning in adults aged 50 years or older; 2) the associations between cumulative handgrip strength and the risk of cognitive and functional impairment in adults aged 50 years or older.
Our study utilized data from ELSA and SHARE. Both ELSA and SHARE are ongoing, biennial longitudinal cohort studies that recruited participants aged 50 years and older from English and several European countries, respectively. Detailed descriptions of the study designs, sampling procedures, and survey methods for ELSA [13] and SHARE [14] have been published elsewhere. Briefly, data were collected biennially by well-trained researchers, including questionnaires using face-to-face interviews, physical examinations, and biological specimens. The London Multicenter Research Ethics Committee approved ELSA, the Ethics Council of the Max Planck Society, and the Ethics Committee of the University of Mannheim approved SHARE. All participants provided written informed consent.
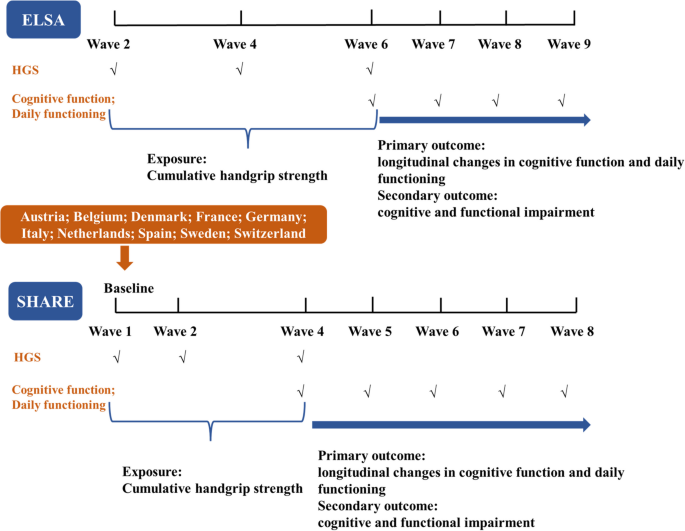
The baseline for our study was defined as wave 2 (2004) in ELSA and wave 1 (2004) in SHARE. The SHARE recruited 12 countries in wave 1 (the year 2004), including Austria, Germany, Sweden, Netherlands, Spain, Italy, France, Denmark, Greece, Switzerland, Belgium, and Israel, but two out of 12 countries did not continuously measure handgrip strength from wave 1 to wave 4 and thus were deleted from our analysis. To explore the association between cumulative handgrip strength and longitudinal changes in cognitive function and daily functioning, the study design is illustrated in Fig. 1. Specifically, handgrip strength was measured in waves 2, 4, and 6 in ELSA, and in waves 1, 2, and 4 in SHARE. Cumulative handgrip strength, the primary exposure, was calculated over an eight-year period in ELSA and a six-year period in SHARE. The outcomes- longitudinal changes in cognitive function and daily functioning-were estimated from wave 6 to wave 9 in ELSA and wave 4 to wave 8 in SHARE. In our study, we included 17,143 participants from the ELSA and SHARE, and 6,122 participants were excluded due to 1) lack of information regarding handgrip strength from wave 2 to wave 6 in the ELSA or from wave 1 to wave 4 in the SHARE; 2) lack of information regarding cognitive function and daily functioning at baseline; 3) had a history of dementia and Alzheimer’s disease; 4) lack of information regarding necessary covariates at baseline; 5) loss to follow-up. Finally, we included 11,021 participants (3,239 from the ELSA and 7,782 from the SHARE) to assess the association between cumulative handgrip strength and temporal changes in cognitive function and daily functioning (Supplementary Figure 1). Additionally, as the secondary outcomes, cognitive function and daily functioning were analyzed as categorical variables, referred to as cognitive and functional impairments. For these analyses, 9,340 participants (2,277 from ELSA and 7,063 from SHARE) were included to examine the association between cumulative handgrip strength and the risk of cognitive and functional impairments.
The study designs. Abbreviations: ELSA, English Longitudinal Study of Aging; HGS, handgrip strength; SHARE, Survey of Health, Ageing, and Retirement in Europe. Note: The ELSA did not collect the handgrip strength in wave 3 and wave 5, and SHARE did not collect the handgrip strength in wave 3
In ELSA, the Smedley handheld dynamometer (Stoelting Co., Wood Dale, IL, USA) was used to measure handgrip strength (kg) three times on each hand in waves 2, 4, and 6. In SHARE, the Smedley handheld dynamometer (TTM, Tokyo, 100 kg) was used to measure handgrip strength twice on each hand in waves 1, 2, and 4. All participants were asked to determine which hand was their dominant hand before handgrip strength testing, and starting with their dominant hand, participants were asked to squeeze the handles with maximal effort. The average of three (in ELSA) or two (in SHARE) measurements of the dominant hand were used to assess handgrip strength [15]. Handgrip strength measurements from 3 visits (wave 2, wave 4, and wave 6) in ELSA and three visits (wave 1, wave 2, and wave 4) in SHARE were used to evaluate cumulative handgrip strength.
To obtain the cumulative handgrip strength over eight-year period for ELSA and six-year period for SHARE, we denoted X1, X2, and X3 as the handgrip strength measured at waves 2, 4, and 6 in ELSA and waves 1, 2, and 4 in SHARE; T1 and T2 as the time-lengths for waves 2→4 and 4→6 in ELSA and waves 1→2 and 2→4 in SHARE, respectively. According to the trapezoid rule, the cumulative handgrip strength was calculated as the area under the curve for handgrip strength throughout the exposure [11]:
$$\frac{\left(X1+X2\right)*T1}{2}+\frac{\left(X2+X3\right)*T2}{2}$$
Since the time intervals for wave 2, wave 4, and wave 6 were approximately four years in ELSA, we assigned T1 and T2 the value of “4 years” for analysis. Since the time intervals for wave 1, wave 2, and wave 4 were 2 and 4 years in SHARE, we assigned T1 and T2 the values of “2 years” and “4 years” for analysis, respectively.
We used the measurement of cognitive function available in both ELSA and SHARE at each wave, including verbal memory and semantic fluency. Verbal memory consisted of immediate and delayed recall of 10 randomly unrelated nouns and was evaluated by summing up correctly recalled words. Semantic fluency was assessed by asking participants to name as many animals as possible within 1 minute. The validity and reliability of the verbal memory and semantic fluency tests have been well-established [16, 17]. For each domain, higher scores indicate better cognition. Z-scores were calculated to allow direct comparisons among cognitive tasks. We standardized the cognitive scores for each test, and z-scores were calculated by subtracting the mean scores at each wave and dividing by the standard deviation (SD) of scores at each wave. The mean of the z-scores of the two tests was used to assess the global cognitive z-scores. Because the semantic fluency for wave 6 in ELSA was not measured, we used semantic fluency measured from wave 5 instead.
The activities of daily living (ADLs) and instrumental ADL (IADLs) were used to assess daily functioning [18]. ADLs in ELSA and SHARE include tasks such as walking across a room, dressing, bathing and showering, eating, getting in or out of bed, and using the toilet. IADLs include using the phone, taking medications, managing money, shopping for groceries, and preparing meals in ELSA, with additional tasks in SHARE, such as using a map and doing house- or garden work for SHARE. We assigned each item 0 (did not report any problems with the activity) or 1 (some difficulty with the activity). We summed up all corresponding items as the total ADL and IADL scores. The sum of total ADL and IADL scores was used to assess the level of daily functioning. The score ranges from 0 to 11 for ELSA and 0 to 13 for SHARE, where higher scores indicate greater impairment in daily functioning.
The primary outcome was longitudinal changes in cognitive function and daily functioning. The secondary outcome was cognitive impairment and functional impairment. According to previous studies, cognitive impairment was defined as a score of 1.5 SDs lower than the mean of the population stratified by educational background [19]. Functional impairment was defined as having limitations in one or more activities, including ADLs and IADLs [20].
Covariates included sociodemographic characteristics (age, sex, marital status, and education level), health-related lifestyle and behaviors (cigarette smoking, alcohol drinking, and physical activity), and history of diseases (hypertension, diabetes, stroke, heart disease, and cancer) at baseline. Sex was grouped as male and female. Marital status was categorized as married, single, and unknown. Education level was grouped into three groups: low (including no qualification, level 1 national vocational qualification (NVQ) or certificate of secondary education, NVQ2 or GCE O-level for the ELSA; lower than upper secondary level of education for the SHARE), high (including NVQ3 or GCE A-level, higher qualification but below degree, and degree level or higher or NVQ4/5 for the ELSA; higher than post-secondary non-tertiary for the SHARE) and unknown. Cigarette smoking was grouped as never smoking, former smoking, and current smoking. Alcohol drinking was defined as drinking alcohol at least once a week. Physical activity was classified into three groups: sedentary behavior (mild physical activity less than once a week), moderate physical activity (moderate physical activity more than once a week), and vigorous physical activity (vigorous physical activity more than once a week). BMI was calculated by dividing weight in kilograms by height in meters squared (kg/m2) and classified into normal weight (<25 kg/m2), overweight (25–29.9 kg/m2), obesity (≥ 30 kg/m2), and unknown. Hypertension was defined as SBP ≥140 mm Hg or DBP ≥ 90 mm Hg, a self-reported physician diagnosis of hypertension, or current use of anti-hypertensive therapy. Diabetes was defined as an HbA1c level ≥ 47.5 mmol/mol (6.5%), a self-reported physician diagnosis of diabetes, or current use of anti-diabetes therapy. Stroke was defined as self-reported doctor-diagnosed stroke. Heart disease was defined as self-reported doctor-diagnosed heart disease (including angina, congestive heart failure, and myocardial infarction). Cancer was defined as self-reported doctor-diagnosed cancer (Supplementary Table 1).
Mean (Standard deviation, SD) and frequency (percentage) were used to summarize continuous and categorical variables. The ANOVA (for continuous variables) and Chi-square tests (for categorical variables) were performed to compare the baseline characteristics across individuals with different sex-specific quartiles of cumulative handgrip strength. The details of sex-specific quartiles of cumulative handgrip strength were represent in Supplementary Table 2.
Given the repeated measurements of cognitive function and daily functioning, linear mixed models were used to account for the within-participant correlation. Linear mixed models were conducted to estimate the coefficient and 95% CIs for the longitudinal association between cumulative handgrip strength and longitudinal changes in cognitive function and daily functioning. The intercept and slope of time were fitted as random effects at the participant level to address between-participant differences at baseline and rates of cognitive function and daily functioning changes. We added the “time*cumulative handgrip strength” term to our models to assess the rates of change in cognitive function and daily functioning over time across different quartiles of cumulative handgrip strength (Supplementary Methods). In addition, Cox regression models were conducted to estimate hazard ratios (HRs) and 95% CIs for associations between cumulative handgrip strength and risk of cognitive impairment and functional impairment. Cumulative handgrip strength was analyzed both as a continuous variable (z-transformed for standardization) and as sex-specific quartiles, with the highest quartile designated as the reference group.
To understand the confounding effects of various covariates, we adjusted for them in three models in a stepwise manner. We adjusted for age and sex in Model 1; additionally adjusted for marital status, education level, physical activity, alcohol drinking, and cigarette smoking in Model 2; and additionally adjusted for hypertension, diabetes, stroke, heart disease, cancer, and obesity in Model 3. Linear trends in the longitudinal associations (dose-response relationships) between cumulative handgrip strength and cognitive function and daily functioning decline were assessed by assigning the sex-specific median cumulative handgrip strength for each cumulative handgrip strength quartile and modeling it as a continuous variable.
In the subgroup analyses, we stratified participants by age (< 70 and ≥ 70 years), sex (male and female), physical activity (sedentary behavior, moderate physical activity, and vigorous physical activity), and BMI <25 kg/m2, 25–29.9 kg/m2, and ≥ 30 kg/m2). We added a product term of the stratifying variable and cumulative handgrip strength to the final models to examine potential effect modifications by the stratifying variables using Wald tests.
We performed three sensitivity analyses to confirm the robustness of our results. Firstly, we evaluated the association between cumulative handgrip strength and the subdomains of cognitive function and daily functioning. Secondly, some studies have utilized the maximum handgrip strength value from multiple measurements, rather than the average value, to assess handgrip strength. To address this methodological variation, we repeated all analyses using an alternative measurement approach: the maximum handgrip strength value derived from three measurements in ELSA and two measurements in SHARE, both taken from the dominant hand. Thirdly, we repeated all analyses among individuals with complete data on cognitive function and daily functioning during follow-up.
All analyses were performed using R version 4.2.1. An α = 0.05 was chosen as the threshold for statistical significance.
A total of 11,021 participants (3,239 participants from ELSA and 7,782 participants from SHARE) were included in the analysis. In our study, the mean age was 69.41 (SD, 8.19) years, and 44.76% were men. 30.12 % had high education level, 13.38% were current smokers, 57.09% were drinkers, and 43.40% had vigorous physical activity (Table 1). Cumulative handgrip strength was lower in those who were older, single, non-drinkers, non-smokers (only for females), had low education level, engaged in sedentary behavior, had BMI < 25kg/m2, or had a history of hypertension, diabetes, stroke, heart disease (All P ≤ 0.02; Supplementary Table 3). Detailed data for ELSA and SHARE are provided in Supplementary Tables 4–5.
As shown in Table 2, lower cumulative handgrip strength was associated with an accelerated decline in global cognitive function. Compared with the highest quartile, participants in the lowest quartile of cumulative handgrip strength exhibited significantly lower global cognitive function (β: −0.244; 95% CI: −0.317, −0.170 for ELSA and β: −0.359; 95% CI: −0.406, −0.311 for SHARE). Additionally, participants in the lowest quartile of cumulative handgrip strength experienced a faster decline in global cognitive function over time (β: −0.025 SD/y; 95% CI: −0.037, −0.013 for ELSA, and β: −0.019 SD/y; 95% CI: −0.026, −0.013 in SHARE) compared with those in the highest after controlling for sociodemographic, lifestyle, and related-diseases covariates. There were linear trends in associations with accelerated global cognitive decline across quartiles of cumulative handgrip strength in both ELSA and SHARE (P for trend < 0.001). When cumulative handgrip strength was treated as a continuous variable, its decline was associated with an accelerated decline in cognitive function (β: −0.008; 95% CI: −0.012, −0.004 for ELSA; −0.005; 95% CI: −0.007, −0.002 for SHARE; Table 2). Similar results were observed in four countries from the SHARE cohort (Table 2). No significant differences were observed after incremental adjustments for sociodemographic and lifestyle covariates (Supplementary Table 6).
Using cognitive impairment as a secondary outcome, participants in the lowest quartile of cumulative handgrip strength had higher odds of cognitive impairment (HR: 1.60; 95% CI: 1.07, 2.39 for ELSA; HR: 2.85; 95% CI: 2.15, 3.77 for SHARE; Table 3). Consistent findings were observed in five countries from SHARE (Table 3).
Sensitivity analyses confirmed the robustness of these results. In the subdomains of cognitive function, participants in the lowest quartile of cumulative handgrip strength exhibited lower verbal memory in both ELSA and SHARE. Additionally, verbal memory declined faster in the lowest quartile compared to the highest quartile of cumulative handgrip strength in SHARE (β: −0.020 SD/year; 95% CI: −0.029, −0.012; Supplementary Table 7). Similarly, semantic fluency declined faster in the lowest quartile in both ELSA (β: −0.034 SD/year; 95% CI: −0.050, −0.018) and SHARE (β: −0.017 SD/year; 95% CI: −0.025, −0.009; Supplementary Table 8). The results remained consistent when handgrip strength was measured as the maximum of several handgrip strength measurements of the dominant hand (Supplementary Table 9), or when we restricted our analyses to participants with complete follow-up data (Supplementary Table 10). The association between cumulative handgrip strength and cognitive impairments remained similar after adjusting for sociodemographic or/and lifestyle covariates (Supplementary Table 11) or using maximum handgrip strength measurements as the alternative measurement (Supplementary Table 12).
Subgroup analyses revealed some heterogeneities, particularly by sex, with lower cumulative handgrip strength having a stronger effect on cognitive function in females (Supplementary Table 13–14).
Participants in the lowest quartile of cumulative handgrip strength had lower daily functioning (β: 0.267; 95% CI: 0.161, 0.374 for ELSA and 0.153; 95% CI: 0.079, 0.227 for SHARE), and experienced a faster decline in daily functioning compared to those in the highest quartile (β: 0.105 point/y; 95% CI: 0.081, 0.129 for ELSA and 0.217 point/y; 95% CI: 0.195, 0.238 for SHARE) after controlling for sociodemographic, lifestyle, and related-diseases covariates. There were linear trends in association with accelerated daily functioning decline across quartiles of cumulative handgrip strength in both ELSA and SHARE (P for trend < 0.001). Similar results were found in all six countries from SHARE (Table 4). No significant differences were observed after adjusting for sociodemographic and lifestyle covariates (Supplementary Table 15). When treated as a continuous variable, declining cumulative handgrip strength was associated with a faster decline in daily functioning (β: 0.027; 95% CI: 0.019, 0.036 for ELSA; β: 0.053; 95% CI: 0.046, 0.061 for SHARE; Table 4).
Using functional impairment as a secondary outcome, participants in the lowest quartile of cumulative handgrip strength had higher odds of functional impairment (HR: 1.51; 95% CI: 1.15, 2.00 for ELSA; 1.64; 95% CI: 1.41, 1.90 for SHARE; Table 5), compared with those in the highest quartile. Similar results were found when cumulative handgrip strength was treated as a continuous variable (Table 5).
Supplementary Tables 16–17 represent the association between cumulative handgrip strength and subdomains of daily functioning (ADLs and IADLs), with results consistent with the main findings. Results remained unchanged when handgrip strength was measured as the maximum of several handgrip strength measurements of the dominant hand (Supplementary Table 18), or when analyses were restricted to participants who had complete follow-up data (Supplementary Table 19). The positive associations between declining cumulative handgrip strength and functional impairment persisted after adjusting for sociodemographic and lifestyle covariates (Supplementary Table 20) or using the maximum handgrip strength measurements as the alternative measurement (Supplementary Table 21). In the sub-scores of ADL and IADL, lower cumulative handgrip strength was significantly associated with increased limitations in dressing, bathing, eating, grocery shopping, and meal preparation (Supplementary Table 22).
Subgroup analyses revealed some heterogeneities in the associations between cumulative handgrip strength and daily functioning, with stronger associations observed among females, individuals with sedentary behavior, and those with a BMI > 30 kg/m2, particularly in SHARE (Supplementary Table 13–14).
In our study, using the data from 11,021 participants (7,782 participants from ELSA and 3,239 participants from SHARE), we found that low levels of cumulative handgrip strength were associated with accelerated declines in cognitive function and daily functioning over time, as well as a higher risk of cognitive and functional impairment. Notably, the association between low levels of cumulative handgrip strength and cognitive and functional impairment was stronger in females than in males. The association between cumulative handgrip strength and an accelerated functional decline was stronger in individuals with sedentary behaviors.
Our study revealed that cumulative handgrip strength was lower among older adults, non-drinkers, non-smokers (only in females), those with low education levels, and individuals who engaged in sedentary behavior. These findings are consistent with a systematic review indicating that older adults (based on 20 studies) and those not engaged in physical activities (based on five studies) tended to have lower handgrip strength [21]. Similarly, a study of 3,634 adults from the Korea National Health and Nutrition Examination Survey also found that lower levels of handgrip strength was associated with reduced physical activity and lower education levels [22]. However, the associations between smoking status, alcohol consumption, and handgrip strength remain inconsistent. For example, a study of 7,649 Korean adults suggested that current smokers had stronger mean handgrip strength compared to ex-smokers and non-smokers, particularly among individuals aged over 60 years [23], In contrast, a study of American adults found no significant association between muscle strength and smoking status [24]. Similarly, the role of alcohol consumption as either a protective or risk factor for handgrip strength decline remains debated [25, 26].
Relationships between handgrip strength and cognitive function were widely reported in previous studies [27]. A recent prospective cohort study of 340,212 participants from UK Biobank showed that a 5 kg increment in absolute handgrip strength was associated with a reduced risk of 14% in all-cause dementia (HR, 0.86; 95% CI: 0.83, 0.88) [28]. However, most existing studies usually used a single time-point measurement of handgrip strength, and did not account for the effects of cumulative exposure for an individual to muscle strength on cognition over time. Recently, a cohort study involving 6,696 participants from the Korean Longitudinal Study of Aging (KLoSA) with multiple measurements of handgrip strength demonstrated that compared with the stable or increased handgrip strength group, the decreased handgrip strength group showed a cognitive decline during follow-up (β: −0.31; 95% CI: −0.41, −0.22 for men; −0.27; 95% CI: −0.37, −0.16 for women) [29]. However, this study was limited to Korean populations, which restricts the generalizability of the results. Our study, using the data from 11 countries (including the UK and 10 European countries), calculated cumulative handgrip strength based on three repeated measurements of handgrip strength over 6–8 years and robustly demonstrated that lower levels of cumulative handgrip strength was related to an accelerated decline in cognitive function over time. Thus, our findings confirmed the predictive value of the repeated handgrip strength measurements for identifying people at a higher risk of cognitive decline. When cognitive impairment was used as the outcome, the findings consistently showed that exposure to lower levels of cumulative handgrip strength was associated with an increased risk of cognitive impairment. However, disparities in this association were observed across countries. For instance, Austria showed a negative but statistically insignificant association, while Belgium exhibited a significantly positive association. These inconsistencies may stem from differences in sample sizes and population characteristics, underscoring the need for further research to explore these heterogeneities in greater depth.
Another important finding of our study was that poor cumulative handgrip strength was strongly associated with accelerated daily functioning decline and functional impairment. Previous studies have investigated the effect of handgrip strength on daily functioning, including ADLs and IADLs, in different populations. For example, a study of 18,391 Americans aged 50 years and over found that lower handgrip strength was associated with a 1.7-times increased risk of IADL disability (OR: 1.70, 95% CI: 1.57, 1.84) and was associated with a 2.26-times increased risk of ADL disability (OR: 2.26, 95% CI: 2.03, 2.51), respectively [30]. The China Health and Retirement Longitudinal Study (CHARLS) illustrated similar results and found functional limitations mediated the relationship between weak handgrip strength and cognitive function impairment [31]. Unlike previous studies that used baseline handgrip strength, our study utilized multiple repeated measurements of handgrip strength to explore the longitudinal relationship between cumulative handgrip strength and daily functioning. Although there were differences in the manners of handgrip strength measurement (single time vs. multiple repeated times) between our study and the previous studies, our results align with the existing literature, confirming the credibility of cumulative handgrip strength in identifying people at high risk for functional disability. Furthermore, our study found that lower levels of cumulative handgrip strength was associated with a decline in each item of both ADLs and IADLs. Specifically, abilities such as dressing, bathing, eating, grocery shopping, and meal preparation were notably affected. This pattern was consistently observed across the ELSA and SHARE datasets, suggesting a broad influence of handgrip strength on functional independence. Similar findings have been reported among American adults [32, 33]. Maintaining normal levels of handgrip strength is essential for independent living and self-care ability [34]. Handgrip strength could reflect the overall muscle strength due to its association with other muscular tissues; declined handgrip strength would restrict the ability of individuals to perform physical activity, which necessitates overall muscle strength [35], potentially impairing tasks demanding greater force generation, such as grocery shopping and meal preparation. Additionally, reduced handgrip strength has been linked to cognitive decline, which may further compromise performance in autonomous living tasks—even those requiring minimal muscular force output. Functional disability is associated with a range of adverse outcomes, including an increased risk of elder abuse, and mortality [36, 37]. Therefore, enhancing handgrip strength to reduce functional disability is crucial for improving the overall health and well-being of adults.
The subgroup analyses revealed that the association between cumulative handgrip strength and cognitive decline was stronger in females than males. Similarly, a previous study [38] found a stronger relationship between low handgrip strength and mild cognitive impairment in females than males. The previous study showed that females experienced greater memory decline relative to males, and APOE genotype influenced the gender differences in neuropathology, which may contribute to the observed disparities in progression of cognitive decline [39]. A stronger association between lower levels of cumulative handgrip strength and a higher risk of functional impairment was also found in females, which was in line with the result from a prospective study of China Health and Retirement Longitudinal Study (CHARLS) [40]. Relatively high susceptibility to decreases in muscle strength and mass in females might partially explain these gender disparities [41]. Moreover, cognition and physical function are strongly related; cognitive decline may contribute to ADL and IADL disability [30]. Our results found that there was a faster cognitive decline in females than males, which may be another plausible explanation for a stronger association between handgrip strength and functional impairment among females. Besides, the association between lower cumulative handgrip strength and accelerated functional decline was stronger in individuals with sedentary behaviors, consistent with previous studies [42]. Aerobic resistance, endurance, and physical flexibility have a mediating effect on the correlation between sedentary behaviour and functional disability including the decline in ADLs and IADLs [43].
Though mechanisms underlying the association of handgrip strength with cognitive function and daily functioning were not fully understood, several potential pathological pathways have been suggested. First, SPARC-related modular calcium-binding protein 1 (SMOC1) was significantly over-expressed in some plaque structures of patients with Alzheimer’s disease [44]. Simultaneously, increased expression levels of SMOC1 with handgrip strength decline have been observed [45], suggesting that SMOC1 or its related signaling pathways may play a role in the associations mentioned above. Second, the association of handgrip strength and cognitive function could result from differences in brain structure. For example, great handgrip strength contributed to increased grey matter volume in the brain, resulting in better cognitive function [46]. Besides, low handgrip strength could accelerate the progression of cerebral white matter hyperintensity (WMH) and thereby increase functional impairment [47]. Third, weak handgrip strength was associated with insufficient serum folate concentrations [48], which has been proposed as the risk factor for the onset of cognitive function impairment and degenerative dementia [49]. Moreover, elevated homocysteine levels were associated with decreased handgrip strength [50] and cognitive decline [51]. Fourth, in terms of the association between handgrip strength and daily functioning, several shared physiopathologic mechanisms, including inflammation (elevated level of C-reactive protein) [52, 53], declined hemoglobin level [54], oxidative stress [55], have been proposed, as all of which may result in both declines in handgrip strength and daily functioning.
The positive association between lower cumulative handgrip strength and cognitive and functional impairment suggested that handgrip strength could be used as a routine screening tool for identifying people at significant risk of dementia and functional disabilities due to its simplicity and low cost. The multiple repeated measurements of handgrip strength in our study further highlighted that dynamic assessments of handgrip strength could be considered for screening individuals for potential neurological function disorders. Previous studies observed that handgrip strength and muscle mass were significantly ameliorated by exercise in older adults [56]. Maintaining handgrip strength at optimum levels or preventing their declines achieved by physical activity could be an effective strategy to prevent dementia and physical dependence onset. Based on our subgroup analyses, associations between handgrip strength and cognitive and functional impairments were stronger among females, suggesting that policymakers should acknowledge gender differences when formulating policies and health services.
The first strength of this study is that we recruited 11,021 participants from two multi-nationally representative longitudinal cohort studies that covered 11 countries to provide robust evidence on the association between cumulative handgrip strength and long-term changes in cognitive function and daily functioning. Second, cumulative handgrip strength in this study was derived from multiple repeated time-point measurements, which allowed us to explore the cumulative exposure effect of handgrip strength on cognitive function and daily functioning trajectories. Nevertheless, we need to acknowledge several limitations. Firstly, our study used questionnaires to measure cognitive function and daily functioning, which might not be sensitive enough to find minor subclinical deficits. While the validity and reliability of verbal memory and semantic fluency tests have been well-established for estimating cognitive function, more precise neuropsychological testing could provide greater accuracy and should be incorporated in future research. Secondly, although our study included several essential covariates, some unmeasured confounding factors, such as nutrition, might have impacted this association and were not included in the analyses due to data limitations. Meanwhile, some covariates, such as physical activity and alcohol drinking, could not be quantitatively determined as the WHO recommended because the data was unavailable, which may influence the results. Thirdly, several individuals were excluded from this study due to loss of follow-up and lack of necessary information, which may lead to biased results. Fourthly, normalized handgrip strength using absolute handgrip strength divided by weight or BMI in our study was not calculated due to the high missing values of weight or BMI, which may influence the precision of the predictive value of handgrip strength on cognition function and daily functioning decline. Fifthly, our study was the lack of a formal sample size calculation, which may affect the generalizability of the findings. However, the use of two multi-nationally representative longitudinal cohort studies with 11,021 participants mitigated this concern to some extent.
Our study suggested that lower levels of cumulative handgrip strength was related to an accelerated decline in cognitive function and daily functioning and a higher risk of incident cognitive and functional impairment. Strengthening muscle strength should be emphasized in preventing neurodegenerative disorders and disabilities in elderly populations.
No datasets were generated or analysed during the current study.
- ELSA:
-
English Longitudinal Study of Ageing
- SHARE:
-
Survey of Health, Ageing and Retirement in Europe
- UK:
-
United Kingdom
- HR:
-
Hazard ratio
- OR:
-
Odds ratio
- ADL:
-
Activities of daily living
- IADL:
-
Instrumental activities of daily living
- SD:
-
Standard deviation
- NVQ:
-
National vocational qualification
- BMI:
-
Body mass index
- DBP:
-
Diastolic blood pressure
- SBP:
-
Systolic blood pressure
- CI:
-
Confidence interval
- Coef:
-
Coefficient
- Q:
-
Quartile
- CHARLS:
-
China Health and Retirement Longitudinal Study
- SMOC1:
-
SPARC-related modular calcium-binding protein 1
- WMH:
-
White matter hyperintensity
- HGS:
-
Handgrip strength
The authors thank the original data creators, depositors, copyright holders, and the funders of the Data Collections for using data from the English Longitudinal Study of Ageing and the Survey of Health, Aging, and Retirement in Europe.
This work was supported by the National Natural Science Foundation of China (Grant No.72204031), the CMC Excellent-talent Program (No. 2024qnGzn17), the Foundation of Sichuan Research Center of Applied Psychology of Chengdu Medical College (Grant No. CSXL-24212 and CSXL-22301), the Research Foundation of Chengdu Medical College(Grant No. CYSYB20-12), the Open Fund of development and Regeneration Key Laboratory of Sichuan Province (Grant No. SYS23-02), the Clinical Science Research Project of Chengdu Medical College & Nanbu County People’s Hospital(Grant No. 202309), the Natural Science Foundation of Sichuan (Grant No. 2022 NSFSC0649).
ELSA was approved by the London Multicenter Research Ethics Committee (MREC/01/2/91). Ethical approval of SHARE from the first to fourth waves was obtained from the Ethics Committee of the University of Mannheim. In 2018, the Ethics Council of the Max Planck Society reviewed and approved the fourth and consecutive waves of the SHARE project. Informed consent was obtained from all ELSA and SHARE participants included in the study.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Han, B., Zeng, Z., Wen, Y. et al. Cumulative handgrip strength and longitudinal changes in cognitive function and daily functioning among people aged 50 years and older: evidence from two longitudinal cohort studies. Arch Public Health 83, 150 (2025). https://doi.org/10.1186/s13690-025-01624-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13690-025-01624-1