BMC Pediatrics volume 25, Article number: 278 (2025) Cite this article
The increasing hypertension (HTN) prevalence in children, largely driven by obesity, highlights the need to investigate its risk factors, including the role of abdominal obesity.
We analysed data from the Korea National Health and Nutrition Examination Survey (2007–2021) to assess the prevalence of overweight and obesity (OW-OB), elevated blood pressure (EBP), and HTN among 11,554 participants aged 10–18 years. EBP and HTN were defined according to the 2017 American Academy of Pediatrics guidelines, and abdominal obesity was defined as a waist-to-height ratio (WHtR) of ≥ 0.5. Secular trends in the prevalence of OW-OB and EBP-HTN were examined across five time periods, and risk factors for EBP-HTN were evaluated in OW-OB children stratified by abdominal obesity status.
The prevalence of EBP, HTN, and OW-OB was 8.22% (95% confidence interval [CI], 7.60–8.86), 10.46% (95% CI, 9.72–11.20), and 25.11% (95% CI, 24.17–26.05), respectively. Among the 3,015 participants with OW-OB, 13.53% (95% CI, 12.03–15.04) and 17.64% (95% CI, 15.98–19.31) were diagnosed with EBP and HTN, respectively. Although the prevalence of OW-OB and EBP-HTN had increasing trends from 2007 to 2009 to 2019–2021, these trends were not statistically significant. In the children with OW-OB, obesity severity, male sex, older age, and paternal HTN were associated with EBP-HTN. The HTN risk factors differed according to abdominal obesity status. In participants with abdominal obesity, male sex (OR 2.32, 95% CI 1.643–3.299; p < 0.0001), older age (OR 1.16, 95% CI 1.102–1.233; p < 0.0001), and severe obesity (OR 3.12, 95% CI 1.991–4.895; p < 0.0001) were significant risk factors; whereas, in those without abdominal obesity, paternal HTN (OR 1.66, 95% CI 1.207–2.303; p = 0.0025), hypercholesterolemia (OR 1.85, 95% CI 1.114–3.083; p = 0.0178), male sex (OR 1.83, 95% CI 1.329–2.530; p = 0.0002), and older age (OR 1.11, 95% CI 1.036–1.198; p = 0.0038) were significant risk factors.
In children with overweight or obesity, the risk factors for EBP-HTN vary depending on the presence of abdominal obesity. These findings highlight the need for differentiated surveillance and prevention strategies based on abdominal obesity status in this high-risk population.
Not applicable
Hypertension (HTN) is one of the most prevalent chronic diseases worldwide and a leading cause of morbidity and mortality. Conversely, paediatric HTN was previously considered rare, with secondary HTN identified as its principal aetiology. However, the rising prevalence of childhood obesity has contributed to a gradual increase in HTN, prompting a reorientation of the research agenda towards primary HTN as a substantial contributory factor [1].
A growing body of evidence indicates that HTN in childhood represents a risk factor for the subsequent development of HTN in adulthood [2]. Furthermore, elevated blood pressure (EBP) in children is associated with various cardiovascular risk factors and outcomes that may later manifest in adulthood [2]. These findings highlight the importance of early identification, prevention, and management of HTN during childhood and adolescence.
Numerous studies have extensively investigated the relationship between body mass index (BMI) and blood pressure (BP) in children and adolescents [3,4,5]. A comparative analysis of 167 children with obesity and 31 non-obese children showed that children with a BMI Z-score of 2.5 or higher exhibited an increased frequency of EBP and degree of systolic BP (SBP) elevation than those with a Z-score of less than 2 [3]. In another study, mean SBP and diastolic BP (DBP) were positively correlated with increasing BMI decile [4]. The pathogenesis of HTN in children with obesity is multifactorial, involving increased sympathetic nervous system (SNS) activity, activation of the renin-angiotensin-aldosterone system, and renal compression by adipose tissue, with visceral adiposity playing a central role [2]. Moreover, the adipose tissue distribution, particularly visceral fat, contributes more significantly to EBP than total body fat through mechanisms including increased insulin resistance, heightened inflammatory responses, and hormonal dysregulation [6, 7]. Studies have reported that abnormalities in cardiovascular rhythms persist in adolescents with hypertension even after BP reduction, with improvements associated with visceral fat reduction [8]. This emphasises the importance of visceral adiposity in childhood HTN. Additionally, individuals with increased visceral fat have been observed to exhibit higher muscle SNS activity, which may further contribute to HTN [9]. Conversely, HTN is less frequent in those with lower basal muscle SNS activity, even in the presence of obesity, suggesting a potential correlation between abdominal obesity and HTN [10].
Previous studies have identified the association between abdominal fat distribution and HTN in children; however, few studies have characterized the difference in HTN risk factors based on abdominal obesity status among children with overweight or obesity. Understanding these differences is essential for developing more effective, tailored strategies for HTN risk assessment and management.
Therefore, this study aimed to ascertain the current prevalence of HTN and obesity in Korean children and adolescents using data from the Korea National Health and Nutrition Examination Survey (KNHANES) and to investigate the factors influencing HTN in children and adolescents with obesity. Particularly, we aimed to identify the differences in HTN risk factors based on abdominal obesity status, to provide insights into individualized surveillance and prevention strategies.
This study was based on data from the KNHANES conducted by the Korean Ministry of Health and Welfare between 2007 and 2021. The KNHANES is a nationally representative cross-sectional surveillance survey conducted annually by the Korea Centers for Disease Control and Prevention and the Ministry of Health and Welfare since 1998. Informed consent was obtained from all participants and from parents or legal guardians for children under 18 years of age. The protocol of the KNHANES was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (2007-02CON-04-P, 2008-04EXP-01-C, 2009-01CON-03–2 C, 2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01–2 C, 2013-07CON-03–4 C, 2013-12EXP-03–5 C). The KNHANES applies sampling weights to account for the complex survey design and to produce nationally representative estimates based on the latest population and housing census. The survey follows a multistage clustered probability design, incorporating health interviews, health examinations, and nutrition surveys. A detailed description of the KNHANES has been published elsewhere [11].
We divided the data obtained from KNHANES 2007–2021 into five distinct periods: period 1 (2007–2009), period 2 (2010–2012), period 3 (2013–2015), period 4 (2016–2018), and period 5 (2019–2021). A total of 12,653 individuals aged 10 to 18 years who participated in the KNHANES between 2007 and 2021 were included in the analysis. The exclusion criteria were as follows: a lack of BP data (n = 979), a DBP below 30 mmHg (n = 990), an absence of anthropometric data (n = 912), and a history of congenital heart disease (n = 78). This study was approved by the Institutional Review Board of CHA University Hospital (IRB No. 2021-12-002).
Measurements of height, body weight, and waist circumference (WC) were conducted by trained medical personnel using calibrated equipment and in accordance with established protocols. Height was measured to the nearest 0.1 cm using a stadiometer (Seca 225, Seca), and weight to the nearest 0.1 kg using an electronic balance (GL-6000-20, G-tech). BMI was calculated by dividing weight in kilograms by the square of height in metres. The resulting value was then transformed into a standard deviation score (Z-score) using the Centers for Disease Control and Prevention (CDC) 2000 Growth Chart. Class I, II, and III obesity were defined as having a BMI between the 95th percentile and 120% of the 95th percentile, between 120% and 140% of the 95th percentile, greater than 140% of the 95th percentile, respectively [12]. In combination, participants who were overweight or obese were designated as the OW-OB group. Abdominal obesity was defined according to a waist-to-height ratio (WHtR) equal to or greater than 0.5, following established criteria validated in children and adolescents [13,14,15].
After five minutes of rest in a seated position, participants’ BP was measured by a team of medical professionals. BP was measured using either a mercury sphygmomanometer (Baumanometer Desk model 0320 in 2007–2012 and Baumanometer Wall Unit 33[0850] in 2013–2019) or a non-mercury sphygmomanometer (Greenlight 300 in 2020). Comparative studies revealed no significant differences between the values obtained from mercury and non-mercury sphygmomanometers [16]. All BP measurements were taken on the right arm three times, using the same instruments at 30-second intervals, with a cuff appropriate for arm circumference. The average values of the second and third measurements of SBP and DBP were used for analysis.
According to the 2017 American Academy of Pediatrics references, age-, sex-, and height-specific BP percentiles were calculated for SBP and DBP and were then classified as normal, EBP, stage 1 HTN, and stage 2 HTN using the CDC growth chart [17]. For participants aged 13 years or older, BP < 120/<80 mm Hg, 120/<80 to 129/<80 mm, 130/80 to 139/89 mm Hg, and ≥ 140/90 mmHg were categorised as normal, EBP, stage 1 HTN, and stage 2 HTN. Participants with EBP or HTN were collectively designated as the EBP-HTN group.
In the KNHANES, participants were asked whether any of their parents had been diagnosed with HTN, dyslipidemia, diabetes, or obesity. In addition to self-reporting family history, the presence of disease in a family member was determined by conducting a comprehensive investigation of the KNHANES dataset using the family identification number provided. If the KNHANES data for each individual met the diagnostic criteria for HTN, dyslipidaemia, diabetes, or obesity, the disease was deemed to be present.
Laboratory data, including levels of fasting blood glucose (FBG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL), high-density lipoprotein cholesterol (HDL), triglycerides (TG), and liver enzymes were analysed. An elevated FBG level was defined as a value exceeding 100 mg/dL, indicating hyperglycaemia. Furthermore, the following criteria were employed to define abnormal laboratory findings: (1) TC ≥ 200 mg/dL, (2) LDL ≥ 130 mg/dL, (3) HDL < 40 mg/dL, (4) TG ≥ 130 mg/dL, (5) aspartate aminotransferase or alanine aminotransferase > 40 mg/dL, and (6) glycosylated haemoglobin A1C (HbA1C) > 5.7% [18,19,20].
Information about health-related lifestyles was obtained via self-reporting using a questionnaire during the interview. Information on the dietary intake of all participants was collected via a 24-hour dietary recall questionnaire conducted by trained interviewers. The dietary questionnaire was conducted in person and included details regarding the type, amount, and frequency of foods and drinks consumed on the previous day. Food item data were converted to nutrient units using the Food Composition Table developed by the National Institute of Agricultural Sciences (7th revision) and the database of the Korean Health Industry Development Institute (for instant and imported foods).
All analyses were conducted using sampling weights to account for the complex multistage probability sampling design. Data are presented as the mean and standard error (SE) or percentages, as appropriate. Trends in the prevalence of overweight or obesity and EBP or HTN were analysed across five periods using the Cochran–Armitage test. A chi-squared test for categorical variables and an analysis of variance test for continuous variables were conducted to evaluate differences in characteristics between participants with normal BP and EBP in the OW-OB group. After dividing the cohort into two groups based on the presence of abdominal obesity within the OW-OB category, comparisons were conducted using either the chi-squared test for categorical variables or analysis of variance for continuous variables. Regression analyses were performed to identify risk factors contributing to the development of EBP-HTN in the OW-OB group. Regression analyses were also performed to identify contributing factors to the presence or absence of abdominal obesity.
Furthermore, a nadir analysis was conducted to investigate age-related BP patterns and identify the age with the lowest mean SBP and DBP. For this, the mean and SE of BP were stratified by age, and trends across ages were analysed.
Statistical analyses were performed using the SAS survey procedure (version 9.4; SAS Institute, Cary, NC, USA) to account for the complex sampling design. Two-sided p-values of < 0.05 were considered to indicate statistical significance.
In total, 11,554 individuals were included in the definitive analysis of OW-OB and EBP-HTN prevalence. The mean age was 14.13 years (SE 0.029), and 53.33% of the participants were male (SE 0.52).
A total of 3,015 participants (25.11%, SE 0.48) were identified as having an OW-OB status, comprising 15.65% (SE 0.38) and 9.46% (SE 0.34) of those with a BMI in the categories of overweight and obese classes 1–3 range, respectively. A total of 2,080 individuals were classified as EBP (n = 927, 8.22%, SE 0.32) or HTN (n = 1,153, 10.46%, SE 0.37).
The mean age of the OW-OB children and adolescents was 13.50 years (SE 0.057), including 66.68% (SE 1.02) males. The mean BMI and BMI Z-score were 25.57 (SE 0.08) and 1.5592 (SE 0.0080), respectively. A total of 883 individuals were classified as having EBP (n = 394, 13.53%, SE 0.77) or HTN (n = 489, 17.64%, SE 0.85). The mean SBP and DBP were 111.84 mmHg (SE 0.24) and 67.40 mmHg (SE 0.23), respectively. Abdominal obesity was identified in 1,514 individuals (51.85%, SE 1.11) within this group.
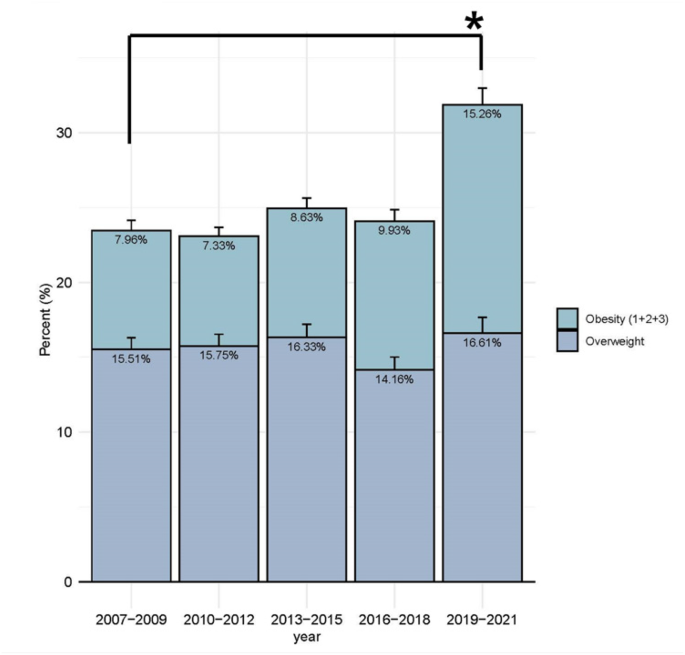
The prevalence of OW-OB was 23.47% (95% CI, 21.55–25.39), 23.08% (95% CI, 21.32–24.84, 24.96% (95% CI, 22.89–27.04), 24.09% (95% CI, 21.89–26.27), and 31.87% (95% CI, 29.14–34.61) in periods 1, 2, 3, 4, and 5, respectively (Fig. 1). Although there was no statistically significant secular trend in the prevalence of OW-OB across periods 1 to 5 (trend-p for overweight = 0.9577, obesity = 0.7212, OW-OB = 0.1964), obesity prevalence increased from 7.96% (95% CI, 6.65–9.28) in period 1 (2007–2009) to 15.26% (95% CI, 31.11–17.42) in period 5 (2019–2021).
The trend in the prevalence of OW-OB in all participants between 2007 and 2021. OW-OB = overweight and obesity trend-p for overweight = 0.9577, trend-p for obesity = 0.7212, trend-p for OW-OB = 0.1964 * P-value for the prevalence of obesity, and OW-OB between 2007–2009 and 2019–2021: p < 0.001
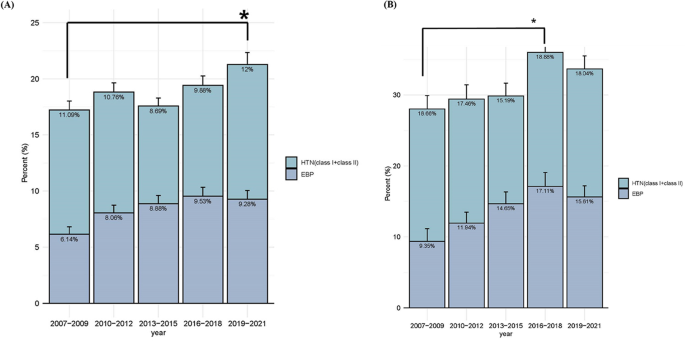
The overall prevalence of EBP-HTN demonstrated an upward trend, although this was not statistically significant (trend-p for EBP = 0.3765, HTN = 0.9232, EBP-HTN = 0.4821). However, the prevalence of EBP-HTN during period 5 exhibited a significant increase compared to period 1, rising from 17.23% (95% CI, 15.24–19.21) to 21.28% (95% CI, 18.65–23.92) (p-value = 0.0143) (Fig. 2A).
The trend in the prevalence of EBP-HTN. (A) The trend in the prevalence of EBP-HTN in all participants from 2007 to 2021. EBP = elevated blood pressure; EBP-HTN = elevated blood pressure or hypertension; HTN = hypertension trend-p for EBP = 0.3756, HTN = 0.9232, EBP-HTN = 0.4821 * P-value for EBP between 2007–2009 and 2019–2021: p = 0.0022 * P-value for EBP-HTN between 2007–2009 and 2019–2021 = 0.0143. (B) The trend in the prevalence of EBP-HTN in the OW-OB group from 2007 to 2021 EBP = elevated blood pressure; EBP-HTN = elevated blood pressure or hypertension; HTN = hypertension; OW-OB = overweight and obesity trend-p for EBP = 0.1044, HTN = 0.988, EBP-HTN = 0.2236 * P-value for EBP between 2007–2009 and 2016–2018: p = 0.0057 * P-value for EBP-HTN between 2007–2009 and 2016–2018: p = 0.0181
In individuals with OW-OB, the prevalence of EBP-HTN was higher in periods 4 and 5 compared to period 1, showing an increasing trend that did not reach statistical significance (trend-p for EBP = 0.1044, HTN = 0.988, EBP-HTN = 0.2236). When comparing periods, the prevalence of EBP-HTN was significantly higher in period 4 than in period 1 (p = 0.0181), while the difference between period 1 and period 5 was not statistically significant (p = 0.0815). Furthermore, there was no significant difference between periods 4 and 5 (p = 0.4692). (Fig. 2B).
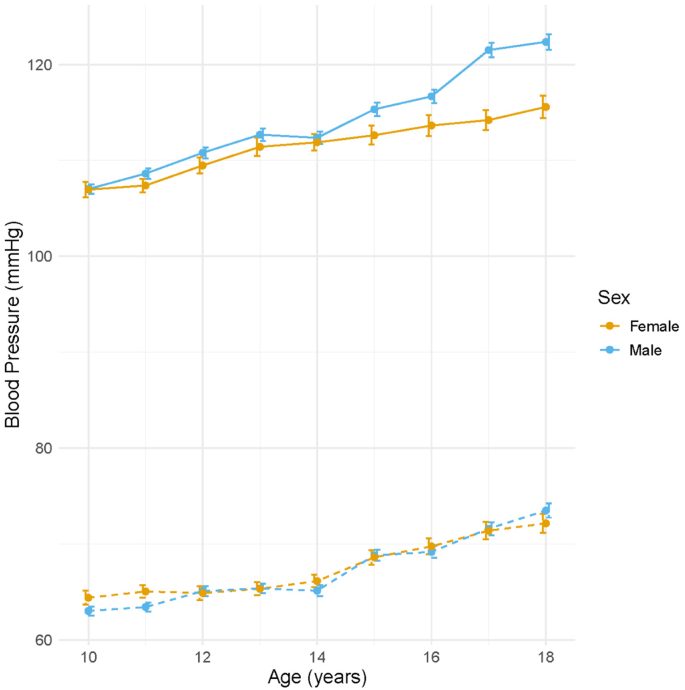
In the OW-OB population, the lowest SBP and DBP were observed at approximately 10 years of age, with nadir values of 72 mmHg (SBP) and 39 mmHg (DBP) in males, and 36 mmHg (SBP) and 36 mmHg (DBP) in females. Following this nadir, both SBP and DBP exhibited a steady increase throughout adolescence. Among males, SBP increased from 107.02 ± 0.51 mmHg at age 10 to 119.66 ± 1.03 mmHg at age 18, while DBP rose from 63.04 ± 0.48 mmHg to 73.93 ± 1.00 mmHg over the same period. Similarly, among females, SBP increased from 106.96 ± 0.81 mmHg at age 10 to 111.62 ± 1.27 mmHg at age 18, and DBP from 64.42 ± 0.72 mmHg to 71.40 ± 1.11 mmHg (Supplementary Table 1, Fig. 3). These findings highlight sex-specific differences in BP trajectories during adolescence and underscore the importance of age- and sex-appropriate evaluation when diagnosing EBP and HTN in children and adolescents.
Age-specific trends in mean systolic blood pressure (SBP) and diastolic blood pressure (DBP). Mean SBP (red-orange line) and DBP (blue line) with standard errors (gray error bars) are presented according to age (10–18 years) in children and adolescents from the Korea National Health and Nutrition Examination Survey (2007–2021)
A comparative analysis was conducted on the anthropometric factors, family history, and health examination data of the participants in the normal BP and EBP-HTN groups (Table 1). Male predominance, older age, and higher BMI Z-scores were observed in the EBP-HTN group (p-value < 0.0001). When the data were stratified by obesity category, a higher proportion of individuals with obesity was observed in the EBP-HTN group. The presence of abdominal obesity was significantly higher in the EBP-HTN group (47.18% [95% CI, 44.46–49.91] vs. 62.14%[95% CI, 58.47–65.83], p < 0.0001). Furthermore, paternal or maternal HTN was significantly more common in the EBP-HTN group (32.58% [95% CI, 30.08–35.10] vs. 41.35% [95% CI, 37.53–45.19], p < 0.0001) (Table 1).
In the laboratory findings, the incidence of abnormal results indicating metabolic disorders, including elevated FBG, TC, TG, and low HDL, was significant in the EBP-HTN group. Regarding dietary factors, no significant differences were observed in total calorie, carbohydrate, fat, protein, sodium, and potassium intake (Table 1).
Abdominal obesity was observed in 1,514 children and adolescents in the OW-OB group. The group with abdominal obesity demonstrated male predominance, a higher mean age, and significantly elevated SBP and DBP compared to the group without abdominal obesity. Furthermore, the abdominal obesity group exhibited a significantly higher proportion of EBP or HTN (elevated BP, 10.88% [95% CI, 9.01–12.76] vs. 15.99% [95% CI, 13.84–18.15, p < 0.0001; stage I HTN, 11.98% [95% CI, 9.99–13.97[ vs. 18.53% [95% CI, 16.04–21.02], p < 0.0001) and higher BMI Z-scores (1.3388 vs. 1.7638, p < 0.0001), and a greater likelihood of having a family history of HTN, obesity, hyperlipidaemia, and diabetes compared to the group without abdominal obesity. Additionally, the laboratory findings revealed more indications of metabolic diseases, such as elevated HbA1c, TC, LDL, and TG, along with decreased HDL levels in the abdominal obesity group. However, no statistically significant differences were observed between the two groups in dietary factors (Table 2).
The factors associated with EBP/HTN in the OW-OB group were analysed (Table 3). Univariable analysis showed male sex, older age, obesity severity, abdominal obesity, and a family history of HTN were associated with EBP-HTN. Furthermore, elevated TC and TG levels and decreased HDL levels were found to be associated with EBP-HTN in blood tests. Multivariable analysis, adjusted for multiple factors, showed male sex (odds ratio [OR], 1.96; 95% CI, 1.534–2.512), advanced age (OR 1.14; 95% CI, 1.091–1.191), and greater obesity (obesity class 2 + 3 OR, 3.41; 95% CI, 2.191–5.327) were associated with an elevated risk of EBP or HTN. Additionally, a family history of paternal HTN (OR, 1.41; 95% CI, 1.136–1.765) was identified as a further risk factor (Table 3).
Given the notable differences in the prevalence of EBP-HTN, anthropometric characteristics, and laboratory results between the abdominal and non-abdominal obesity groups, an analysis was conducted using multivariable logistic regression based on the presence of abdominal obesity.
In individuals with abdominal obesity, male sex (OR, 2.32; 95% CI, 1.643–3.299), advanced age (OR, 1.16; 95% CI, 1.102–1.233), and greater degrees of obesity, such as class II or III obesity (OR, 3.12; 95% CI, 1.991–4.895), were identified as risk factors for EBP-HTN. In contrast, in individuals without abdominal obesity, a family history of paternal HTN (OR, 1.66; 95% CI, 1.207–2.303) and elevated TC (OR, 1.85; 95% CI, 1.114–3.083), in addition to male sex (OR, 1.83; 95% CI, 1.329–2.53), advanced age (OR, 1.11; 95% CI, 1.036–1.198), and class I obesity (OR, 2.31; 95% CI, 1.485–3.612), were associated with EBP-HTN (Table 4).
In our study, the frequency of obesity was 9.46%, representing a significant increase from 7.96% in period 1 (2007–2009) to 15.26% in period 5 (2019–2021).(Fig. 1) The prevalence of obesity among Korean children and adolescents was 9.98% (95% CI, 8.45–11.41) during the period 2016–2018, lower than the 21.8% reported in the 2013–2016 NHANES in the United States (US) [21] but higher than the global average in 2016 (5.6% for girls and 7.8% for boys) [22]. The prevalence of obesity increased globally from 1975 to 2016; however, it plateaued in high-income countries [22]. The prevalence of childhood obesity in Korea also exhibited minimal change from period 1 to period 4. However, our study revealed a notable increase in the prevalence of obesity between periods 1 and 5. This finding suggests that the recent increase in obesity, particularly during the COVID-19 pandemic, may have contributed to worsening cardiovascular risk profiles in Korean Youth. In 2020, social distancing was enforced due to the global pandemic caused by SARS-CoV-2, which significantly impacted the physical activity levels of children and adolescents [23]. A similar trend was observed in the prevalence of EBP and HTN among the total study participants (Fig. 2A). However, in the OW-OB group (Fig. 2B), the EBP-HTN prevalence slightly decreased in period 5 than in period 4, despite the obesity increase. This suggests that factors beyond obesity, including behavioural or environmental changes during the pandemic, may have influenced BP trends, warranting further investigation. Song et al. reported an increase in HTN prevalence from 7.1 to 12.5% between 2018 and 2020 using KNHANES data, with a notable rise among individuals with normal BMI, indicating that obesity alone may not completely explain the recent increase in HTN [24]. The prevalence of HTN in Korean children and adolescents was 10.46% over the study period and 8.69% in period 3 (2013–2015), higher than that in the US or globally but lower than that in China. According to NHANES data from 2013 to 2016, HTN prevalence among US adolescents aged 12–19 years was 4.11% overall and 9.43% in those with obesity [21]. A meta-analysis of 47 studies revealed a pooled global prevalence among children aged 19 years and younger of 4.0% (95% CI, 3.29–4.85%) and 6.02% (95% CI, 4.38–7.91%) during 2010–2014 [25]. In contrast, the prevalence among Chinese children aged 7–17 years in 2015 was 19.2%, the highest among these comparisons [26]. The differences in study methodologies should be carefully considered when comparing childhood HTN prevalence across countries. Variations in BP measurement techniques, including the number of measurements, auscultatory versus oscillometric device uses, and the intervals between repeated assessments, may significantly influence prevalence estimates. Moreover, diagnostic criteria and population characteristics differ across studies, likely contributing to discrepancies in reported HTN prevalence between Korea, the US, and China. Notably, KNHANES obtains three BP measurements during a single visit; however, HTN diagnosis typically requires elevated readings across three separate visits. Additionally, the interval between repeated BP measurements in KNHANES was approximately 30 s, which is shorter than that recommended in clinical practice. This may have contributed to measurement variability or overestimation of BP values, representing a potential source of bias in the prevalence estimates of HTN. This methodological difference may lead to HTN prevalence overestimation, as BP tends to decrease with repeated measurements [27]. Finally, although the increasing trends in obesity and HTN during the study period did not reach statistical significance, these patterns may still have clinical relevance. Even modest increases in childhood obesity and HTN can have meaningful public health implications, highlighting the need for continuous monitoring and preventive strategies.
In this study, we observed that the prevalence of HTN in the OW-OB group was 1.5 to 2 times higher than in the total group. As our research demonstrated, BMI is positively related to SBP and DBP. Chorin et al. observed an increase in SBP by 10 mmHg and DBP by 3–4 mmHg from the 1st to the 10th decile of BMI [4]. A school-based screening study conducted in Houston revealed a prevalence of HTN in children with obesity of 11%, compared to 2.6% in children with normal BMI [27]. Additionally, data from NHANES in the US indicate a high prevalence of HTN in children with obesity [21]. The prevalence range among overweight children and adolescents with obesity in Europe is estimated to be between 27% and 47%, depending on the reference values used [28].
Various studies have identified overweight, obesity, family history, gender, and dietary habits as risk factors for HTN in children and adolescents [29]. The findings of our study indicate that the severity of obesity is associated with the development of HTN, even among children with obesity. A study examining the correlation between BP and BMI in a cohort of 111,618 children and adolescents aged 6–17 years revealed a prevalence of severe obesity (BMI ≥ 120% of the 95th percentile) of 5.6% [30]. As the severity of obesity increased, the risk of HTN rose, being 1.8 times and 5.7 times higher in those with a BMI of 120–129% and 140% of the 95th percentile, respectively, than in those with a BMI of 100–109% of the 95th percentile. These results suggest that regular monitoring and management of BP are crucial in healthcare for children with obesity, and weight management should be a significant therapeutic objective to prevent further increases in the prevalence of obesity.
The analysis revealed no statistically significant differences in dietary patterns between individuals with normal BP and EBP-HTN in the OW-OB groups, particularly regarding salt intake, which was high in both groups, exceeding 3000 mg per day. This finding challenges previous reports suggesting that salt intake is a significant factor for HTN in children and adolescents [31]. However, salt intake is likely to be associated with a higher prevalence of HTN in this study than global prevalence. Salt intake was higher in both the normal BP and EBP-HTN groups with OW-OB than the mean salt intake of 2,840 mg/day observed in individuals aged 4–17 years in the US NHANES from 2003 to 2016 [32]. In the US, the prevalence of HTN has been shown to decrease after a low-salt diet [21]. The findings of these studies indicate that the elevated prevalence of HTN in children and adolescents in Korea is likely attributable to elevated levels of salt intake, and the importance of a low-salt diet should be emphasised in Korea.
Furthermore, in our study, the nadir of BP was observed at approximately 10 years of age. Subsequently, both SBP and DBP progressively increased throughout adolescence. This pattern suggests that puberty-related physiological changes, including growth acceleration, hormonal shifts, and increased body mass, may contribute to the upward trend in BP with age. Monitoring this age-related BP trajectory is essential in the early identification and management of EBP in children and adolescents.
Abdominal obesity is a well-known risk factor for cardiometabolic disease development as it reflects insulin resistance and ectopic fat accumulation, leading to metabolic syndrome and diabetes [33]. However, the potential of overall adiposity, typically assessed by BMI, or central adiposity, measured by indices including WC, waist-to-hip ratio (WHR), and WHtR, in better predicting HTN development, remains controversial [34,35,36,37]. A meta-analysis of ≥ 2.3 million participants from 57 prospective cohort studies revealed that increments in various obesity indices, including BMI, WC, WHR, and WHtR, were associated with a one- to two-fold increased HTN risk [38]. In paediatric populations, central obesity has been closely associated with increased cardiovascular risk [39]. Conventionally, WC has been used as an abdominal obesity marker; however, age- and sex-specific percentiles are needed, and its accuracy may be influenced by growth-related changes in height during adolescence. In contrast, WHtR has several advantages in growing children: it adjusts for height and applies a uniform cut-off (≥ 0.5) across ages and sexes [40,41,42,43]. While some studies suggested that WC may be better for identifying trunk fat mass in children [44, 45], the overall data, particularly from large-scale pediatric studies, supports WHtR use as a simple, reliable, and effective tool for estimating abdominal obesity in children with consistently high diagnostic accuracy [35, 40,41,42,43, 46]. For instance, a recent meta-analysis involving ≥ 180,000 children and adolescents confirmed that a WHtR cut-off of approximately 0.49 provides excellent diagnostic accuracy for identifying abdominal obesity [40].
In our study, the presence of abdominal obesity, represented by WHtR ≥ 0.5, was a significant influencing factor for EBP-HTN in OW-OB children. We divided the group into subgroups according to the presence of abdominal obesity and analysed factors predicting EBP-HTN. Male sex, age, and severity of obesity, represented by higher BMI Z scores, were significant in both groups, correlating well with the results of previous studies [47].
However, our study provides new evidence in children with OW-OB, the risk factors for EBP-HTN risk factors differ according to abdominal obesity status. In the group without abdominal obesity, paternal history of HTN and hypercholesterolemia, in addition to the aforementioned factors, emerged as significant risk factors for EBP-HTN. Several studies have indicated that parental HTN is associated with an increased risk of cardiometabolic disorders in adolescents [48, 49]. Yoo et al. demonstrated that parental HTN was associated with an increased risk of overweight, obesity, abdominal obesity, and higher BP in children [47]. Zhao et al. found that the prevalence of HTN was higher in both obese and healthy individuals with a family history of HTN, indicating that a family history of HTN is an important factor for developing HTN in adolescence [49]. The potential role of genetic factors as important mechanisms should be considered. Genetic contributions to HTN are estimated to be between 30% and 60% [50]. A family history of HTN encompasses at least two influencing factors—genetic and environmental—both of which are influenced by lifestyle and socio-economic status. These findings highlight the need for differentiated risk assessment and surveillance strategies in children with obesity, considering abdominal obesity status. Our study is insufficient to establish a causal relationship with genetic factors due to its cross-sectional design. Therefore, further research in this area is necessary.
This study has some limitations. First, abdominal obesity was defined solely by WHtR due to the lack of WHR and WC percentile data, likely limiting a more comprehensive assessment. Second, BP was measured thrice during a single visit, not across multiple visits as recommended, potentially leading to HTN prevalence overestimation due to transient elevations or white-coat effects. Third, we could not exclude participants with diagnosed HTN, antihypertensive medication use, or endocrine disorders, likely influencing the results. Fourth, potential confounding factors, including physical activity and diet, were not available in the dataset. Although physical activity has been reported to affect both obesity and BP, its omission may have introduced residual confounding, though its impact may be partially mediated through body weight and fat distribution. Lastly, exclusions based on missing data or congenital heart disease may have introduced selection bias, although these criteria were necessary to ensure data validity. Despite these limitations, this study’s strengths include the use of a large, nationally representative sample and the identification of differential risk factors for HTN according to the presence or absence of abdominal obesity, providing valuable insights for targeted prevention strategies.
In conclusion, according to KNHANES data, OW-OB and EBP-HTN increased in 2019–2021 compared to 2007–2009. EBP and HTN were more common in the OW-OB group, with obesity severity, male sex, paternal HTN, and older age identified as key associated factors. Risk factors differed by abdominal obesity status, with obesity-related factors being more influential in children with abdominal obesity, while genetic factors, including paternal HTN and hypercholesterolemia, were more important in those without abdominal obesity. These findings suggest that abdominal obesity status could be an important factor to consider when assessing HTN risk in children with obesity and may help inform more individualized surveillance and prevention strategies in this high-risk population. Further longitudinal studies are warranted to confirm these associations.
The datasets used during the current study are publicly available from the Korea National Health and Nutrition Examination Survey repository, https://knhanes.kdca.go.kr/.
- BMI:
-
Body Mass Index
- BP:
-
Blood Pressure
- CDC:
-
Centers for Disease Control and Prevention
- DBP:
-
Diastolic Blood Pressure
- EBP:
-
Elevated Blood Pressure
- FBG:
-
Fasting Blood Glucose
- HDL:
-
High-Density Lipoprotein Cholesterol
- HbA1C:
-
Glycosylated Haemoglobin A1C
- HTN:
-
Hypertension
- IRB:
-
Institutional Review Board
- KNHANES:
-
Korea National Health and Nutrition Examination Survey
- LDL:
-
Low-Density Lipoprotein Cholesterol
- OW-OB:
-
Overweight and Obese
- SBP:
-
Systolic Blood Pressure
- SE:
-
Standard Error
- SNS:
-
Sympathetic Nervous System
- TC:
-
Total Cholesterol
- TG:
-
Triglycerides
- WC:
-
Waist Circumference
- WHR:
-
Waist-to-Hip Ratio
- WHtR:
-
Waist-to-Height Ratio
This research did not receive any financial support.
Informed consent was obtained from all participants and from parents or legal guardians for children under 18 years of age. The protocol of the KNHANES was approved by the Institutional Review Board of the Korea Centers for Disease Control and Prevention (2007-02CON-04-P, 2008-04EXP-01-C, 2009-01CON-03–2 C, 2010-02CON-21-C, 2011-02CON-06-C, 2012-01EXP-01–2 C, 2013-07CON-03–4 C, 2013-12EXP-03–5 C). The study followed guidelines stated in the declaration of Helsinki. This study was approved by the Institutional Review Board of CHA University Hospital (IRB No. 2021-12-002).
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Jeong, S.I., Kim, HR. & Kim, S.H. Different risk factors for elevated blood pressure according to abdominal obesity in overweight children and adolescents. BMC Pediatr 25, 278 (2025). https://doi.org/10.1186/s12887-025-05634-4