Archives of Public Health volume 83, Article number: 174 (2025) Cite this article
Osteoarthritis (OA), a leading cause of disability worldwide, is increasingly recognized for its systemic impact. Despite its prevalence, the age-dependent effects of OA remain underexplored, particularly regarding its association with comorbidities across the life course.
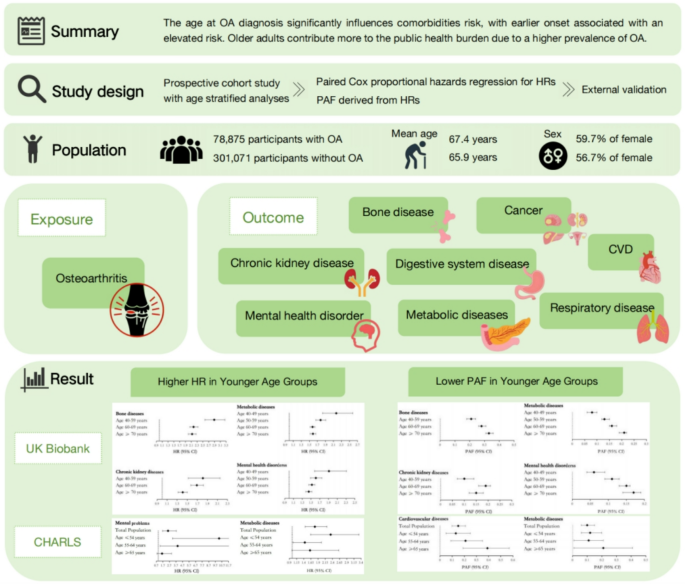
Using the UK Biobank (78,825 incident OA cases; 301,071 age-/sex-matched controls; age range: 40–86 years) and the CHARLS cohort (China, 5,735 participants, age range: 45–92 years), we employed Cox models with age as the time scale to estimate hazard ratios (HRs) for OA-associated comorbidities, including bone diseases, metabolic disorders, and mental health conditions, stratified by age at diagnosis. Analyses adjusted for sociodemographic factors and population-attributable fractions (PAFs) were calculated to quantify preventable burdens.
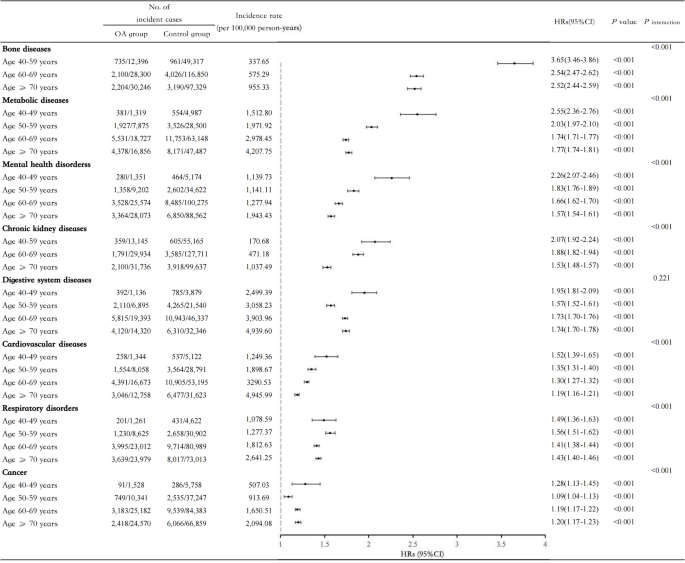
OA was associated with significantly increased risks across multiple comorbidities. All comorbidities exhibited an age-dependent gradient: relative risks were highest in younger individuals and declined with age, while absolute risks increased due to OA’s higher prevalence in older populations. For example, HRs for metabolic disorders declined from 2.55 (95% CI: 2.36–2.76) in those aged 40–49 to 1.77 (95% CI: 1.74–1.81) in those aged ≥ 70. Validation in CHARLS confirmed these patterns.
The systemic effects of OA vary substantially by age at onset. Younger individuals face elevated proportional risks likely shaped by behavioral, occupational, and structural factors, while older adults bear a larger absolute burden. These findings underscore the need for age-sensitive strategies to reduce long-term health consequences of OA and promote healthy aging.
Text box 1. Contributions to the literature |
|---|
• Osteoarthritis (OA) diagnosed at younger ages may have different long-term health implications, yet this dimension is often overlooked in public health research. |
• This study provides new evidence on how age at OA diagnosis shapes comorbidity risks using data from large population-based cohorts in both the UK and China. |
• Findings reveal that early-onset OA disproportionately affects disadvantaged groups and contributes to widening health disparities. |
• Results support more equitable and age-tailored chronic disease prevention and management strategies for OA across diverse populations. |
Osteoarthritis (OA) is a major global health challenge and the most prevalent form of arthritis, significantly contributing to disability and reduced quality of life worldwide [1]. In 2020, OA affected approximately 595 million people, and its prevalence is projected to reach 11% of the global population by 2050 [2]. Although traditionally viewed as a degenerative joint disease primarily affecting older adults, the rising prevalence of early-onset OA has emerged as a critical concern. Recent studies indicate that early-onset OA, defined as OA diagnosed before the age of 55, now accounts for an increasing proportion of cases, with substantial implications for long-term health outcomes and economic costs [3].
Patients diagnosed at younger ages endure prolonged exposure to chronic pain, constrained mobility, and mental health challenges, all of which significantly impair quality of life, social productivity, and overall well-being [ 3, 4 ]. Emerging data highlight a doubling of early-onset OA incidence over the past two decades, with 52% of newly diagnosed cases in 2019 occurring in individuals under the age of 55 [ 3 ]. This demographic shift is likely driven by lifestyle factors, including rising rates of obesity, sedentary behavior, and repetitive joint trauma [ 5, 6 ]. Early-onset OA accounted for an estimated $46 billion in healthcare expenditures and $61 billion in productivity losses in 2019 alone [ 3 ]. These figures underscore the significant personal and societal costs associated with early-onset OA, raising urgent questions about its broader health impacts.
Beyond its direct effects on joint function, OA has been linked to an elevated risk of comorbid conditions, including cardiovascular disease (CVD), diabetes, and mental health disorders. Notably, up to 67% of OA patients report at least one comorbidity, a prevalence far exceeding that of the general population [7, 8]. Constrained mobility and reduced physical activity caused by OA are well-recognized risk factors for many systemic diseases [9]. While evidence on OA-associated comorbidities has primarily focused on older adults, younger individuals with early-onset OA may face unique and heightened risks. These patients experience longer disease durations and earlier exposure to harmful factors that contribute to the development and progression of OA, such as systemic inflammation, obesity, and metabolic disorders—factors that are also associated with an increased risk of subsequent comorbidities [10, 11]. Moreover, they are more likely to experience OA-related anxiety, depression, and reduced physical activity due to fear of joint pain at an earlier stage [12]. These combined factors may further exacerbate their vulnerability to chronic diseases and increase the risk of premature mortality [13, 14].
Despite these observations, the age-dependent effects of OA on comorbidities remain poorly understood. Current studies offer conflicting findings, with some suggesting that younger OA patients face disproportionately higher risks of specific comorbidities, while others report no significant differences [15,16,17]. These discrepancies may stem from several methodological challenges, including differences in cohort selection (e.g., clinical vs. population-based samples), reliance on self-reported diagnoses or varying clinical definitions, inconsistent classification of age at onset, and incomplete adjustment for socioeconomic or behavioral confounders. Heterogeneity in healthcare systems, occupational exposures, and access to care across populations may further obscure true age-related patterns. These limitations highlight the complexity of disentangling age-specific risks and underscore the need for harmonized, large-scale analyses across diverse settings. Critically, few studies have explicitly examined how age at OA diagnosis modifies the risk of comorbidities and mortality, leaving a significant knowledge gap. Addressing this gap is essential for developing targeted prevention and management strategies tailored to the unique needs of different age groups.
To better contextualize this gap, several hypotheses have been proposed to explain why early-onset OA may confer a greater burden of comorbidities. Biologically, early-onset OA may represent a more aggressive phenotype marked by prolonged systemic inflammation and early metabolic dysfunction, both implicated in the development of cardiovascular and endocrine disorders [11, 18]. Behaviorally, younger OA patients may be more likely to engage in physical inactivity or adopt maladaptive coping strategies—such as poor diet or substance use—in response to pain and functional limitations [12]. Occupational exposures may also contribute, as early-onset OA is more prevalent in individuals with physically demanding jobs, who may face increased joint stress and reduced access to healthcare [19]. Psychosocial stressors, including employment disruption, financial strain, and social isolation, may further elevate both mental and physical health risks [12]. These interconnected factors highlight the need for a clearer understanding of age-related patterns of risk in OA populations.
To this end, this study aims to investigate whether the age at OA diagnosis modifies the risk of developing comorbidities, using data from two large and demographically diverse population-based cohorts: 379,896 middle-aged and older adults from the UK Biobank (UKB) and 5,735 adults from the China Health and Retirement Longitudinal Study (CHARLS). These cohorts differ substantially in socioeconomic structures, healthcare access, and occupational exposures, offering a unique opportunity to assess whether age-related patterns in OA-associated risks are consistent across distinct populations. By providing robust evidence on the age-dependent patterns of disease risk in OA patients, our findings may inform strategies to mitigate the systemic health burdens of OA across age groups.
This study employed a retrospective matched cohort design with long-term follow-up to explore the associations between OA and various health outcomes. The primary analysis utilized the UKB cohort, while external validation was conducted using the CHARLS, facilitating the generalization of findings across different populations. To ensure transparent and comprehensive reporting, this study adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROE) guidelines for cohort studies.
The UKB is a large-scale population-based study that recruited over 500,000 participants aged 38–69 between 2006 and 2010 [20]. CHARLS, a nationally representative cohort, enrolled adults aged 45 and above to study aging-related health outcomes in China [21]. Participants from both cohorts provided written informed consent, and ethical approvals were obtained (UK Biobank: North West Multicentre Research Ethics Committee [REC reference for UK Biobank 11/NW/0382]; CHARLS: Peking University Biomedical Ethics Review Committee [IRB00001052-11015]).
For both populations, individuals with a prior OA diagnosis, pre-existing outcome diseases were excluded; however, these exclusions were applied only when analyzing the corresponding specific outcomes. Incident OA cases were identified after the initial cohort baseline (enrollment period). Each OA participant was matched to four non-OA controls based on birth year and gender, ensuring comparability between groups. For OA cases, the date of diagnosis was defined as their baseline, while matched controls were assigned the same baseline date as their corresponding OA cases. In the UKB, the final study population included 78,825 OA cases and 301,071 matched controls, representing the cohort before exclusions for specific outcomes. For CHARLS, the population was similarly matched, adhering to the same criteria, comprising 1,147 OA cases and 4,588 matched controls (Supplemental Fig. 1).
Participants were followed from baseline to the first occurrence of an outcome disease, death, loss to follow-up, or the study end date. The follow-up periods varied depending on cohort-specific data availability: UKB: Follow-up ended on October 31, 2022 (England), August 31, 2022 (Scotland), or May 31, 2022 (Wales); CHARLS: Follow-up ended with the latest available wave of data collection (31 December 2018).
OA was identified using hospital inpatient records from the UKB, including data from Hospital Episode Statistics (England), Morbidity Records (Scotland), and the Patient Episode Database (Wales). These records were supplemented by death register data (NHS Digital, NHS Central Register, and National Records of Scotland). Incident OA cases were defined based on International Classification of Diseases 10th Revision (ICD-10) codes M15-M19. Only participants with OA diagnosed after baseline were included as exposure cases in this study.
In the external validation cohort (CHARLS), arthritis was identified through self-reported physician diagnoses during follow-up. The self-reported cases were validated against available medical documentation when possible, ensuring consistency and reliability.
Outcome diseases were selected based on their clinical relevance, public health impact, prevalence, and guidance from prior literature and expert opinion [14, 22]. These selections reflect conditions with established associations with OA and broader implications for public health. The following disease categories were identified as outcomes of interest: Bone diseases (ICD-10 codes M80–M94), Cancer (C00–C97), Cardiovascular diseases (I00–I79), Chronic kidney diseases (N18), Digestive system diseases (K00–K95), Mental health disorders (F00–F98), Metabolic diseases (E00–E90), Respiratory diseases (J00–J99). For the UKB cohort, outcome data were obtained from hospital inpatient records. Incident cases were defined as the earliest recorded diagnosis of any of the specified diseases following baseline. In the CHARLS cohort, 7 disease outcomes, such as CVD, digestive system diseases, kidney diseases, and mental problems, were ascertained based on self-reported diagnoses during follow-up. Medical documentation was used to validate these cases whenever possible. A detailed definition and code of the included outcome diseases of interest was reported in Supplemental Tables 1 and Table 2.
We included a range of covariates in our analyses, based on prior evidence, expert opinion, and their relevance to the study’s objectives [23,24,25,26]. These covariates were selected to account for demographic, socioeconomic, clinical, and lifestyle factors, and their potential confounding effects. The covariates were defined consistently across both the primary cohort (UKB) and the external validation cohort (CHARLS), with some cohort-specific adjustments.
Demographic characteristics
The demographic characteristics analyzed in this study included age at baseline, sex assigned at birth, ethnicity, heavy physical work, educational level, Townsend Deprivation Index (TDI), and residential area. Age at baseline was determined based on the year of birth, with the date of OA onset serving as the baseline for OA patients. To ensure comparability between the OA and non-OA groups, sex and age were matched at a 1:4 ratio using the MatchIt package in R. Sex was categorized as male or female. Ethnicity was classified into two groups: White and non-White. Heavy physical work was divided into two groups: with and without heavy physical work. Educational level was categorized as college/university degree versus other. The TDI is an established area-level measure of material deprivation derived from census data. It combines four standardized variables—households without a car, overcrowded households, non-owner-occupied households, and unemployment rates—to generate a composite score reflecting socioeconomic deprivation [27]. Positive TDI values indicate higher deprivation, while negative values indicate relative affluence. Residential area referred to the participants’ reported area of residence, with 27 predefined options including locations such as ‘Barts,’ ‘Birmingham,’ and ‘Bristol.’
Clinical covariates
Clinical covariates included Body Mass Index (BMI), joint diseases, joint pain, Charlson Comorbidity Index (CCI), medication use, biomarkers, and hand grip strength. BMI was calculated from weight (kg) and height (m²). Joint diseases were classified as present or absent based on medical records from baseline. Joint pain was a self-reported variable categorized as present or absent. The CCI was calculated using the Comorbidity R package and included chronic conditions such as stroke, diabetes, and heart disease. Information regarding the use of Nonsteroidal Antiinflammatory Drugs (NSAIDs), lipid-lowering medications, and vitamin supplements was self-reported and categorized as yes or no. Triglyceride and cholesterol levels were included as covariates, with values adjusted for Fasting time. Finally, hand grip strength was measured in kilograms using a standardized procedure.
Lifestyle covariates
Lifestyle factors were used to create a healthy lifestyle score, with higher scores reflecting healthier behaviors. Seven modifiable lifestyle factors were considered: smoking, physical activity, alcohol consumption, diet, sleep duration, sedentary behavior, and social connection [28, 29]. A healthy lifestyle was defined as follows: smoking (non-smokers were assigned a score of 1); physical activity (≥ 150 min of moderate activity per week or 75 min of vigorous activity per week); alcohol consumption (≤ 14 units per week); diet (adhering to at least 50% of American Heart Association dietary recommendations); sleep duration (7–8 h per night); sedentary behavior (< 4 h per day); social connection (non-socially isolated individuals). Each factor was scored as either 0 (unhealthy) or 1 (healthy), resulting in a total score ranging from 0 to 7. Higher scores correspond to healthier lifestyles.
For the external validation cohort (CHARLS), given the differences in data availability, the covariates adjusted for in the validation analysis included education, smoking, alcohol consumption, sleep, joint pain, mobility difficulties, hand grip strength, and BMI. These covariates were selected to align with those in the primary cohort and reflect the available data within CHARLS. A detailed definitions of these covariates, along with their corresponding UKB data fields, are provided in Supplemental Tables 1 and Table 2.
We began by examining baseline characteristics, presenting continuous variables as means with standard deviations (SDs) and categorical variables as proportions. We used multiple imputations by chained equations implemented via the mice package in R to impute missing values for covariates. The imputation model included all covariates as predictors, with 5 imputations performed and the results pooled across datasets using Rubin’s rules to obtain the final effect estimates [30]. Participants were grouped into four baseline age categories (40–49, 50–59, 60–69, and ≥ 70 years) to facilitate stratified analyses. Incidence densities for each disease outcome were calculated as the number of events per total person-years of follow-up within each age group.
To evaluate the association between OA and various disease outcomes, we employed paired Cox proportional hazards regression models. These models utilized baseline age and follow-up time as time scales [31] and incorporated a strata statement to account for matched pairs, allowing for heterogeneity in baseline hazards [32]. Adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated, controlling for confounders, including ethnicity (white or non-White), residential area (England, Scotland, or Wales), heavy physical work (yes or no), educational level (college/university or other), TDI, healthy lifestyle score, BMI, CCI, joint diseases (yes or no), joint pain (yes or no), the use of NSAIDs (yes or no), the use of lipid-lowering medications (yes or no), the use of vitamin supplements (yes or no), triglycerides, cholesterol, fasting time, and hand grip strength. The proportional hazards assumption was tested using weighted Schoenfeld residuals [33] and no violations were observed (p > 0.05).
We assessed potential interactions between OA and age using both multiplicative and additive models. For the multiplicative interaction, we included an interaction term (age×OA) in the Cox models. For the additive interaction, we calculated the relative excess risk due to interaction (RERI) and its 95% CI [34]. Additive hazards models were also applied to estimate the absolute differences in disease risk associated with OA across age groups, quantified as the number of additional cases attributable to OA.
To explore the potential public health impact, we calculated the population-attributable fraction (PAF) [35] estimating the proportion of disease outcomes preventable if OA were eliminated. PAFs were derived using the formula \(\:PAF=\frac{P\ast\:(HR-1)}{p\ast\:\left(HR-1\right)+1}\), where P represents the population prevalence of OA and HR represents the age-specific HR for each outcome. Nationally representative prevalence data from the Global Health Data Exchange were incorporated for age-specific PAF estimates.
We performed an array of sensitivity analyses to test the robustness of our findings. First, we replaced composite measures of healthy lifestyle scores with individual factors, mutually adjusting for these variables. Second, we excluded outcomes occurring within the first two years of follow-up to minimize reverse causality [36]. Third, we employed the Fine-Gray sub-distribution hazard model to examine the association between OA and the occurrence of its comorbidities, treating all-cause mortality as a competing risk [37]. Finally, after excluding all study subjects with missing data, the results were reanalyzed. All analyses were conducted using R software (version 4.4.1). Statistical significance was defined as a two-sided p-value < 0.05.
Our analysis included 379,896 participants from the UKB, with 78,825 diagnosed with OA after a median follow-up of 6.59 years. Participants who developed OA were older (67.4 vs. 65.9 years), more likely to be female subject, had higher BMI, lower educational level, and poorer health metrics (e.g., lower hand grip strength and higher CCI). OA patients also had a higher prevalence of joint pain, joint-related diseases, and greater use of lipid-lowering drugs and NSAIDs (Table 1 and Supplemental Table 3).
OA was associated with a significantly increased risk of comorbidities, including bone diseases (HR 2.67, 95% CI 2.62–2.72), metabolic diseases (HR 1.82, 95% CI 1.80–1.84), chronic kidney disease (CKD) (HR 1.82, 95% CI 1.78–1.86), digestive system diseases (HR 1.71, 95% CI 1.69–1.73), and mental health disorders (HR 1.67, 95% CI 1.65–1.70). These findings were consistent across multiple outcome diseases (Table 2).
The association between OA and disease risk varied by age. For metabolic disease, the HR decreased progressively with age: (HR 2.55, 95% CI 2.36–2.76) (40–49 years), (HR 2.03, 95% CI 1.97–2.10) (50–59 years), (HR 1.74, 95% CI 1.71–1.77) (60–69 years), and (HR 1.77, 95% CI 1.74–1.81) (≥ 70 years). Similar trends were observed for bone diseases, mental health disorders, CKD, CVD, and respiratory disorders. However, no age-dependent pattern was found for cancer or digestive system diseases (Fig. 1). Sensitivity analyses confirmed the robustness of these findings (Supplemental Tables 4–7). The PAF was higher in older age groups, reflecting the higher OA prevalence (Supplemental Table 8). While we did not find evidence of a significant additive interaction between OA and age (Supplemental Table 9), the relative risk of comorbidities increased with age. For instance, in the ≥ 70 years age group, OA was associated with an additional 16 cases of metabolic diseases per 100,000 person-years (Supplemental Table 10).
We externally validated these findings using data from the CHARLS cohort, comprising 5,735 participants after excluding those with pre-existing arthritis. Consistent with the UKB cohort, arthritis was positively associated with most comorbidities. For instance, notable associations were observed for digestive diseases (HR 3.17, 95% CI 2.78–3.61), mental health disorders (HR 2.89, 95% CI 2.41–3.47), and cardiovascular diseases (HR 2.68, 95% CI 2.40–2.99). Associations were strongest in younger age groups, with the highest HR observed in the ≤ 54 years age group, progressively declining in older age groups (Supplemental Table 11). These findings corroborate the age-modified gradient initially identified in the UKB cohort (Supplemental Tables 11–13).
This large cohort study revealed robust associations between OA and a diverse spectrum of comorbidities, including CVD, CKD, bone diseases, and metabolic disorders. Importantly, these associations displayed a clear age-dependent gradient: younger individuals with OA exhibited the highest relative risks, reflecting OA’s early systemic impact, while older individuals experienced a greater absolute risk due to OA’s higher prevalence in later life. This dual pattern underscores OA’s distinct clinical and public health implications across the lifespan, emphasizing the need for tailored prevention and management strategies.
Our study advances the field by providing a nuanced, age-stratified analysis that highlights the evolving impact of OA across the life course. While previous studies broadly characterized OA’s association with chronic diseases such as diabetes, thromboembolic disease, and osteoporosis [14, 38] they often used coarse age groupings that obscured finer patterns. By examining risks continuously across age strata, we revealed a striking gradient: younger individuals face disproportionately high relative risks, likely driven by the early and systemic effects of OA, while older populations experience a cumulative absolute burden owing to OA’s widespread prevalence. These findings not only confirm OA’s systemic nature but also redefine its role as a multisystem disease with far-reaching implications beyond joint health.
Notably, our analysis reconciles the seemingly paradoxical trends of declining HRs and increasing PAFs with age. While relative risks decrease in older adults—potentially due to competing risks or attenuated systemic effects—the absolute burden of OA grows, driven by its prevalence and contribution to multimorbidity. This divergence underscores the dual challenge posed by OA: younger individuals require interventions to mitigate systemic risks early, while older adults need comprehensive strategies to manage the cumulative burden of comorbidities.
The external validation of these findings in the CHARLS cohort further reinforces their robustness and generalizability. Despite differences in population characteristics such as socioeconomic status and healthcare access, the observed age-modified gradient persisted. This consistency across cohorts highlights OA’s systemic impact across diverse contexts, strengthening confidence in our conclusions. Moreover, the validation underscores the relevance of these findings for varied populations and healthcare systems, making a compelling case for integrating OA management into broader public health strategies.
The clinical implications of these findings are profound. For younger individuals, the elevated relative risks of systemic comorbidities observed in this study suggest the importance of early identification and management strategies. While further research is needed to establish causality and intervention efficacy, targeted screening for conditions such as CVD and metabolic syndrome may be beneficial in this population. Encouraging joint-friendly physical activity and promoting lifestyle modifications that reduce metabolic risks could complement these efforts. For older adults, the higher absolute burden of comorbidities highlights the need for integrated care approaches that address both OA and its associated conditions. Multidisciplinary management strategies focused on pain relief, mobility enhancement, and mental health support are likely to improve quality of life and functional independence. Evidence from existing literature suggests that interventions such as physical therapy, tailored exercise programs, and fall prevention strategies may be particularly relevant for this group [39, 40].
These age-specific considerations underscore the need for a deeper understanding of the mechanisms linking OA to systemic health outcomes. Shared pathways, including systemic inflammation, metabolic dysregulation, psychological changes, and biomechanical stress, may play a role and warrant further exploration [10, 41,42,43]. Low-grade systemic inflammation, evidenced by elevated levels of C-reactive protein and pro-inflammatory cytokines, is a well-documented contributor to OA progression and comorbidities such as cardiovascular disease and diabetes [11, 18]. Similarly, metabolic dysregulation, including obesity-driven lipid metabolism alterations and mitochondrial dysfunction, is strongly associated with both OA and conditions like metabolic syndrome and neurodegeneration [10, 44]. Psychological factors, such as depression and chronic pain-related stress, may exacerbate comorbidity risks through behavioral and neuroendocrine pathways [12]. Biomechanical stress from joint malalignment or overuse may also contribute to local and systemic effects, particularly in weight-bearing joints [45]. While all these mechanisms are plausible, the inflammation–metabolic axis is currently the most widely supported in the literature due to its consistent association with OA and multiple comorbidities across diverse populations [46, 47]. Nevertheless, the relative contributions of these pathways remain uncertain, highlighting the need for further mechanistic studies. Identifying effective therapeutic targets within these pathways could help refine interventions for both younger and older individuals with OA.
This study also highlights the importance of integrating relative and absolute risk measures into clinical and public health decision-making. While relative risks draw attention to vulnerable younger populations, absolute risks underscore the public health burden in older adults. Tailoring interventions to these complementary perspectives can maximize their impact. Public health initiatives should prioritize early diagnosis, education on OA’s systemic implications, and access to multidisciplinary care. Simultaneously, personalized care strategies that address shared risk factors across OA and its comorbidities could mitigate their collective burden.
This study has several strengths. The use of two large, diverse cohorts allowed for robust and generalizable analyses, while the age-stratified approach provided nuanced insights into the OA-comorbidity relationship. Our external validation in the CHARLS cohort confirmed the consistency of observed trends, highlighting the broad applicability of our findings across different populations. However, some limitations should be acknowledged. The UKB and CHARLS cohorts, while extensive, may not fully represent certain subgroups, particularly in terms of racial and ethnic diversity, potentially limiting the generalizability of our findings. The CHARLS data, including OA and comorbidities, are primarily based on self-reports from study participants, which introduces potential recall bias and measurement error. This reliance on self-reported data may lead to outcome misclassification, such as under- or over-reporting of OA or comorbidities, which could attenuate or exaggerate the observed associations. Additionally, the observational nature of our study precludes definitive causal inference, and residual confounding may persist despite adjustments for a range of covariates. Lastly, differences in follow-up duration between the cohorts may also influence the observed associations.
Our findings underscore the systemic and age-dependent nature of OA, establishing its role as a significant driver of multimorbidity across the lifespan. Younger individuals with OA face elevated relative risks of comorbidities, while the absolute burden is more pronounced in older populations, emphasizing OA’s dual impact on individual and public health. These results highlight the critical need for tailored, age-specific strategies in OA management, ranging from early interventions to mitigate systemic effects in younger patients to comprehensive care addressing multimorbidity in older adults. Moving forward, longitudinal studies are needed to establish causality and evaluate the long-term efficacy of interventions, such as lifestyle modifications or physical therapy programs. Additionally, biomarker-based research, such as examining inflammatory cytokines or metabolic markers, is essential to elucidate shared biological mechanisms and develop targeted therapies. These efforts, combined with assessments of intervention impacts across diverse populations, will be critical to reducing OA’s multifaceted burden and improving population-level health outcomes.
1) Data sharing: https://biobank.ndph.ox.ac.uk/showcase/index.cgi (UKB). 2) Resource sharing: https://biobank.ndph.ox.ac.uk/showcase/index.cgi (UKB). This research has been conducted using the UK Biobank Resource under Application Number 95180. 3) Data sharing: https://charls.pku.edu.cn (CHARLS).
- BMI:
-
Body mass index
- CCI:
-
Charlson comorbidity index
- CHARLS:
-
China health and retirement longitudinal study
- CI:
-
Confidence interval
- CKD:
-
Chronic kidney disease
- CVD:
-
Cardiovascular disease
- HR:
-
Hazard ratio
- ICD-10:
-
International classification of diseases 10th revision
- NSAID:
-
Nonsteroidal antiinflammatory drug
- OA:
-
Osteoarthritis
- PAF:
-
Population-attributable fraction
- RERI:
-
Relative excess risk due to interaction
- SD:
-
Standard deviation
- STROE:
-
Strengthening the reporting of observational studies in epidemiology
- TDI:
-
Townsend deprivation index
- UKB:
-
UK biobank
This research has been conducted using the UKB Resource under Application Number 95180. The authors would like to acknowledge the support of all paper participants to this paper.
This study was supported by Key Discipline of Zhejiang Province in Public Health and Preventive Medicine (First Class, Category A), Hangzhou Medical College; Natural Science Foundation of Zhejiang Province (No.LTGY23H260009), China; Zhejiang Province Key Science and Technology Plan for Medicine and Health (No. WKJ-ZJ-2333); and Zhejiang Provincial Medical and Health Science and Technology Program (Grant No. 2023KY653, 2024KY928).
UK Biobank: North West Multicentre Research Ethics Committee [REC reference for UK Biobank 11/NW/0382].
CHARLS: Peking University Biomedical Ethics Review Committee [IRB00001052-11015].
The informed consent was signed by all participants.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Ding, L., Dai, R., Jin, D. et al. Age-specific comorbidity risks in osteoarthritis: implications for healthy aging across diverse populations. Arch Public Health 83, 174 (2025). https://doi.org/10.1186/s13690-025-01670-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13690-025-01670-9