BMC Women's Health volume 25, Article number: 307 (2025) Cite this article
Obesity has been linked to chronic inflammation and insulin resistance and oxidative stress, which are all associated with the pathogenesis of endometriosis (EMs). This study aimed to investigate the associations between the emerging metabolic markers-lipid accumulation product (LAP) and visceral adiposity index (VAI)-with the EMs.
This study utilized data from the National Health and Nutrition Examination Surveys (NHANES) 1999–2006. Weighted univariate and multivariate logistics regression models were conducted to explore the associations between LAP and VAI with EMs, with the results expressed as odds ratios (ORs) and 95% confidence intervals (CIs). Moreover, the subgroup analyses based on age, the history of pregnancy, chronic kidney disease (CKD), and female hormones use were further performed to verify whether theses associations remain robust.
Totally 5,188 eligible women were included, with a mean age of 37.19 ± 0.18 years. Among them, 359 (6.92%) had EMs. After adjusted all covariates, we observed both highest LAP and VAI are associated with EMs (LAP: OR = 1.56, 95%CI: 1.02–2.39; VAI: OR = 1.54, 95%CI: 1.09–2.18). Subgroup analyses shown that the associations between LAP and VAI with EMs were more significant among women aged ≥ 35 years, with the female hormones use, with the history of pregnancy, and without the history of CKD.
We observed both high LAP and VAI were associated with EMs among reproductive women in the United States. However, further large-scale, well-designed, prospective cohort studies still need in the future to confirm these findings.
Endometriosis (EMs) is a chronic gynecological disorder characterized by multiple clinical manifestations, including severe dysmenorrhea, dyspareunia, difficulty in defecation or urination, and long-term chronic pelvic pain [1, 2]. Globally, this condition affects approximately 10% (~ 190 million) of reproductive-aged women [3, 4]. Accumulating epidemiological evidence has established significant associations between EMs and various health complications, including infertility [5], cancer [6], autoimmune disease [7] and cardiovascular disease) (CVD) [8]. The current clinical diagnosis of EMs predominantly relies on invasive procedures, contributing to significant diagnostic delays. Notably, approximately 60% of affected women experience a mean diagnostic latency of 7 years following symptom onset [9], resulting in delayed therapeutic interventions for most patients. The World Health Organization recognizes the important impact of EMs on human reproductive health and quality of life [10]. Therefore, exploring potential associations between easily accessible clinical indicators and Ems is of great significance for the early diagnosis and treatment of EMs.
Emerging evidence suggests a complex, bidirectional relationship between obesity and metabolic disorders, with dysregulated lipid metabolism frequently coexisting with persistent inflammation [11]. Inflammation represents a physiological defense mechanism mediated by the immune system in response to various stimuli. The inflammatory response can lead to a temporary decline in tissue function, which is also one of the mechanisms of many diseases [12]. Chronic inflammation may affect estrogen regulation and endometrial receptivity in women [13], and a study reported that approximately two-third of embryo implantation failures are related to insufficient endometrial receptivity [14]. In other hand, obesity also leads to insulin resistance and oxidative stress, which may jointly affect endometrial stromal cells [15]. All these physiological mechanisms were associated with the occurrence and development of EMs. Current epidemiological evidence regarding the obesity-EMs remains inconsistent. Notably, most studies have reported inverse correlations between conventional anthropometric indicators [body mass index (BMI) and waist circumference (WC)] and EMs. Multiple large-scale studies, including meta-analyses [16] and population-based cohorts [17, 18], have consistently reported inverse associations between conventional adiposity measures (BMI/WC) and EMs. This apparent “obesity paradox” in endometriosis poses challenges for establishing clear clinical guidelines for reproductive endocrine health management. Furthermore, both BMI and WC, as traditional obesity indicators, present inherent methodological limitations that may contribution to these contradictory observations. BMI only represents over obesity it fails to differentiate between body composition components [19]. Similarly, WC provides limited information about visceral adipose tissue deposition [20]. These inherent limitations may obscure the true direction of the association between obesity and EMs. To address these shortcomings, Kahn et al. [21] proposed the concept of lipid accumulation product (LAP), which combines the anatomical index WC reflecting excessive body fat accumulation and the physiological index triglyceride (TG), and then transforms it into a continuous variable according to gender. Notably, LAP demonstrates superior performance to BMI in predicting diabetes and CVD risk, while more accurately reflecting visceral lipid accumulation. Another innovative adiposity indicator, the visceral adiposity index (VAI), specifically captures both the distribution and metabolic activity of visceral fat [22]. Previous studies have verified the potential predictive ability of LAP and VAI in other metabolic diseases including CVD, type 2 diabetes, insulin resistance, and metabolic-associated fatty liver disease [23,24,25]. However, the potential associations between these metabolic indices and Ems remain largely unexplored.
Building upon this scientific foundation, we hypothesized that LAP and VAI would associate with EMs. Utilizing data from the National Health and Nutrition Examination Survey (NHANES), this study investigated the associations between LAP/VAI and EMs. Our study aims to identify readily available clinical biomarkers to enable earlier risk stratification and more effective management of high-risk populations for EMs.
Data of this cross-sectional study were extracted from the NHANES database 1999–2006 (only 1999–2006 contained information on EMs assessments). The NHANES is a large-scale, long-running epidemiological study designed to assess the health and nutritional status of the residents of the United States. It collects a wealth of detailed information on demographic characteristics, lifestyle, health status and nutritional intake. This research received approval from the National Center for Health Statistics Research Ethics Review Board, and all subjects signed informed consent forms. The detailed NHANES study design and data are available at (https://www.cdc.gov/nchs/nhanes/).
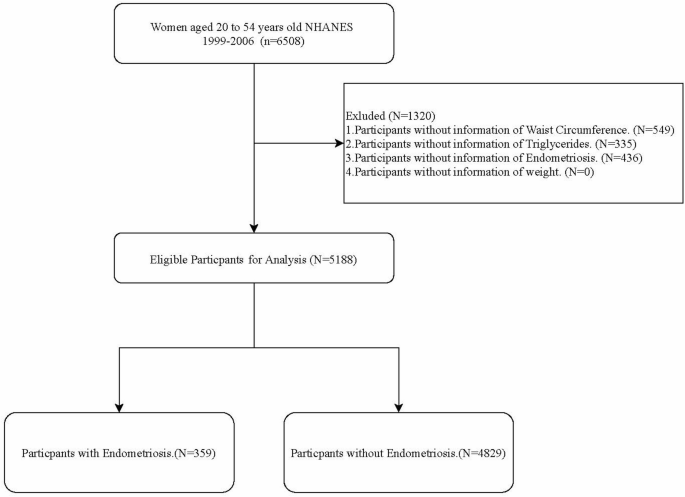
In this cross-sectional study, we explored the associations between two metabolism-related indictors LAP and VAI with EMs. Initially, 6,508 women aged 20–54 years old were extracted from the NHANES 1999–2006. Among them, 549 women without the measurement information of WC, 335 women without the measurement information of TG and 436 women without the assessment information of EMs were excluded. Finally, 5,188 eligible women were included for further analysis. The participant selection process was detailed in Fig. 1.
A questionnaire survey in NHANES was utilized to diagnose the EMs. The NHANES questionnaire was administered by experienced clinicians or trained professionals. Participants who answered “yes” to the question “Has a doctor or other health professional ever told you that you had endometriosis (age at interview 20–54 years) were considered to have EMs, otherwise they were considered to have non-EMs (https://wwwn.cdc.gov/Nchs/Nhanes/Search/default.aspx).
The computational equations of LAP and VAI were based on the study of Kahn [21] and Amato et al. [26], respectively:
LAP = [WC (cm) − 58] × [TG (mmol/L)]
VAI = [WC (cm)/36.58 + 1.89 × (BMI)] × (TG (mmol/L)/0.81) × [1.52/HDL (mmol/L)]
Note, WC, waist circumference; TG, triglycerides; BMI, body mass index; HDL, high density lipoprotein.
Covariates were adjusted to reduce deviation, which included demographic information [age, race, poverty to income ratio (PIR) and education level], lifestyle (drinking, smoking status and physical activity), physical examination [WC, height, weight, BMI, systolic blood pressure (SBP), and diastolic blood pressure (DBP)], reproductive information (pregnancy history, age at menarche, pregnancy, hysterectomy), treatment information (female hormones use and steroids drug), complications [diabetes, dyslipidemia, hypertension, CVD [5] and chronic kidney disease (CKD)], and laboratory values [total cholesterol (TC), glycated hemoglobin (HbA1c), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C) and TG]. In the NHANES study, the blood samples are collected by trained phlebotomists for fasting venous blood collection at Mobile Examination Centers (MEC), then transported cold-chain to the National Center for Environmental Health (NCEH) laboratory and processed with 24 h. All laboratory values are collected and processed following the Clinical Laboratory Improvement Amendments (CLIA) standardized protocols.
PIR was calculated as the ratio of family income to the federal poverty threshold, adjusted for family size and survey year. A PIR ≤ 1 indicates poverty status, while higher values reflect greater economic security [27]. Diabetes was defined as HbAlc ≥ 6.5%, fasting glucose ≥ 126 mg/dL, 2 h oral glucose tolerance test (OGTT) ≥ 200 mg/dL, taking hypoglycemic agent or diagnosing as diabetes by clinicians. Hypertension was defined as SBP ≥ 130 mmHg and/or DBP ≥ 80 mmHg, taking antihypertensive drugs or diagnosing as hypertension by doctors. Dyslipidemia was defined as TC ≥ 200 mg/dL (5.2 mmol/L) or TG ≥ 150 mg/dL (1.7 mmol/L) or LDL-C ≥ 130 mg/dL (3.4 mmol/L) or HDL-C ≤ 40 mg/dL (1.0 mmol/L), or self-reported hypercholesterolemia or current treat of cholesterol-lowering drugs/lipid-lowering drugs. CVD was defined by question “Ever told you had angina/heart attack/coronary heart disease/stroke/heart failure?”. CKD was defined as estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 and eGFR was determined by the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation [28].
Smoking status was divided into former smoker (more than 100 cigarettes in lifetime and quit smoking now), current smoker (more than 100 cigarettes in lifetime and was still smoking at the time of the survey), and never smoker (less than 100 cigarettes in lifetime). Drinking status was assessed by “How often drink alcohol over past 12 months?” and divided into never, light, moderate and heavy according to 0, < 1, 1–8 and ≥ 8, respectively. Physical activity was expressed as the metabolic equivalent task (MET) and calculated as follows: physical activity (met·min/week) = recommended MET × exercise time for corresponding activities (min/day) × the number of exercise days per week (day) [29]. Pregnancy history was assessed by “Ever been pregnant?” (yes/no). Age at menarche refers to the first menstrual period and assessed by “Age range at first menstrual period?” Pregnancy was assessed “Think that you are pregnant now?” (yes/no). Women who answered “yes” to “Had a hysterectomy?” were considered as having hysterectomy history.
Both LAP and VAI were categorized by quintiles. The measurement data were described as mean and standard error [mean (± SE)], whereas categorical data were reported as number and percentage [n (%)]. Weighted t-tests or weighted chi square test were utilized to compare differences between EMs and non-EMs groups. Missing data were filled using multiple imputation, and sensitivity analysis before and after imputation was performed (Table S1). All potential covariates were included into weighted univariate logistics regression model analysis, and those statistically associated with EMs were identified (all P < 0.05). Then, the selected covariates (age, race, PIR, education, female hormones, steroids drug, hypertension, dyslipidemia and CVD) were included into the weight multivariate logistics regression models to further explore the association between LAP and VAI with EMs, with odds ratio (OR) and 95% confidence interval (CI) (Table S2). Subgroup analyses based on age, pregnancy history, use of female hormones and the CKD history were performed to explore whether the association between LAP and VAI with EMs remain robust.
All data were analyzed via SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Two-sided P < 0.05 was considered as statistically significant.
Finally, this study included 5,188 eligible women, comprising 4,829 (93.08%) non-endometriosis cases and 359 (6.92%) EMs cases. Table 1 exhibits the characteristics of study women in non-EMs and EMs groups. The proportion women with the highest LAP level (> 77.81) in EMs group was significantly higher than in the non-EMs group (26.53% vs. 19.31). The differences were found in age, race, the level of PIR and education, smoking, the use of steroids drug and female hormones, as well as the history of dyslipidemia, hypertension and CVD between non-EMs and EMs groups (all P < 0.05).
We employed two logistics regression models to explore the association between LAP, VAI and EMs, as depicted in Table 2. After adjusted the potential covariates affecting EMs, we observed that women with highest LAP level were associated with EMs (OR = 1.56, 95%CI: 1.02–2.39); women with highest VAI level were also associated with EMs (OR = 1.54, 95%CI: 1.09–2.18).
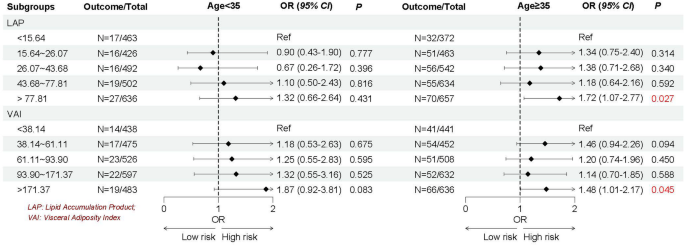
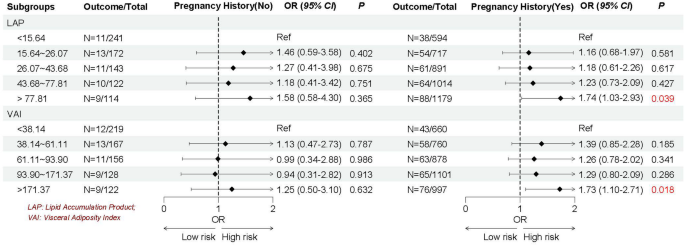
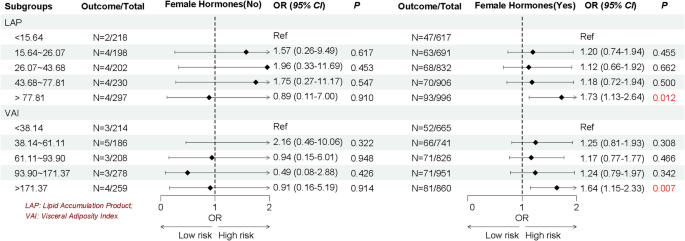
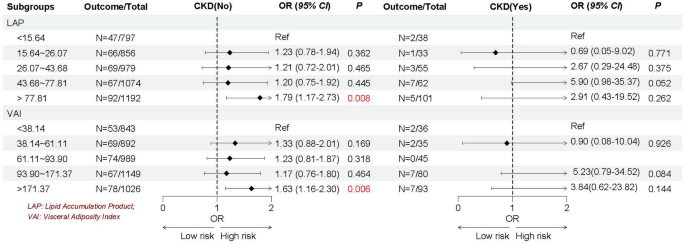
Given the heterogeneity of EMs, we stratified by age, the history of pregnancy and CKD, and female hormones use to further explore whether the association between LAP and VAI with EMs remain robust. As shown in Figs. 2, 3, 4 and 5, we found the association between LAP and VAI with EMs remain robust, especially among women age ≥ 35 years old (LAP: OR = 1.72, 95%CI: 1.07–2.77; VAI: OR = 1.48, 95%CI: 1.01–2.17), with the history of pregnancy (LAP: OR = 1.74, 95%CI: 1.03–2.93; VAI: OR = 1.73, 95%CI: 1.10–2.71), with female hormones use (LAP: OR = 1.73, 95%CI: 1.13–2.64; VAI: OR = 1.64, 95%CI: 1.15–2.33), and without the history of CKD (LAP: OR = 1.79, 95%CI: 1.17–2.73; VAI: OR = 1.63, 95%CI: 1.16–2.30).
Association between VAP and VAI with EMs based on the history of CKD
Note, VAP, lipid accumulation index; VAI, visceral adiposity index; EMs, endometriosis; CKD, chronic kidney diseases
The population-based cross-sectional study suggest that elevated levels of LAP and VAI are associated with EMs. These associations remained robust across subgroup analyses, especially among reproductive women aged ≥ 35 years old, with female hormones use, with the history of pregnancy and without the history of CKD. To the best our knowledge, this was the first NHANES-based study to assess the association between noninvasive, easily accessible, and gender-specific metabolic indicators of LAP and VAI with EMs among reproductive women in the United States.
In modern society, obesity has evolved beyond a mere lifestyle concern into a widespread and multifaceted disease entity that significantly disrupts physiological and metabolic homeostasis, with projections indicating sustained prevalence in the foreseeable future [30]. Epidemiology research have revealed that obesity-related changes in metabolic profiles often precede disease onset [31]. Concurrently, the health of reproductive women has emerged as a critical global public health priority, with obesity-through its extensive systemic pathophysiological effects -being recognized as a key modifiable risk factor [32]. Previous studies have revealed that obesity may contribute to endometrial dysfunction through multiple pathways, including insulin resistance, oxidative stress, high leptin levels, and high estrogen levels, ultimately impairing endometrial receptivity and ovarian dysfunction [13, 15]. However, current epidemiological findings regarding the obesity-EMs association remain inconsistent. Several studies have found a negative correlation between BMI and WC and EMs [33,34,35,36]. In these studies on body fat distribution and EMs, except for the reasons of ethnicity and sample sizes of the research population, the researchers used traditional anthropometric indicators. While BMI serves as a convenient anthropometric measure for obesity screening, it has several limitations. As a simple ratio of weight to height squared, BMI cannot differentiate between adipose and muscle tissue, nor does it provide information about regional fat distribution [19]. WC offers improved clinical utility by specifically assessing central adiposity; however, this metric is subject to significant measurement variability and cannot be reliably applied to special populations including children, pregnant women, and people with abdominal water accumulation [20]. Currently, computerized tomography (CT) and magnetic resonance imaging (MRI) represent the reference standards for quantifying visceral obesity. Both can well identify the volume of visceral and subcutaneous adipose tissue. However, due to reasons such as high cost, time-consuming examination and the presence of radiation, they are not routinely used in clinical and community screening and large-scale research on obesity-related diseases [37]. These methodological constraints in body composition assessment may consequently obscure the true nature of the association between obesity and endometriosis.
Emerging evidence suggests that adipose tissue distribution patterns, rather than overall adiposity alone, serve as independent risk factors for numerous metabolic disorders. This recognition has propelled visceral fat assessment to the forefront if obesity research in recent years. The elevated TG levels correlate strongly with distinct fat distribution. Therefore, researchers have proposed new evaluation indicators LAP and VAI by combining traditional obesity evaluation indicators WC, BMI and blood lipids through further calculation [21, 22]. Compared to traditional obesity evaluation indicators, these innovative indices suggest superior accuracy in quantifying visceral adiposity and lipid accumulation patterns. Importantly, multiple studies have validated their clinical utility in predicting the risk of CVD, diabetes and metabolic syndrome [23,24,25,26]. However, evidence for the association between LAP or VAI and EMs is scarce. The present study included 5,188 eligible reproductive women in United States aimed to explore the association between LAP and VAI with EMs. After comprehensive adjustment for potential confounders, women in the highest LAP and VAI suggested significantly associated with EMs (56% and 54% higher odds, respectively; both P < 0.05). Considering that women with EMs may be affected by demographic, complications or concomitant medication, we further stratified the study women according to age, the history of pregnancy and CKD and female hormones use to verify whether the association between LAP and VAI with EMs remain robust. Subgroup results continued to show robust associations between LAP and VAI with EMs, especially among women aged ≥ 35 years, with the history of pregnancy, with the female hormones use, or without CKD history. Our findings align with previous NHANES-based research suggesting a significant independent association between insulin resistance [assessed via triglyceride-glucose index (TyG) index] and EMs (OR = 1.58, 95% CI: 1.17–2.14, and overall P < 0.001 [38]. This growing body of evidence suggests that metabolic dysfunction contributes to EMs pathogenesis, highlighting the potential benefits of glycemic and lipid control in high-risk populations. As fat intake increases, the amount of triglycerides converted into fat in the body increases, which increases the output of low-density lipoprotein, thereby increasing the risk of insulin resistance. Among the pathogenesis of EMs that have been elucidated so far, insulin resistance, oxidative stress, and inflammation levels are considered to be important physiological mechanisms, all of which may be caused by obesity [13]. Throughout reproductive processes, precisely regulated inflammatory responses are essential. However, excessive inflammation impairs endometrial receptivity and negatively impacts reproductive outcomes the pregnancy process. Obesity exacerbates this through elevated pro-inflammatory cytokine production, macrophage activation in adipose tissue and disruption of immune homeostasis [39]. Oxidative stress, as an imbalance between reactive oxidative species (ROS) and biological antioxidants, plays a key role in the development of EMs. ROS molecules can activate apoptotic mechanisms, leading to cell damage or death. Previous studies have reported that women with EMs have higher levels of malondialdehyde and ROS and lower total antioxidant capacity compared with healthy controls [40].
Therefore, our study provides robust epidemiological evidence supporting the association between LAP and VAI and EMs based on a large, representative database such as NHANES. To enhance the statistical power of the results, we adjusted for covariates related to exposure and outcome, including demographic information, medical history, health behaviors, and medication history. In addition, we further stratified the study population according to its characteristics and conducted subgroup analyses based on age, history of CKD and pregnancy, and female hormones use. The persistent diagnostic delays in gynecology underscore the urgent need to shift from invasive surgical diagnostics to progressive, non-invasive assessment strategies. Our study suggests that compared to imaging modalities such as ultrasound, MRI, and CT, VAP and VAI offer greater convenience, cost-effectiveness, and accessibility, potentially serving as primary screening tools for EMs. Particularly for high-risk populations such as reproductive-aged women ≥ 35 years, those with a history of pregnancy, or hormone users, our findings support the initiation of more intensive gynecological follow-ups. Furthermore, for patients with abnormally elevated LAP/VAI, our results provide a theoretical basis for clinicians to implement lifestyle medical interventions, such as dietary guidance, to mitigate potential risks. However, our study also has several limitations. The cross-sectional study is a method for studying characteristics, phenomena, or associations of human or social groups at different time points or in different regions. The design of a cross-sectional study has some inherent limitations. Data of cross-sectional are collected at a single point in time, and it is difficult to determine the temporal order of events or to determine whether a particular variable directly affects another variable. Therefore, our study cannot infer a causal association between LAP and VAI with EMs. Additionally, cross-sectional studies are only based on known variables and may not be able to fully control the inference of external factors on variables. Third, EMs were assessed using the NHANES questionnaire, which may be subject to recall bias, thereby reducing the incidence of EMs. Future studies could strengthen diagnostic accuracy by linking electronic health records (EHRs) or incorporating biomarkers specific to EMs. Finally, the NHANES database included the representative American population, so the associations between LAP and VAI with EMs needs to be verified with caution in other ethnic groups. In summary, the associations between LAP and VAI with EMs observed in this study still needs to be confirmed by future large, well-designed, multicenter prospective cohort studies.
In conclusion, we found that both higher LAP and VAI were associated with EMs among reproductive women in the United States. Future large, multicenter, well-designed prospective cohort studies are still needed to further investigate the clinical utility of LAP and VAI as potential noninvasive and cost-effective indicators associated with EMs.
The datasets generated during and/or analyzed during the current study are available in the NHANES database, https://wwwn.cdc.gov/nchs/nhanes/.
- EMs:
-
Endometriosis
- CVD:
-
Cardiovascular disease
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- LAP:
-
Lipid accumulation product
- TG:
-
Triglyceride
- VAI:
-
Visceral adiposity index
- NHANES:
-
National Health and Nutrition Examination Surveys
- PIR:
-
Poverty to income ratio
- SBP:
-
Systolic blood pressure
- DBP:
-
Diastolic blood pressure
- TC:
-
Total cholesterol
- HbA1c:
-
Glycated hemoglobin
- HDL-C:
-
High density lipoprotein cholesterol
- LDL-C:
-
Low density lipoprotein cholesterol
- CKD:
-
Chronic kidney disease
- eGFR:
-
Estimated glomerular filtration rate
- CKD-EPI:
-
Chronic Kidney Disease-Epidemiology Collaboration
- CLIA:
-
Clinical Laboratory Improvement Amendments
- MEC:
-
Mobile Examination Centers
- NCEH:
-
National Center for Environmental Health
- SE:
-
Standard error
- OR:
-
Odds ratio
- CI:
-
Confidence interval
- TyG:
-
Triglyceride-glucose
The requirement of ethical approval for this was waived by Hospital of Fudan University, because the data was accessed from NHANES (a publicly available database). The need for written informed consent was waived by the Institutional Review Board of Hospital of Fudan University due to cross-sectional nature of the study. All methods were performed in accordance with the relevant guidelines and regulations.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Ma, N., Hu, Y. & Xu, Y. Association between lipid accumulation product and visceral adiposity index with endometriosis: evidence from NHANES 1999–2006. BMC Women's Health 25, 307 (2025). https://doi.org/10.1186/s12905-025-03862-5