Critical Care volume 29, Article number: 261 (2025) Cite this article
The weaning of critical care patients with acute kidney injury (AKI) from renal replacement therapy (RRT) lacks predictive criteria. The urinary urea excretion index (UUEI) based on the urine urea concentration and diuresis may be a relevant prognostic criterion. The aim of this study was to assess the value of utilising a UUEI-based weaning protocol from a RRT catheter.
This was a multicentre before-after study including patients with non-obstructive AKI requiring RRT during their intensive care unit (ICU) stay. A before cohort (2013–2015) was compared to an after cohort (2017–2019) in the interval of which a UUEI-based weaning protocol was implemented. In the after cohort, as soon as the UUEI exceeded 1.35 mmol/kg/24 h, physicians were encouraged to stop RRT and withdraw the catheter, whereas in the before cohort the catheter was withdrawn at the physician’s discretion. The primary outcome was the number of RRT catheter-free days on day 28 after initiating RRT.
In total, 179 and 130 patients were included in the before and after cohorts, respectively. The median numbers of catheter-free days in the before and after cohorts on day 28 were 13.0 (IQR 0.0–21.0) vs. 16.5 (IQR 4.3–24.0), respectively (p = 0.02). The adjusted rate ratio for the number of catheter-free days over the number of days at risk was 1.11 (95% CI 1.03–1.19; p = 0.006) in favour of the after cohort. Catheters were reinserted to resume RRT in 3 vs. 5 patients in the before and after cohorts, respectively.
These results confirm that a UUEI-based protocol is a safe technique to prompt withdrawal of the RRT catheter in patients with AKI requiring RRT during their ICU stay.
Acute kidney injury (AKI) in intensive care unit (ICU) patients requires renal replacement therapy (RRT) in 10–15% of cases [1]. Several recent large international prospective trials have found that delayed vs. early initiation of RRT is not associated with lower survival while decreasing the use of RRT by 38–49% [2,3,4]. Weaning becomes a relevant issue once RRT is initiated. Only a few studies have investigated optimisation of RRT weaning to reduce RRT-associated complications, including catheter-associated infections, thrombocytopenia, and others [5,6,7]. RRT is involved in kidney injury via RRT-associated hemodynamic instability and other mechanisms, leading to the “artificial kidney induces acute kidney injury” hypothesis [8]. This can translate into further delays or incomplete renal recovery. However, weaning from RRT too early is associated with fluid accumulation and can further delay weaning from respiratory support, significantly lengthening the ICU stay and increasing mortality.
The different RRT weaning protocols have created challenges [9]. The most popular criterion to trigger weaning is daily urine output, with important variations according to studies (from spontaneous diuresis > 400 mL/24 h to > 2000 mL with diuretics) [2,3,4]. In a post-hoc analysis of a prospective multicentre observational study, Uchino and colleagues reported that daily urine output (436 mL/24 h without diuretics) was an efficient criterion to predict weaning from RRT (specificity of 80.9% sensitivity of 46.5%) [10]. Functional biomarkers, such as cystatin C, or damage biomarkers, such as neutrophil gelatinase-associated lipocalin (NGAL), do not provide better accuracy and are costly [11, 12]. To date, no consensus guidelines have been developed for RRT weaning, and objective criteria are needed to guide this crucial step [9].
In a large recent meta-analysis, Katulka and colleagues reported the daily urine urea excretion index (UUEI) as a criterion with the best discrimination value (AUROC 0.96) for successful weaning from RRT [13]. They drew from a small study by Aniort and colleagues, who found that a UUEI > 1.35 mmol/kg/24 h was a better marker for RRT weaning than urine output or urine urea alone, with a sensitivity of 89.2%, specificity of 96.7%, and positive predictive value of 97.1% [14]. This criterion has the advantage of being standardised to the weight and urine output and is less affected by diuretics. Subsequently, this index has been introduced into the routine practices of several ICU teams.
The present study assessed whether routine use of an RRT weaning protocol, including systematic RRT catheter withdrawal when UUEI ≥ 1.35 mmol/kg/24 h is reached, is associated with an increase in the number of catheter-free days at 28 days from initiating RRT without harm compared with standard practice.
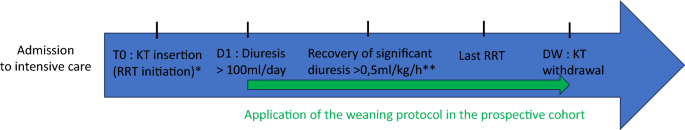
D-STOP is a multicentre before-after study (NCT03763188). Patients were included in either the before cohort with standard practices (October 2013–October 2015) or in the after cohort (September 2017–October 2019) while a weaning protocol (detailed below) was implemented. The study included one medical ICU at the University Hospital of Bordeaux and three medical and surgical ICUs at hospitals in Pau, Bayonne, and Libourne, France.
Consecutive ICU patients > 18 years old with AKI requiring RRT during their ICU stay were eligible.
Exclusion criteria were:
The following protocol was applied to the after cohort:
We used ICU admission weight measured on a bed scale rather than daily weight to ensure less dependence on volume variation.
The protocol used in the after cohort was shared and implemented over a two-year period at the medical centres. Some of the physicians participating in the study at the three centres (Bayonne, Pau and Libourne) had moved from the main investigating centre (Bordeaux) to pursue their professional careers; they participated at their new locations to implement the weaning protocol using UUEI criteria as a way to wean the patients from RRT and a catheter.
Withdrawal of the catheter in the before cohort was at the physician’s discretion, which meant that the physician withdrew the catheter once the decision to wean RRT was made. No mention of the UUEI was made to physicians as these data were collected before Aniort and colleagues published their results [14].
The management of other medical issues was left to the physician’s discretion.
The primary outcome was the number of days without an RRT catheter (RRT catheter-free days) beginning 28 days from RRT initiation. The number of RRT catheter-free days was the difference between the number of days in the observation period (28 days) and the number of days with an RRT catheter. A patient who died with a catheter was considered to have zero catheter-free days. In the specific case of the need for reinserting the catheter within the first 28 days, the number of RRT catheter-free days was defined as the difference between the observation period (28 days) and the total number of days with an inserted catheter. In the case of a patient discharged from the ICU before the end of the observation period, the occurrence of death or new initiation of RRT was determined by examining the medical records or calling the general practitioner. We performed a survival analysis in which the event of interest was the first catheter withdrawal within 28 days since initiating RRT. The date of RRT initiation was the date of catheter insertion, and censoring occurred at D28 of insertion.
Secondary outcomes included:
Baseline characteristics and the clinical and biological data were expressed as mean (± standard deviation), median (interquartile range; IQR), or percentage, as appropriate. Qualitative data were compared using the X2 or Fisher test. Quantitative data were compared using Student’s t-test. P-values < 0.05 were considered significant.
We used first a Poisson regression model to compare the primary endpoint, i.e., the number of RRT catheter-free days on day 28 between the two cohorts. This model is appropriate for quantitative data and allows the inclusion of an offset term, considering that the observation period might be different between subjects (in case of death within 28 days from the insertion of a catheter). Because the observed data were heterogeneous in the form of excess zeros (patients without any catheter-free days) (Additional File 1), we finally used a zero-inflated Poisson regression model with the period (after vs. before) as the main exposure [15]. The model estimated:
$$\text{Rate Ratio}= \frac{\frac{\text{Number of the catheter free days}}{\text{Number of days at risk }}\text{In the PROSPECTIVE cohort }}{\frac{\text{Number of the catheter free days }}{\text{Number of days at risk}}\text{In the RETROSPECTIVE cohort}}$$
This analysis was adjusted for centre, age, active neoplasia, immunosuppression (defined by haematological malignancy, allogeneic stem cell transplantation, active solid cancer, leukopenia < 1 G/L or neutropenia ≤ 0.5 G/L induced by chemotherapy, solid organ transplantation, acquired immunodeficiency syndrome, systemic corticosteroids ≥ 0.5 mg/kg per day of prednisone equivalent for at least 3 weeks, or immunosuppressive or immunomodulatory drugs), CKD stage, SOFA score at RRT initiation, diuresis and serum creatinine KDIGO criteria at RRT initiation, nephrotoxic drugs, ACE inhibitors, iodine, non-steroid anti-inflammatory drugs, diuretics, aminoglycosides, glycopeptides, or amphotericin B-based components delivered at any time between AKI and catheter withdrawal. Spline functions were used to model continuous variables when appropriate. In the sensitivity analysis, patients who had a catheter withdrawn followed by a reinsertion for subsequent RRT within 7 days after weaning (weaning failure) were considered to have had no catheter withdrawn; days of interruption were not considered catheter-free days. In the secondary analysis, we estimated the cumulative incidence of first catheter withdrawal in each group, utilising the Aalen-Johansen estimator to take into account competing risks [16]. Death or hospital discharge without prior catheter withdrawal were considered competing events. Groups were compared using Gray’s test. Analyses were performed using R version 4.2.0 (The R Foundation for Statistical Computing, Vienna, Austria) (Fig. 1).
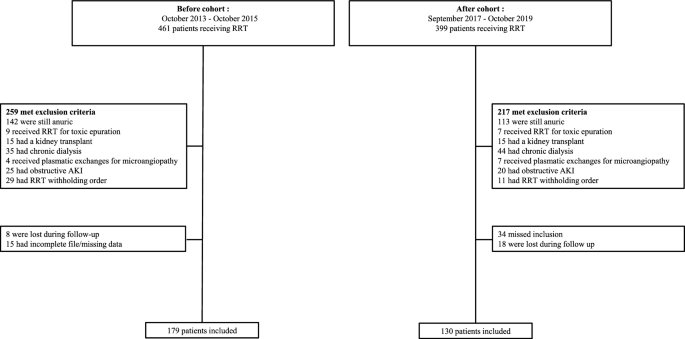
In total, 179 (58%) and 130 (42%) patients were included in the before and after cohorts, respectively (Fig. 2. flow chart). The most frequent exclusions were patients who were anuric (≤ 100 mL/d urine output) from RRT initiation to death, discharge from the ICU, or 28 days from the insertion of a catheter.
No data were missing. The family physicians of 12 patients without a previous serum creatinine dosage confirmed that they had no history of CKD and an estimated glomerular filtration rate (eGFR) of 90 mL/min. Both groups were similar in age, sex, and comorbidities (Table 1). Fewer patients with CKD (Additional File 2 provides more details about CKD), lower baseline serum creatinine, higher SAPS II and SOFA scores, and an increased rate of mechanical ventilation or a vasopressor requirement were observed in the after vs. the before cohort.
Additional File 3 presents the distribution of the UUEI, and Additional File 4 presents the results for obese patients (n = 48). We used actual body weight, but Additional File 5 provides a comparison of the UUEI with actual vs. adjusted body weight. The median UUEIs of obese patients (BMI ≥ 30; n = 41) with actual vs. adjusted weight were 1.8 [1.4–2.7] and 2.2 [1.8–3.3], respectively. Additional File 6 presents the results according to male vs. female.
In total, 15% of patients in the after cohort deviated from the protocol (Additional File 7). The most frequent deviation was a 24-h delay in catheter withdrawal because of no available urinary analysis on that specific day (n = 8 patients). The catheter was removed for technical reasons or suspected infection in 6 other patients, despite the urinalysis not reaching UUEI > 1.35 mmol/kg/24 h, but none of these catheters were reinserted. No patient was catheterised for a longer period than indicated by the UUEI.
Within 28 days after initiating RRT, 121/179 patients (67.6%) in the before cohort and 102/130 (78.5%) in the after cohort had their catheters withdrawn. Forty-nine (15.9%) patients died with their catheter within 28 days: 34 (19.0%) in the before cohort and 15 (11.5%) in the after cohort. Two patients (0.6%) in the before cohort were discharged from the hospital with their catheter before day 28 (bridge to chronic intermittent haemodialysis). Finally, 35 patients (11.3%) still had their catheter on day 28: 22 (12.3%) in the before cohort and 13 (10%) in the after cohort. Thirteen patients (4.2%) with a first catheter withdrawal required re-insertion of the catheter within 28 days (6 vs.7 in the before vs. after cohort).
The median number of catheter-free days on day 28 was 13.0 (IQR 0.0–21.0) in the before cohort vs. 16.5 (IQR 4.3–24.0) in the after cohort (p = 0.02). The adjusted rate ratio (RR) for the number of catheter-free days was 1.11 (95% CI 1.03–1.19; p = 0.006) in favour of the after cohort, meaning that the rate of the number of catheter-free days over the number of days at risk increased by 11% in the after cohort compared to the before cohort. The adjusted odds ratio of having no catheter-free days was 0.45 (95% CI 0.24–0.84; p = 0.01) in favour of the after cohort.
The sensitivity analysis (in which patients with their first catheter withdrawal followed by a catheter re-insertion < 7 days were considered to have no catheter withdrawal) revealed similar results.
A centre effect emerged, as the adjusted RR of each centre (Bordeaux: RR = 1.14 (1.00, 1.29); Libourne: RR = 1.01 (0.88, 1.16); Pau: RR = 0.97 (0.78, 1.19); Bayonne: RR = 1.24 (1.08, 1.42) presented a significant interaction (p = 0.01). One centre reported no difference in the adjusted RR between the two periods (Additional File 8).
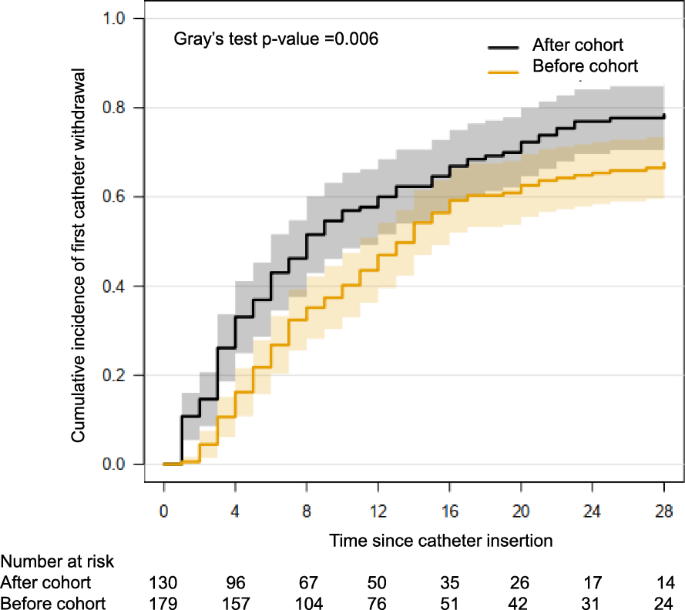
The cumulative incidence rates of first catheter withdrawal were 46% (95% CI 38–55) and 79% (95% CI 71–86) on days 7 and 28 from initiating RRT, respectively, in the after cohort vs. 32% (95% CI 26–39) and 68% (95% CI 61–75) in the before cohort (p = 0.005) (Fig. 3. Allen-Johnson survival analysis of the risk of catheter withdrawal).
Secondary endpoints (Table 2).
The time between the last RRT and catheter withdrawal was shorter in the after cohort, but the time to recovery of diuresis was longer than in the before cohort (p < 0.001). In the after cohort, 17/114 patients (14.9%) had their catheters withdrawn before significant diuresis recovery vs. 6/139 (4.3%) in the before cohort (p < 0.01). Only 8 patients failed catheter weaning: 1.7% in the before cohort vs. 3.8% in the after cohort (p = 0.29) (specificity of 96.2%). Additional File 8 presents the reasons for resuming RRT. No differences in RRT complications or catheter infections were observed between the groups. Table 2 lists other secondary endpoints.
The results of this study revealed that daily assessment of the UUEI to guide the decision to withdraw the RRT catheter was associated with an increased number of catheter-free days 28 days from RRT initiation compared to the routine weaning strategy. The rate of weaning failure, defined as catheter reinsertion within the 7 subsequent days, did not differ between the groups, suggesting that this marker is safe, and no complications were reported after catheter reinsertion. The specificity of the UUEI withdrawal protocol was 96.2%, consistent with that reported in the original study by Aniort and colleagues (96.7%) [14]. Survival analysis confirmed a higher probability of catheter withdrawal in the after cohort. Differences in timing were assessed in the groups: if the duration of RRT did not differ (6 days (3–14) vs. 7 days (3–16) p = 0.66 in the before vs. after cohort), patients in the after cohort had their catheter removed earlier than those in the before cohort. The reduced time from significant diuresis recovery (> 0.5 mL/kg/h) to catheter withdrawal illustrates the efficiency of the UUEI even at a very early stage of the weaning process: more patients had their catheters withdrawn, yet did not exhibit significant diuresis recovery. Thus, diuresis is not sensitive enough for RRT and catheter weaning, as previously suggested (sensitivity 0.66) [13]. Despite creatinine excretion, urea may have a more interesting pathophysiological profile. Urea is passively reabsorbed at the proximal tubule in the cortex and the external medulla and then travels to the internal medulla, which is a specifically focused area during septic aggression, where it plays a role in osmotic movement and urine concentration. The UUEI is based on a routine dosage and two easily monitored sets of clinical data at the bedside in the ICU (diuresis and admission weight). It is not yet clear whether adjusted weight could lead to earlier catheter withdrawal without reinsertion in obese patients. We observed excellent adhesion to the protocol, with catheters always being withdrawn as soon as the index reached 1.35 mmol/kg/24 h. Combining clinical information (diuresis) with biological information helped physicians gain confidence. The results were not consistent at the four centres, as one of the centres reported an RR of 1.01 (0.88–1.16) between periods. Catheter withdrawal was quite early in the before period at this centre, which was explained by very early initiation of RRT (drastically reduced during the after period, after the publication of RRT-initiation randomised controlled trials). Hence, patients with lower renal severity in the before period had very early catheter RRT weaning due to early renal recovery. However, this biased the results toward the absence of a difference between the before and after groups in the entire study population. Our results were robust to this bias.
The rate of complications associated with RRT, particularly catheter infections, was similar between groups due to no difference in RRT duration. The RRT duration during the before period was explained by an early RRT initiation with the possibility of early weaning because of less severe AKI. In the after period, late RRT initiation was applied as a result of recently published studies, and more severe AKI may have been selected. Hence, some patients had late initiation of RRT but needed more prolonged RRT due to more severe AKI. However, we are confident that in the current period, in which late RRT initiation is now recommended, the duration of RRT will also be reduced by this protocol, thus reducing complications associated with exposition [2, 3, 7]. Moreover, more femoral catheters were used during the before period, which may have confused the results. Finally, we were unable to determine modifications in the incidence rate of catheter infection, as this study was underpowered to detect such adverse effects. However, the decrease in catheter exposure in the after cohort may have resulted in lower infection rates. Another harmful effect of the before-period strategy may be related to the longer conservation of catheters, prompting physicians to maintain RRT longer than required, leading to a kind of self-fulfilling prophecy. Unfortunately, we could not provide data on RRT sessions spared by the use of the UUEI as we only recorded RRT duration. The number of RRT sessions was challenging to compare between groups, as patients engaged in both continuous renal replacement therapy (CRRT) and intermittent haemodialysis. Overall, both late initiation timing and earlier weaning from CRRT may decrease the number of complications, save human and material resources, and improve renal recovery [6,7,8].
Our study had some limitations. First, we compared the after cohort with a historical before cohort. Clinicians may have changed their practice during the periods, as several studies have reported modifications to the timing of initiating CRRT [2, 3], and other differences between groups, such as the severity of illness, may have confused the results. As clinicians waited for late CRRT criteria in the after cohort, patients who had no CRRT at the end were excluded, creating a selection bias with the historical cohort. However, less severe patients in the before cohort may have biased the results toward a protective effect on the outcome. On the other hand, better renal recovery in the late CRRT initiation group was observed in previous studies [2,3,4], which would affect our results in the other direction. As a second limitation, we cannot rule out a halo effect of the protocol in the after cohort. However, our results are consistent with those of Aniort and colleagues, which were not subject to this effect because of the retrospective nature of their study [14]. Third, 15% of cases in our after cohort had protocol deviations. Most were represented by a 24-h delay of withdrawal because of a forgotten urinary analysis (which questions the feasibility of the protocol) or by an earlier withdrawal before the UUEI was > 1.35 mmol/kg/24 h either for technical reasons or a suspected infection, which may have biased the results in favour of the tested protocol. The urea index may have been affected by sex, muscle loss, protein catabolism, or nutrition. Additionally, we modified how Aniort and colleagues’ original index was used: we used both intermittent haemodialysis and CRRT, whereas the index was developed for intermittent haemodialysis only. To simplify the calculation, we used weight at admission rather than current weight, and we did not capture modifications in fluid balance. However, the index in our study maintained the same predictive accuracy as in the original study. Finally, even if not statistically significant, the difference in catheter weaning failure rate may be considered clinically significant and should be studied in a larger cohort. Another weaning approach would consist of maintaining the catheter until further renal recovery occurs, which would avoid mechanical complications related to catheter insertion. In our opinion, removing the RRT catheter is more demanding; it is more efficient to stop RRT. When a catheter is still inserted, clinicians tend to use RRT more, particularly because no clear recommendation regarding RRT weaning has been reported. For example, maintaining the catheter when significant diuresis recovery has not been reached (this situation was more frequent in the after group) may prompt unnecessary resumption of RRT while the clinician waits. Differences between the various approaches should be studied in the future.
Use of a catheter-weaning protocol based on the UUEI was associated with an increase in the number of catheter-free days from initiation to day 28 compared to standard practice. A randomised controlled trial should be performed to confirm these results.
Data is provided within the manuscript or supplementary information files. The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
- AKI:
-
Acute kidney injury
- AUROC:
-
Area under receiving operator curve
- CKD:
-
Chronic kidney disease
- CRRT:
-
Continuous renal replacement therapy
- DR:
-
Diuresis recovery
- DW:
-
Day of withdrawal
- eGFR:
-
Estimated glomerular filtration rate
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- KDIGO:
-
Kidney disease improving global outcomes
- RRT:
-
Renal replacement therapy
- SDR:
-
Significant diuresis recovery
- SOFA:
-
Sequential organ failure assessment
- UUEI:
-
Urinary urea excretion index
We would like to thank the patients and the clinical and clerical teams involved in this study. The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/5mDyhE
The study was approved by the French commission for individual freedom “Commission Nationale des Libertés Individuelles (CNIL)” and the ethic committee of the intensive care unit French society “Société de Réanimation de langue Française (SRLF)”.
All patients accepted their personal data to be registered and used for clinical research purpose.
LLG: none CG: none JP: none AM: none CR: none HG: received fees from Baxter, Freseniusnone AR: none AD: received grant support from MSDavenir; fees for conferences symposium from MSD, Pfizer, Novartis, Baxter, Cytosorb, ISep; fees for participation to advisory board from Ocean Dx DG: none RP: none SR; received fees from AstraZeneca, Servier, Novartis, Fresenius, Meditor, Alexion AO: none AB none.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Bodot, S., Gros, A., Le Gall, L. et al. Effects of the urinary urea excretion index on the decision to wean ICU patients with acute kidney injury from renal replacement therapy: a before-after multicentre study (D-STOP). Crit Care 29, 261 (2025). https://doi.org/10.1186/s13054-025-05289-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-025-05289-8