The Licensed Avian Influenza Vaccines: The Informed Consent Form Models - Part 3
We come to the informed consent forms, all 20 of them, which were provided to us under the FOIA.
According to our AI tool, Matt, the primary purpose of informed consent is to ensure that individuals understand the risks, benefits, and potential consequences of participating in a medical procedure or research study before making a decision. It protects their autonomy, promotes transparency, and encourages informed decision-making.
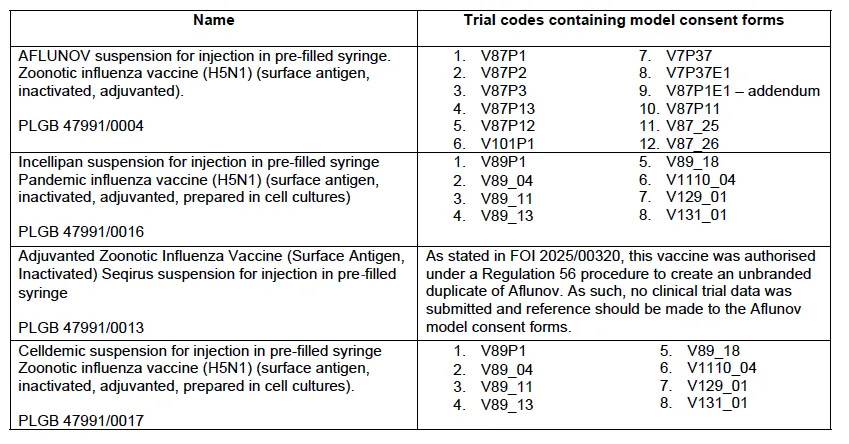
The concept of informed consent dates back to the Nuremberg Trials and is enshrined in the Declaration of Helsinki. As the form's content is fairly repetitive, we thought we’d summarise what we found in a Table. Remember the very helpful synopsis provided by our secret squirrel enablers:
So, each of these model forms, templates used in the trials, served as building blocks for licensing the three vaccines and for the Adjuvanted Zoonotic Influenza Vaccine reference. This is a duplicate, or perhaps not (Matt is still working on it), of Aflunov, so Adjuvanted Zoonotic Influenza Vaccine does not require registration trials.
Take a look at our Introduction for an explanation of the context.
All clear? Let’s go.