Journal of Hematology & Oncology volume 18, Article number: 71 (2025) Cite this article
Antibody–drug conjugates (ADCs) aim to enhance the therapeutic index of cytotoxic agents but can cause unexpected toxicities. This study evaluated adverse events (AEs) from phase 1 trials at The Royal Marsden Drug Development Unit (DDU) over a decade and pivotal phase 2 and 3 trials leading to FDA registration, correlating AEs with ADC components such as target, antibody, linker, payload, and Drug-to-Antibody Ratio (DAR).
We performed a retrospective cohort analysis of patients treated with ADCs in phase 1 trials (January 2014 to January 2024) compared to published phase 2–3 trials of FDA-approved ADCs. Univariate and multivariate logistic regression analyzed ADC components and treatment toxicities.
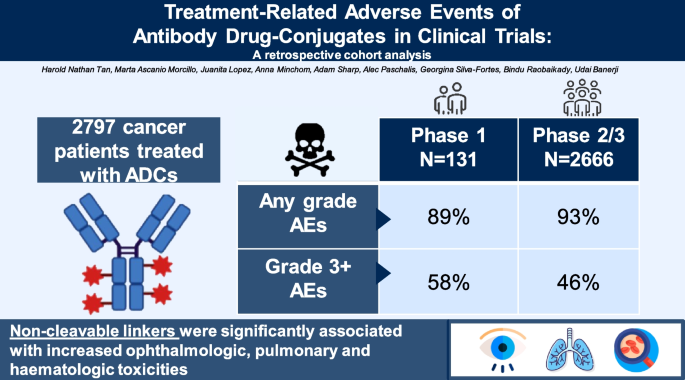
One hundred thirty one phase 1 trial patients and 2666 phase 2–3 trial participants were included. High incidences of any-grade treatment-related AEs were observed (89% in phase 1, 93% in phase 2–3), with 58% experiencing grade 3 or higher toxicities in phase 1 and 46% in later phases. Major AEs included fatigue, hematologic toxicities, nausea/vomiting, ocular toxicities, and peripheral neuropathy. Antibody targets were linked to neuropathy, non-cleavable linkers to ocular, pulmonary, and hematologic toxicities, and tubulin-binding payloads to peripheral neuropathy. ADCs with DAR > 4 were associated with higher pulmonary and hematologic AEs.
Despite their design to minimize toxicity, ADCs were linked to significant AEs. Specific ADC components may contribute to distinct toxicities, necessitating more robust trial data to inform future ADC design.
Antibody–drug conjugates (ADCs) are engineered to improve the therapeutic index of chemotherapy by coupling cytotoxic agents to antibodies that recognize tumor-specific surface antigens. This targeted delivery enables selective release of cytotoxic payloads at tumor sites while avoiding damage to non-target cells. Over the past two decades, the clinical development of ADCs has experienced rapid growth. Currently, 14 ADCs have been approved by the US Food and Drug Administration (FDA), with 189 ADCs being tested in various stages of clinical development [1].
Recent pivotal trials have demonstrated the favorable risk–benefit profile of ADCs by demonstrating superior efficacy with comparable toxicity compared to standard chemotherapy, even in heavily pretreated populations. In the phase 3 DESTINY-Breast06 trial, trastuzumab deruxtecan (T-DXd) nearly doubled progression-free survival (14.0 vs 6.5 months; HR 0.38) and achieved higher objective response rates (68% vs 25%) than chemotherapy in hormone receptor–positive, HER2-low metastatic breast cancer [2]. Similarly, sacituzumab govitecan extended median overall survival in the phase 3 TROPiCS-02 trial (14 vs 11 months; HR 0.79; p= 0.02) with corresponding improvements in response rates and patient-reported outcomes [3]. The ASCENT trial further confirmed sacituzumab govitecan’s efficacy in triple-negative breast cancer, delivering durable survival gains with a manageable toxicity profile [4]. Collectively, these findings highlight ADCs as potentially transformative therapies that provide meaningful survival advantages over cytotoxic chemotherapy.
While designed to minimize off-target toxicity, ADCs can still cause significant adverse events (AEs). Major treatment-related AEs associated with ADCs include hematologic, neurologic, and ophthalmologic toxicities, which can be dose-limiting. A recent meta-analysis demonstrated that 91% of patients developed all-grade treatment-related AEs, with 46% experiencing grade ≥ 3 AEs [5]. The most frequent all-grade AEs were lymphopenia (53%), nausea (44%), and neutropenia (44%) [5]. Further exploration of these treatment-related AEs is essential to develop effective mitigation strategies.
Previous studies have indicated that ADCs are generally well-tolerated, with the majority of AEs being mild to moderate [6]. Nonetheless, serious side effects have been documented, resulting in treatment discontinuation or interruption [6]. Moreover, treatment-related deaths have been reported, with pneumonitis and pneumonia identified as the most common causes [5]. As ADCs gain wider use, it is crucial for clinicians to have a comprehensive understanding of their associated toxicities.
ADC toxicity may occur due to limited stability in the systemic circulation, resulting in premature release of payloads at non-target sites [6, 7]. Notably, previous studies estimate that only about 1–2% of the total administered ADC payload is actually delivered to the target tumor cells, while the remaining 98–99% circulates systemically and is ultimately degraded in off-target healthy tissues [8]. This inefficiency arises from multiple factors, including the chemical stability of the linker, the structure of the antibody and the site of payload conjugation [6, 7]. Linker instability can cause early payload release, while tumor-specific challenges, such as high interstitial pressure, poor vascular access and heterogenous antigen expression, further hinder payload delivery [8]. Even when ADC binds to the target antigen, internalization can be inefficient, and normal cells expressing the same or similar antigen may also take up the ADCs, leading to on-target, off-tumor toxicity [9,10,11]. Additionally, ADCs, through their antibody or payload, may elicit a heightened immune response, potentially leading to unintended immune-mediated side effects [8]. Furthermore, hydrophobic linkers coupled to hydrophobic payloads can promote ADC aggregation, which can incite immunogenic AEs [9, 10]. Together, these diverse mechanisms underscore the importance of careful ADC design to improve tumor-specific delivery and reduce systemic toxicity.
A significant proportion of ADC-related side effects are presumed to be caused by the cytotoxic payload [12]. Previous studies linked monomethyl auristatin E (MMAE) payload with peripheral neuropathy, mertansine (DM1) with thrombocytopenia, and monomethyl auristatin F (MMAF) with ocular toxicity [12, 13]. Payload type can predispose to specific toxicities.
Nonetheless, toxicities arising from ADCs are not always predicated on their payload. For example, brentuximab vedotin and tisotumab vedotin share the same payload but have distinct serious AEs, with progressive multifocal leukoencephalopathy for brentuximab and ocular toxicities for tisotumab [7]. This suggests that other ADC components, such as the target, antibody, or linker, may influence AE profiles.
On-target, off-tumor toxicity may arise from the expression of target antigens on healthy tissues [14]. For example, enfortumab vedotin can cause skin rash due to moderate Nectin-4 expression in normal human skin [7]. Additionally, ADCs with the same target antigen but different payloads may have distinct toxicity profiles. Tastuzumab deruxtecan (T-DXd) and trastuzumab emtansine (T-DM1) both target the HER2 protein but have dissimilar payloads, with T-DXd employing a topoisomerase I inhibitor and T-DM1, a tubulin-targeting agent. Patients treated with T-DXd experience more pulmonary toxicities, such as pneumonitis, while those who receive T-DM1 develop more thrombocytopenia and peripheral neuropathy [15]. Thus, the different components predispose to particular ADC-related AEs, requiring further analysis of available data.
This study had two main objectives: first, to benchmark the frequency of side effects associated with various ADCs at our institution to optimize service delivery and support for phase 1 trial patients. This involved characterizing adverse events in patients treated with a range of ADCs at The Royal Marsden Drug Development Unit (DDU) over a 10-year period (January 2014 to January 2024). Due to confidentiality, specific ADC drugs cannot be named. The second aim was to assess ADC-related toxicity rates in publicly available data from pivotal phase 2 and 3 trials that led to FDA approval, analyzing adverse events in relation to ADC components such as target, antibody, linker, payload, and Drug-to-Antibody Ratio (DAR).
We included all patients who received at least one dose of an ADC in a phase 1 clinical trial at the Royal Marsden DDU between January 2014 and January 2024. Patient demographics, tumor type, and treatment toxicities were collected from hospital electronic medical records, with data accuracy verified by two independent observers (HNT and MAM). Toxicities were assessed using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 5.0. Ethical approval was granted by the Committee for Clinical Research of The Institute of Cancer Research and The Royal Marsden Hospital NHS Foundation Trust (SE1324).
Phase 1 data were compared with findings from a review of phase 2 and phase 3 trials involving FDA-approved ADCs. The included studies assessed hematologic and solid malignancies, used ADCs as monotherapy, and reported TRAEs. Trials involving ADCs combined with other anticancer agents or those published only as meeting abstracts were excluded from analysis.
Baseline patient demographics, ADC characteristics, and adverse events were collected from data available up to January 31, 2024. In the phase 2–3 data set, univariate and multivariate logistic regression were performed to evaluate associations between ADC components and TRAEs of special interest, including ophthalmologic, pulmonary, neurologic, and hematologic toxicities. ADC target antigens were categorized as hematological or non-hematological targets, linkers as cleavable or non-cleavable, and payloads as tubulin binding, DNA-damaging, or protein toxin agents.
Odds ratios with 95% confidence intervals were used to measure associations. Variables with a P-value below 0.20 in univariate analysis were included in multivariate analysis, with statistical significance set at a two-sided P-value of less than 0.05. All analyses were conducted using R Statistical Software (v4.4.1; R Core Team, 2024) [16].
A total of 131 patients (81 females, 50 males) from 12 phase 1 ADC trials were analyzed (Table S1). The median age was 63 years, with 62% of participants being female. The most common malignancies were lung cancer (20%), breast cancer (17%), and ovarian cancer (15%). Nearly all patients (94%) had prior chemotherapy, with a median of three lines of treatment.
The phase 2/3 analysis included 2666 patients across 15 ADC trials, with no overlap of investigational agents between the phase 1 and later-phase cohorts (Table S2). Details of each trial are presented in Table S3. The cohort consisted of 67% females, and most studies were phase 2 (87%). Trastuzumab emtansine (18%) accrued the largest proportion of patients.
In the phase 2/3 data set, the most common ADC characteristics included the target, HER2 (32%), peptide-based cleavable linkers (55%), IgG1 antibodies (87%), and tubulin-binding payloads (72%). Most ADCs had a DAR of 2 to 4 (72%), and half of the patients received an ADC dose exceeding 3 mg/kg (50%) (Table S4).
The overall incidence of all-grade TRAEs was 89% in the phase 1 cohort and 93% in phase 2–3 trials (Table 1). Grade 3 or higher AEs occurred in 58% of phase 1 patients and 46% in phase 2–3 trials. In the phase 1 cohort, there were two treatment-related deaths (1.5%), one from capillary leak syndrome and the other due to pneumonitis. In the phase 2/3 cohort, 31 deaths were reported, mainly due to infections (10 cases) and respiratory events (6 cases). Fatigue was more prevalent in phase 1 trials (48%) compared to 30% in phase 2/3 trials, and eye toxicity was also higher in phase 1 (25% vs 10%). However, hematological toxicities were more frequent in phase 2/3 (42% vs 27%). The incidence of gastrointestinal toxicities and peripheral neuropathy was similar between groups.
Ophthalmologic Toxicity
Univariate analysis found that the type of linker was the only factor significantly associated with ocular toxicity (Table 2). Non-cleavable linkers, particularly the maleimidocaproyl linker used in belantamab mafodotin, increased risk, while no ocular events were noted with the non-cleavable thioether linker in T-DM1.
Pulmonary Toxicity
Significant factors for pulmonary toxicity in univariate regression included ADC target, antibody, linker, payload, and DAR (Table 3). Multivariate analysis revealed that IgG1 antibodies, non-cleavable linkers, tubulin-binding payloads, and a DAR greater than 4 were linked to higher risks of respiratory AEs. IgG1 antibodies increased pulmonary AE odds by 107 times, and non-cleavable linkers had 3.4 times higher odds. Specifically, 20% of patients receiving a non-cleavable thioether linker developed pulmonary toxicity. Tubulin-binding payloads reduced lung toxicity risk by 86%, while a DAR > 4 increased odds by 1.8 2.6 times.
Hematologic Toxicity
Univariate analysis identified significant associations between hematologic toxicity and several ADC components, including the target, antibody, linker, payload, and DAR (Table 4). In multivariate analysis, non-cleavable linkers and a DAR greater than 4 remained independently associated with increased hematologic AEs. Non-cleavable linkers raised the odds of myelotoxicity by 3.2-fold, with thioether linkers demonstrating a 52% probability of inducing hematologic adverse events. A DAR greater than 4 was associated with an 18.3-fold increase in the odds of hematologic toxicity.
Univariate analysis showed that the ADC target, linker, and payload were significant factors for neuropathy (Table 5). Multivariate analysis correlated non-hematologic targets and non-cleavable linkers with decreased neuropathy odds by 35% and 85%, respectively. However, ADCs with tubulin-binding agents had a 198-fold increase in the odds of developing neuropathy.
Reviewing data from phase 1 trials in our institution alongside later-phase trials of FDA-licensed ADCs revealed that ADCs pose a considerable risk of toxicity. Specifically, 89% of patients in phase 1 trials and 93% in phase 2/3 trials experienced any-grade TRAEs with ≥ grade 3 toxicities occurring in 58% of phase 1 patients and 46% in later-phase cohorts. These findings align with a previous meta-analysis encompassing 169 studies [5]. Although ADCs have expanded treatment options for multiple tumor types, their associated toxicities remain a significant concern, potentially restricting combination with other therapies such as targeted agents, immunotherapy, or chemotherapy, due to overlapping AEs.
Common treatment-related adverse events (TRAEs) across both cohorts included fatigue, hematologic toxicities, nausea and vomiting, ophthalmologic toxicity, neuropathy, and dermatologic events. While both groups primarily consist of patients with solid tumors (65% in Phase 2/3 and 100% in Phase 1), fatigue and ophthalmologic toxicity were notably more frequent in Phase 1 trials compared to later phases, likely reflecting the higher disease burden and extensive pretreatment histories typical of early-phase study populations. Another contributing factor is that some agents evaluated in Phase 1 did not progress to later development stages due to unfavorable safety profiles, thereby skewing the Phase 1 cohort toward a higher toxicity burden. Furthermore, Phase 1 trials are designed to explore a broad range of dosing levels, often exposing patients to doses above the eventual recommended Phase 2 dose, which increases the risk of severe toxicities. The early-phase setting also frequently involves the first-in-human evaluation of novel, first-in-class agents, where the safety profiles, pharmacodynamics, and mechanisms of off-target toxicity are not yet fully characterized, further elevating the likelihood of serious adverse events. In contrast, Phase 2 and 3 trials tend to enroll patients using refined dosing regimens based on Phase 1 data, which could contribute to lower SAE rates in later phases of development. Collectively, these factors may explain why grade ≥ 3 TRAEs were observed more frequently in the Phase 1 cohort (58%) compared to the Phase 2/3 cohort (46%).
In contrast, hematologic toxicities were more prominently observed in later-phase trials. One plausible explanation is the inclusion of patients with hematologic malignancies in the Phase 2 and 3 data set, inherently predisposing these cohorts to increased rates of myelosuppression and cytopenias. In such populations, baseline marrow function is often compromised either by prior lines of intensive chemotherapy or by the underlying disease itself, rendering patients more vulnerable to hematologic adverse events, even in the absence of direct marrow targeting by the ADC. Moreover, the cumulative marrow toxicity associated with repeated ADC exposure across treatment cycles may become more apparent in later-phase trials, where dosing schedules are standardized and treatment durations are prolonged to optimize efficacy outcomes. By contrast, the Phase 1 cohort analyzed in our study was composed solely of patients with solid tumors, a population in which marrow reserve tends to be relatively preserved at baseline. Additionally, Phase 1 trials often involve shorter treatment exposures due to dose-limiting toxicities, early progression, or trial discontinuation, which may mitigate the cumulative risk of hematologic toxicity. Taken together, these differences in disease biology, baseline patient characteristics, and treatment exposure dynamics likely account for the higher incidence of hematologic adverse events observed in later-phase trials relative to Phase 1 studies.
Moreover, previous reports have highlighted the cumulative nature of ADC-associated hematologic and neurologic toxicities, suggesting that repeated exposure—even at optimized doses—can lead to an incremental rise in adverse events over time [8]. This underscores the critical need to carefully tailor dosing strategies to maximize efficacy while minimizing long-term toxicities.
Our analysis highlighted that the overall AE profile of ADCs aligns with prior reports, with myelotoxicity, nausea, ocular issues, and neuropathy being common [17,18,19]. Grade 5 events were rare, with pulmonary complications being a major cause of ADC-related fatalities in both cohorts, a finding supported by other research indicating respiratory AEs as a leading cause of ADC-related deaths [5, 20].
Non-cleavable linkers were unexpectedly associated with higher odds of ocular toxicity in our analysis. These linkers, by design, confer greater plasma stability and are intended to minimize premature payload release in circulation [21]. However, emerging evidence suggests that the biology may be more complex. The high incidence of ocular events reported in belantamab mafodotin trials likely contributed significantly to this association, underscoring the need for careful interpretation and prospective validation. Belantamab mafodotin employs a non-cleavable maleimidocaproyl linker, which, despite its intended stability, may be prone to a retro-Michael reaction under physiological conditions, leading to premature drug release and unintended off-target effects [22, 23]. This reaction could facilitate local payload exposure in highly perfused, delicate tissues such as the corneal epithelium, where regenerative turnover and vulnerability to microvascular damage are high.
Furthermore, intracellular processing of ADCs plays a critical role in the risk of ocular toxicity. Since non-cleavable linkers require complete lysosomal degradation of the antibody to release the active payload, any inefficiency in this pathway can result in intracellular payload accumulation, particularly in proliferative tissues like the cornea. Intracellularly, lysosomal processing of ADCs, such as Belanatmab mafodotin, liberates an ionized cytotoxic metabolite that cannot easily diffuse across cell membranes, leading to localized entrapment and exacerbated toxicity within corneal epithelial cells [24]. In addition, Fc receptor–mediated internalization of intact ADCs by non-target ocular cells may further concentrate payload exposure in these sensitive tissues [25]. The chemical structure of ADCs, including hydrophobic or positively charged residues, facilitates this internalization process, increasing the likelihood of ocular damage. Belantamab mafodotin’s payload, monomethyl auristatin F (MMAF), is a potent microtubule inhibitor. When locally released in the cornea, MMAF directly damages proliferative epithelial cells, contributing to ocular toxicity [26].
Thus, while non-cleavable linkers offer important pharmacokinetic advantages in terms of systemic stability, their intracellular fate, linker-payload chemistry, and tissue-specific vulnerabilities, particularly within the eye, warrant deeper mechanistic exploration. A clearer understanding of how linker design, payload properties, and local tissue biology interact will be essential for mitigating these off-target toxicities in next-generation ADC development.
To address these challenges, several innovative strategies are being explored to enhance ADC stability and minimize off-target toxicities. One promising approach involves the development of self-hydrolyzing maleimide-based linkers [27]. These linkers are designed to undergo a controlled hydrolysis reaction, which improves the stability of the ADC in circulation while preventing premature payload release. By minimizing this early release, the risk of off-target toxicity, including ocular and other systemic side effects, can be reduced. Additionally, the use of self-hydrolyzing linkers helps to mitigate the risk of retro-Michael reactions, which have been implicated in the unwanted release of the payload in non-target tissues.
Another strategy gaining traction is site-specific conjugation, which allows for precise attachment of the payload to the antibody at a predetermined site, thus ensuring a more uniform distribution and more predictable pharmacokinetic behavior [28,29,30]. Site-specific conjugation not only enhances the stability of the ADC but also minimizes the potential for heterogeneity in the conjugation process, which can lead to unintended toxicities. This approach allows for better control over the DAR, which is critical in reducing off-target toxicities while maintaining therapeutic efficacy. Moreover, the ability to fine-tune the conjugation site and DAR ratio can optimize the balance between stability, potency, and safety, further advancing the development of safer ADCs. Together, these strategies represent promising avenues for improving the therapeutic index of ADCs by enhancing their pharmacokinetics, reducing premature payload release, and minimizing the risk of toxicities associated with conventional linker-payload designs. These advances will be crucial in enabling the next generation of ADCs to achieve their full therapeutic potential while mitigating the risks of serious adverse events.
A review of the Phase 2/3 dataset revealed that tubulin-binding payloads are associated with an elevated risk of neuropathy, which is likely driven by peripheral axonopathy resulting from the release of free payloads. This finding aligns with neuropathy patterns observed with conventional microtubule-targeting agents, such as taxanes and vinca alkaloids, which similarly cause damage to peripheral nerve fibers [7, 31, 32]. Furthermore, preexisting neuropathy from prior treatments, particularly those involving platinum-based or taxane regimens, may exacerbate the risk of developing neuropathy in patients receiving ADCs with tubulin-binding payloads. As a result, there is a growing focus on the development of novel payloads designed to minimize such toxicities. Among these, emerging agents include apoptosis-promoting payloads, immune-agonists, and RNA inhibitors, which are being explored for their potential to induce targeted therapeutic effects while sparing peripheral nerves, thereby reducing the risk of neuropathic side effects [33].
Higher drug-to-antibody ratios (DARs) exceeding 4 were linked to increased pulmonary and hematologic toxicities, a well-established trade-off between enhanced therapeutic efficacy and heightened systemic risks in ADCs. While elevated DAR values improve tumor-targeting potency by delivering a higher payload per antibody, they also amplify the potential for off-target toxicity. This relationship is intricately shaped by how DAR interacts with key ADC components: the stability of the linker, the properties of the payload, and the pharmacokinetic behavior of the conjugate.
The stability of the linker plays a pivotal role in determining how and when the payload is released. As DAR increases, the number of payload molecules attached to each antibody rises, making it even more critical for the linker to maintain its integrity to prevent premature payload release. When linkers are less stable or prone to premature hydrolysis, such as in the case of certain cleavable linkers, higher DAR values may lead to faster dissociation of the payload in circulation. This can result in off-target exposure, especially in tissues like the lungs and bone marrow, where the payload could exert toxic effects before it reaches the tumor. On the other hand, more stable linkers, while preventing early drug dissociation, might delay intracellular release, reducing the therapeutic effect of the ADC in tumor cells and requiring careful optimization of linker stability relative to DAR to balance efficacy and toxicity.
The nature of the payload is equally influenced by DAR. As the number of payloads per antibody increases with higher DAR values, the pharmacokinetic properties of the ADC change, which can amplify toxicity. Hydrophobic payloads, such as MMAF, can accumulate in non-target tissues, leading to dose-limiting toxicities in sensitive organs like the lung, liver, and bone marrow. The increased drug load per antibody can also increase the chances of non-specific uptake by off-target cells, particularly those with high Fc receptor expression, exacerbating off-target toxicities. Additionally, the payload’s intrinsic properties, such as its ability to inhibit tubulin or induce apoptosis, are magnified by the increased DAR, potentially enhancing both efficacy and off-target harm. Hence, optimizing payload characteristics is essential to minimize the adverse effects associated with elevated DAR values.
The interaction between DAR and pharmacokinetics also plays a crucial role in ADC toxicity. With higher DAR values, the ADC tends to have a longer circulation time, which can increase exposure of the conjugate—and its toxic payload—to non-target tissues. This prolonged circulation, coupled with the higher payload load, leads to greater opportunities for off-target binding and toxicity in organs not targeted by the ADC. The altered pharmacokinetics also affect the biodistribution of the ADC, increasing the concentration in tissues such as the lungs, liver, and bone marrow, resulting in increased risk for pulmonary, hepatic and hematologic toxicities.
To optimize ADC safety and efficacy, careful consideration must be given to how DAR interacts with each of these components. Linker stability must be fine-tuned to avoid premature release of the payload, especially as DAR increases. Similarly, payload characteristics, such as hydrophobicity and receptor affinity, should be balanced with DAR to minimize off-target toxicity. Finally, understanding how DAR influences conjugate pharmacokinetics will be essential in minimizing systemic exposure and ensuring efficient tumor delivery. By addressing the complex interplay of these factors, ADC design can be refined to enhance the therapeutic index, maximizing anti-tumor efficacy while minimizing toxicities.
The reliance on aggregate data from later-phase trials limits the analysis by obscuring individual variability and nuances in AE reporting. Additionally, the retrospective nature of data collection may contribute to incomplete or inconsistent reporting, while differences in trial design, patient populations, dosing schedules, and AE grading standards may further impact the findings. Importantly, the inclusion of trials across a wide range of tumor types, therapeutic targets, payloads, and linker technologies introduces substantial heterogeneity, which may confound observed toxicity patterns. Limited sample size and categorical complexity prevented inclusion of interaction terms and detailed sub-analyses by tumor type, as these approaches risk overfitting and unstable estimates given the current dataset’s power and distribution. Future studies leveraging prospective, patient-level data with harmonized reporting frameworks will be critical to address these limitations and refine our understanding of ADC safety profiles.
Despite being designed to minimize toxicity, ADCs were associated with significant AEs, including unexpected toxicities unrelated to payloads, limiting their therapeutic indices. Interestingly, specific ADC components correlated with distinct toxicities in phase 2/3 data analysis. Understanding the contribution of each component of complex molecules, such as ADCs, to toxicity will help inform the design of future ADCs.
Research data supporting the findings in the phase 1 section of this study are confidential and not publicly available due to restrictions imposed by the sponsors of the trials. However, the data from the phase 2 to 3 trials analyzed in this study are publicly accessible through published journal articles referenced in the manuscript.
The abstract of this paper was presented as an online publication at the 2024 Annual Meeting of the American Society of Clinical Oncology (ASCO), Chicago, IL, 31 May – 4 June, 2024.
The authors acknowledge infrastructural funding from the Experimental Cancer Medicine Centre and National Institute of Health and Care Research Biomedical Research Centre initiatives to the Institute of Cancer Research and The Royal Marsden Foundation Trust. The authors also acknowledge the funding provided by Cancer Research UK Convergence Science Centre to The Institute of Cancer Research and Imperial College of London.
Ethical approval to collect, anonymize, and tabulate toxicities caused by ADCs on phase I trials was approved by the Committee for Clinical Research of The Institute of Cancer Research and The Royal Marsden Hospital NHS Foundation trust; SE1324. The study was performed in accordance with the Declaration of Helsinki.
HNT, MAM, GSF, BR have no conflicts of interest to declare. UB has attended advisory boards and been compensated by Ellipsis Pharma, PharmEnable, and Carrick Therapeutics. He has received preclinical research funding from Verastem and Avacta and research funding to run academic trials from Verastem, Chugai, BTG phamaceuticals and Carrick Therapeutics. JL has served as consultant or an advisory role for Genmab, Novartis, Basilea, Roche-Genentech, CureTeq, Ellipses Pharma, Pierre Fabre and GSK. She has received grant/research funding from Astex Pharmaceuticals, Basilea, and Roche-Genentech. She has received travel support from Basilea and Roche-Genentech. A.M. has served on advisory boards for Janssen, Merck, Takeda, MSD, Genmab, Merck, Pfizer, Astrazaneca and Immutep. She has received honoraria from Chugai, Faron, Merck, GSK, Seagen, Takeda and Janssen. A.M has received travel support from Amgen and Janssen. She has received research funding from Astex, Merck and MSD. A. S. is an employee of the ICR, which has a commercial interest in abiraterone, PARP inhibition in DNA repair defective cancers, and PI3K/AKT pathway inhibitors (no personal income). A.S. has received travel support from Sanofi, Roche-Genentech and Nurix, and speaker honoraria from Astellas Pharma and Merck Sharp & Dohme. He has served as an advisor to DE Shaw Research, CHARM Therapeutics, Ellipses Pharma and Droia Ventures. A. S. has been the CI/PI of industry-sponsored clinical trials. A.P. is named on a patent held by the Institute of Cancer Research (ICR) together with Cancer Research UK identifying JMJD6 as a therapeutic target in advanced prostate cancer.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Tan, H.N., Morcillo, M.A., Lopez, J. et al. Treatment-related adverse events of antibody drug-conjugates in clinical trials. J Hematol Oncol 18, 71 (2025). https://doi.org/10.1186/s13045-025-01720-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13045-025-01720-3






