BMC Nutrition volume 11, Article number: 117 (2025) Cite this article
Vitamin D deficiency has become a common problem globally. The present study aimed to evaluate the effects of different vitamin D3 regimens on maternal concentrations of vitamin D metabolites during pregnancy. Subjects were ≤ 14 weeks gestation pregnant women with 25(OH)D serum level < 30 ng/mL]. Two intervention groups were randomly assigned: 5,000 IU of vitamin D3 daily or 50,000 IU weekly of vitamin D3. Maternal blood samples were collected before and after four weeks of intervention to assess changes in serum concentrations of 25-hydroxyvitamin D (25(OH)D), 1,25-dihydroxyvitamin D (1,25(OH)2D), vitamin D binding protein (VDBP), and 24,25-dihydroxyvitamin D (24,25(OH)2D). Sixty subjects were randomized into two groups, and eight subjects were dropped out. There were no differences in the baseline demographics or baseline levels of any of the vitamin D metabolites between the two groups. In the 50,000 group, the 25(OH)D levels increased from 15.3 ± 4.7 ng/mL to 26.9 ± 6.1 ng/mL (p < 0.001) and 34.6% of the subjects achieved vitamin D sufficiency. While in the 5,000 group, the 25(OH)D levels increased from 14.5 ± 4.3 ng/mL to 27.9 ± 9.3 ng/mL (p < 0.001) and 23.1% of the subjects achieved vitamin D sufficiency. Both groups showed an increasing trend in the total levels of 25(OH)D, 1,25(OH)2D, VDBP, and 24,25(OH)2D. However, the increment of all vitamin D metabolites were not significantly different between two groups. Vitamin D3 50,000 IU weekly is equally effective and safe as 5,000 IU daily in increasing vitamin D levels in pregnant women with vitamin D deficient or insufficient. This randomized controlled trial was retrospectively registered at ClinicalTrials.gov (NCT06054919) on 22nd September 2023.
Vitamin D deficiency has become a common problem globally and is associated with adverse maternal and neonatal health outcomes. It has been estimated that 18–84% of pregnant women worldwide are vitamin D deficient [1]. Despite being located in a warm climate region, Indonesia has a high prevalence of vitamin D deficiency. Several reasons pregnant women in Indonesia experience deficiencies, namely the habit of most women in Indonesia wearing closed clothing such as the hijab, and concerns about sun exposure so they use sunblock and umbrellas to avoid the sun. A study conducted in Jakarta revealed that 99.6% of first-trimester pregnant women had vitamin D (25(OH)D) serum level < 30 ng/mL [2].
Vitamin D and its metabolites are hydrophobic, and > 99% are transported in the blood bound to VDBP. In the liver, vitamin D3 is converted into 25(OH)D, which is the primary vitamin D compound in the circulation. In the renal proximal tubules of the kidney, 1α-hydroxylase hydroxylates 25(OH)D to yield 1,25(OH)2D. This kidney-generated 1,25(OH)2D plays a crucial role in mediating the classical functions of vitamin D in calcium homeostasis and bone mineralization. The production of 1,25(OH)2D from 25(OH)D occurs in immune cells, the skin, the placenta, and other tissues and may contribute to health in nonpregnant and pregnant women. Both 1,25(OH)2D and 25(OH)D are inactivated by CYP24A1, to 1,24,25-trihydroxyvitamin D and 24,25-dihydroxyvitamin D [3, 4].
Adequate vitamin D intake is essential for maternal and fetal health during pregnancy. Recent work has emphasized the importance of vitamin D in pregnancy and the placenta. The human placenta expresses all the components required for vitamin D signaling. Vitamin D regulates key target genes associated with implantation, trophoblast invasion, and implantation tolerance. Vitamin D also plays an important role in promoting the shift to a T-helper 2-dominated immune response pattern, which is vital for successful pregnancy outcomes. Vitamin D has immune-modulatory and anti-inflammatory effects that are important for preventing microbial invasion intrauterine. Maternal vitamin D deficiency has been associated with adverse pregnancy outcomes such as preterm labor, preeclampsia, gestational diabetes mellitus, and fetal growth restriction [5,6,7,8].
According to the 2010 Institute of Medicine Report, the recommended daily allowance of vitamin D during pregnancy is 600 IU. However, this amount may not be enough to maintain sufficient vitamin D in pregnant women [9, 10]. Recent studies have shown that pregnant women should take 4,000 IU/day of vitamin D3 starting from 12 to 16 weeks of gestation to achieve sufficient vitamin D levels. The Endocrine Society Clinical Practice Guideline recommends treating all vitamin D deficient adults with 50,000 IU of vitamin D3 once a week for 8 weeks, or an equivalent daily dose of 6,000 IU of vitamin D3, to achieve a blood level of 25(OH)D above 30 ng/mL [11, 12].
However, there are no available data regarding vitamin D therapy in pregnant women in Indonesia, which vitamin D deficient is prevalent. The present study aimed to evaluate the effects of different vitamin D3 regimens on maternal 25(OH)D, 1,25(OH)2D, VDBP, and 24,25(OH)2D concentrations during pregnancy.
We conducted a randomized controlled trial of vitamin D therapy of 5,000 IU daily or 50,000 IU weekly during pregnancy. The study was conducted at Cipto Mangunkusumo National Center General Hospital and Koja District Hospital in Jakarta, Indonesia, within a period of 1 year. All the pregnant women were screened for eligibility when they presented to the clinic for antenatal care visits and were offered enrolment if they met the following inclusion criteria: gestational age of ≤ 14 weeks, serum 25(OH)D level < 30 ng/mL], and a positive fetal heart rate from ultrasound examination. The exclusion criteria were: multiple pregnancy, pregnancy with congenital anomaly, hyperemesis gravidarum, diarrhea, complicated medical history (hypertension, diabetes mellitus, heart, kidney, or liver disease), or use of any dietary supplement containing vitamin D prior to enrolment. Informed consent was obtained from all participants. This study was approved by the Ethical Committee for Research in Humans from the Faculty of Medicine University of Indonesia (257/UN.2F1/ETIK/PPM.00.02/2023) and was retrospectively registered at ClinicalTrials.gov (NCT06054919) on September 22, 2023. This study also adhered to CONSORT guidelines.
Participants were randomly assigned to one of two parallel intervention groups. The allocation was concealed based on a computer-generated list with a 1:1 allocation ratio. The groups received either vitamin D3 (Cholecalciferol) 5,000 IU (Imedco) daily or 50,000 IU (Imedco) weekly. Vitamin D3 was prepared and labeled by the pharmacy specifically for this study.
The randomization list was developed using permuted blocks of size 6. All participants were provided with a standard prenatal multivitamin (Emineton) that contained fumarate Fe 90 mg, folic acid 0.4 mg, vitamin B6 3 mg, vitamin B12 5 mcg, sulphate cupric 0.35 mg, sulphate cobalt 0.15 mg, sulphate mangan 5 mcg, vitamin C 60 mg, vitamin E 5 mg, and phosphate calcium 60 mg. Both groups received the intervention for four weeks.
At the beginning of the study, participants underwent baseline blood tests to measure serum concentrations of 25(OH)D, 1,25(OH)2D, VDBP, and 24,25(OH)2D. A medical history, physical, and ultrasound examination were also performed. Additionally, they were asked to complete a questionnaire about sunlight exposure. The sun exposure score was calculated using a validated questionnaire by Hanwell (2010) [13].
Throughout the study, weekly follow-up was conducted to evaluate their adherence to the supplement regimen and to monitor any symptoms related to interventions. Maternal self-report and capsule counts were used to assess adherence.
After four weeks of interventions, maternal venous blood was collected to assess changes in serum concentrations of 25(OH)D, 1,25(OH)2D, VDBP, and 24,25(OH)2D. The study had safety thresholds in place to prevent hypervitaminosis D, which was defined as a serum concentration of 25(OH)D ≥ 100 ng/mL.
The concentrations of different forms of vitamin D in the blood were measured using various tests. Serum 25(OH)D was quantified by direct competitive Chemiluminescence Immunoassay (CLIA) using LIAISON®. Deficiency was defined < 20 ng/mL, insufficiency as 20–29 ng/mL, and sufficiency ≥ 30 ng/mL. Serum 1,25(OH)2D was quantified by Liquid Chromatography-tandem Mass Spectrometry (LC-MS/MS) using ImmuTube®. The reference range for 1,25(OH)2D is 18–72 pg/mL. Serum VDBP was quantified by Enzyme-Linked Immunosorbent Assay (ELISA) using Quantikine®. The reference range for VDBP is 168–367 mcg/mL. Serum 24,25(OH)2D was quantified by Acquity I Class Binary Solvent Manager FTN, Xevo TQXS Tandem Mass Spectrometry (Waters Corporation). There was no established reference value for serum 24,25(OH)2D. All of these laboratory tests were conducted at the Prodia Laboratory in Jakarta.
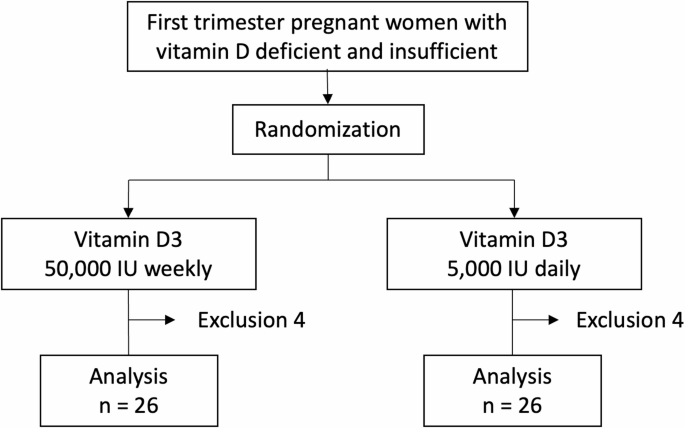
Sixty pregnant women who were eligible to participate were randomly assigned to two groups as shown in Fig. 1. However, after allocation, 5 subjects discontinued participation for any specific reason, and 3 subjects dropped out due to COVID-19 infection. As a result, 52 patients completed the study and were available for analysis. Of these 52 participants, 26 were in the 5,000 group and the other 26 were in the 50,000 group.
The median maternal age and gestational age of the study participants were 30 years and 8 weeks, respectively. The distribution of demographics was equal between the control and treatment groups. Approximately half of the study participants (55.8%) were obese, and 35 (67.3%) were multiparous. All of the subjects had low sun exposure scores (< 33.6). Table 1 shows that demographic data were equally distributed between the two groups.
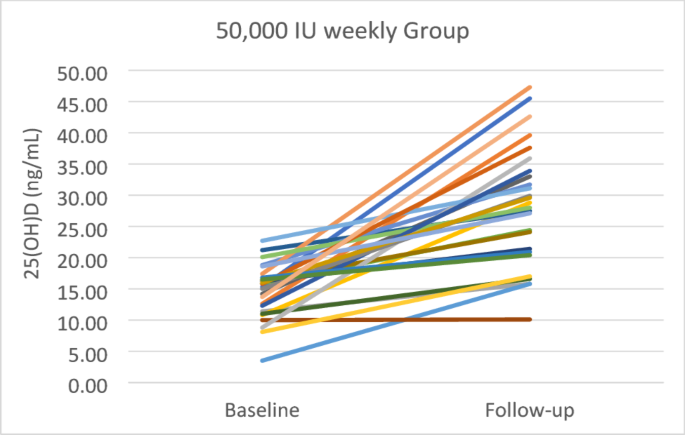
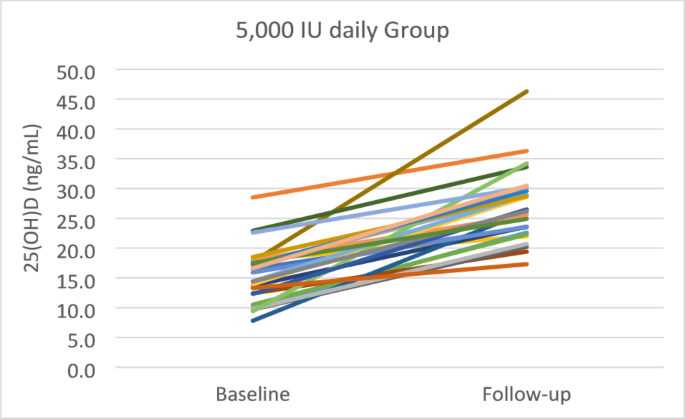
Table 2 demonstrates that the baseline levels of 25(OH)D, 1,25(OH)2D, VDBP, and 24,25(OH)2D were equal between 50,000 and 5,000 groups. The baseline 25(OH)D levels were 15.3 ± 4.7 ng/mL and 14.5 ± 4.3 ng/mL in the 50,000 and 5,000 groups, respectively. After four weeks of treatment, the levels of 25(OH)D were significantly improved in both groups, but the increment was not statistically significant (p = 0.649) between two groups. In the 50,000 group, 25(OH)D levels increased from 15.3 ± 4.7 ng/mL to 26.9 ± 6.1 ng/mL (p < 0.001) and 34.6% of the subjects achieved vitamin D sufficiency (> 30 ng/mL). While in the 5,000 group, the 25(OH)D levels increased from 14.5 ± 4.3 ng/mL to 27.9 ± 9.3 ng/mL (p < 0.001) and 23.1% of the subjects achieved vitamin D sufficiency. The highest 25(OH)D levels recorded were 46.3 ng/mL in the group receiving 50,000 IU and 47.3 ng/mL in the group receiving 5,000 IU. Figures 2 and 3 illustrate the individual changes in 25(OH)D levels in groups of 50,000 and 5,000, respectively.
Both groups showed an increasing trend in the total levels of 1,25(OH)2D, VDBP, and 24,25(OH)2D. However, we only found a statistically significant difference in the levels of 1,25(OH)2D between baseline and follow-up in the 5,000 group (p = 0.042). Additionally, we found a statistically significant difference in the follow-up levels of VDBP between the 50,000 and 5,000 groups (p = 0.013). There was no statistically significant difference in the increment of 1,25(OH)2D, VDBP, or 24,25(OH)2D between the two groups.
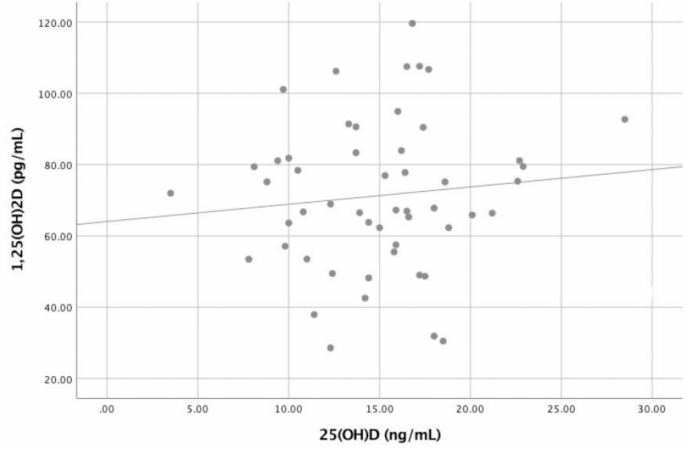
Pearson correlation analysis revealed no correlation between baseline levels of 25(OH)D and 1,25(OH)2D (r = 0.105, p = 0.458) as can be seen in Fig. 4.
To the best of our knowledge, this is the first study to explore the impact of high-dose oral vitamin D on serum vitamin D metabolites among first trimester pregnant women in Indonesia. Among the subjects we studied, 55.8% were obese with a median early pregnancy BMI of 26.23 kg/m2. Previous research conducted in Malaysia (Palaniveloo, et al., 2020) revealed that early pregnancy BMI (OR = 2.95, 95% CI = 1.03–8.47) was significantly associated with vitamin D deficiency [14]. BMI can affect vitamin D metabolism because vitamin D is a fat-soluble vitamin that can be sequestered in adipose tissue, leading to lower bioavailability in the obese state. Alternatively, obesity may suppress the hepatic enzyme 25-hydroxylation of vitamin D to 25(OH)D, thereby reducing bioactivity [15].
The daily time spent on the sun and skin exposure over one week was assessed. Previous research in Minangkabau, Indonesia, showed that pregnant women who spent less than one hour outdoors had a tenfold increase in the risk of developing vitamin D deficiency (OR 9.659, CI 95% 1.883–49.550; p-value = 0.007) [16]. According to a study conducted in Jakarta, Indonesia, our findings are consistent with the fact that 99.6% of first-trimester pregnant women in Jakarta suffer from vitamin D deficiency and insufficiency [2]. The primary source of vitamin D is sunlight exposure, particularly UVB light, which accounts for 90% of the body’s vitamin D requirements. Consequently, inadequate sunlight exposure is the leading cause of vitamin D deficiency.
This study revealed that the administration of 50,000 IU/week of vitamin D3 for 4 weeks significantly increased 25(OH)D serum levels. These results are consistent with a randomized controlled study conducted by Bimson, et al., which demonstrated that giving vitamin D3 50,000 IU/week to treat vitamin D deficiency in pregnant women for 8 weeks significantly increased 25(OH)D levels. However, the 25(OH)D increment in that study was much greater than that in the present study [17]. Additionally, in our study, only 34.6% of the subjects achieved vitamin D sufficiency. Bimson, et al. reported that 84.4% of subjects achieved vitamin D sufficiency [17]. This difference might be attributed to the shorter duration of intervention in our study. A longer duration of study might be needed to evaluate the optimal duration of vitamin D3 therapy.
Our study also found that taking a daily dose of 5,000 IU of vitamin D3 for 4 weeks significantly increased 25(OH)D levels. However, only 23.1% of the subjects were able to achieve normal levels of 25(OH)D. A study conducted by Bokharee, et al. on pregnant women in Pakistan demonstrated that administering 5,000 IU/day of vitamin D increased 25(OH)D levels by 23.14 ± 11.18 ng/mL in 8 weeks [18]. Another study by Yap, et al. found that 90% of pregnant women who took a vitamin D dose of 5,000 IU/day for 12 weeks achieved a sufficient vitamin D concentration of 32 ng/mL [19]. As a result, a longer intervention duration of high-dose vitamin D is needed for further studies to determine the optimal vitamin D dose and duration for pregnant women.
The mean baseline 25(OH)D levels were equivalent between the 5,000 and 50,000 groups. After receiving intervention for 4 weeks, there was a significant increase in 25(OH)D levels in both groups. However, there was no significant difference in delta 25(OH)D levels between the two groups. These findings are consistent with previous research indicating that a weekly dose of vitamin D2 or D3 at 50,000 IU for 8 weeks is equivalent to a daily dose of vitamin D2 or D3 at 6,000 IU in achieving serum 25(OH)D levels of more than 30 ng/mL in adults with vitamin D deficiency [12]. Sophia, et al. showed there were no significant differences in serum 25(OH)D with vitamin D3 supplementation 1,500 IU daily, 10,500 IU once weekly, and 45,000 IU once every 28 days for 2 months [20].
Although 25(OH)D is not the biologically active form of vitamin D, 25(OH)D is the major circulating form of vitamin D with a half-life of approximately 2–3 weeks [12]. A pharmacokinetic study showed that the level of 25(OH)D continues to rise through day 14 and by day 28 after administering a single dose of 50,000 IU of vitamin D3 [21]. This finding indicates the potential of intermittent high doses of vitamin D therapy, as shown in this study. Weekly vitamin D therapy potentially improves patient compliance with long-term therapy.
After 4 weeks of intervention, the 5,000 group showed a significant increase in 1,25(OH)2D levels, while the 50,000 group showed no significant change. However, in terms of increment 1,25(OH)2D, there was no significant difference between the two groups. This finding is consistent with previous studies showing that vitamin D supplementation was effective at increasing 25(OH)D levels but did not alter 1,25(OH)2D levels [22, 23]. In addition, this study showed no correlation between 25(OH)D and 1,25(OH)2D levels. This finding is consistent with previous studies [24, 25]. The concentration of 1,25(OH)2D in the bloodstream is tightly regulated by various factors, including parathyroid hormone, calcium, phosphate, and the growth factor FGF23 [26]. However, metabolic changes in vitamin D during pregnancy remain unexplained. Large amounts of 1,25(OH)2D begin to be produced and released into the maternal circulation as soon as placental implantation occurs. During pregnancy, production of large amounts of 1,25(OH)2D may occur in the maternal kidneys, fetal kidneys, or placenta [27].
This study found that the administration of vitamin D3 for 4 weeks increased VDBP levels in both groups, with the follow-up level being greater in the 5,000 group than in the 50,000 group. Total VDBP levels increase during pregnancy, which indicates an increase in the amount of 1,25(OH)2D bound to VDBP to prevent pregnant women from developing hypercalcemia. The higher follow-up level found in the 5,000 group might reflect the significant increase in the 1,25(OH)2D level that was found only in the 5,000 group. However, using linear regression, no relationship was observed between circulating VDBP and 1,25(OH)2D. This finding is consistent with a previous study showing that the level of DBP is not influenced by serum vitamin D level, even if it is deficient [28].
At the end of our study, no signs or symptoms of vitamin D toxicity, such as anorexia, diarrhea, constipation, nausea, or vomiting, were observed. None of the participants had hypervitaminosis D (≥ 100 ng/mL) after interventions. Additionally, no remarkable allergic reactions or side effects were observed in either group. These findings are consistent with previous research by Bimson, et al., who reported that administering vitamin D3 50,000 IU/week for 8 weeks to treat vitamin D deficiency during pregnancy is both effective and safe [17]. Therefore, this research supports that vitamin D3 doses of 5,000 IU/day and 50,000 IU/week given for 4 weeks can be considered safe and tolerable for pregnant women in the first trimester with vitamin D deficiency and insufficiency.
This study is the first to explore the impact different dose of vitamin D therapy on serum vitamin D metabolites among pregnant women in Indonesia. The findings of the current study may provide the basis for formulating guidelines and recommendations for vitamin D therapy among pregnant women in Indonesia. This study has also certain limitations. First, we did not assess the nutritional intake of each subject which is a confounding factor in this study. Additionally, we did not measure the levels of PTH, calcium, or phosphate, which affect the concentration of 1,25(OH)2D in the bloodstream. Furthermore, the duration of the intervention was not long enough to determine the optimal duration of therapy.
Vitamin D3 50,000 IU weekly is equally effective and safe as 5,000 IU daily in increasing 25(OH)D serum levels in pregnant women with vitamin D deficient or insufficient. Future research with larger samples and longer duration is needed to determine the optimal duration of the therapy required to achieve vitamin D sufficiency.
Data is provided within the manuscript.
- 1,25(OH)>2 D:
-
1,25-dihydroxyvitamin D
- 24,25(OH)2D:
-
24,25-dihydroxyvitamin D
- 25(OH)D:
-
25-hydroxyvitamin D
- LC-MS/MS:
-
Liquid Chromatography-tandem Mass Spectrometry
- ELISA:
-
Enzyme-Linked Immunosorbent Assay
- VDBP:
-
Vitamin D binding protein
The authors would like to express gratitude to all participants and all the medical staff in the Department of Obstetrics and Gynecology, Faculty of Medicine, Universitas Indonesia/Cipto Mangunkusumo National General Hospital.
This research was funded by a 2020 Ministry of Research, Technology and Higher Education of Republic of Indonesia Grant.
The study was conducted according to the guidelines of the Declaration of Helsinki, and was approved by the Ethical Committee for Research in Humans from the Faculty of Medicine, Universitas Indonesia ((257/UN.2F1/ETIK/PPM.00.02/2023), and was retrospectively registered at ClinicalTrials.gov (NCT06054919) on 22nd September 2023.
Not Applicable.
Informed consent was obtained from all subjects involved in the study.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Syafitri, I., Irwinda, R., Saroyo, Y.B. et al. Maternal concentrations of vitamin D metabolites in response to high-dose oral vitamin D during first trimester pregnancy: a randomized controlled trial. BMC Nutr 11, 117 (2025). https://doi.org/10.1186/s40795-025-01104-3