BMC Complementary Medicine and Therapies volume 25, Article number: 219 (2025) Cite this article
Although exercise is a first-line approach to treating chronic low back pain (CLBP) in clinical practice, passive techniques such as dry cupping therapy are considered complementary to this condition. However, knowledge about the effects of combining dry cupping therapy with active exercises in people with non-specific CLBP is shallow.
To evaluate the effects of dry cupping therapy with the McKenzie method (MDT) on disability and functional outcomes in people with non-specific CLBP.
Eighty-eight people with non-specific CLBP aging 18 to 59 years will be recruited and evaluated for inclusion and exclusion criteria. Participants will be randomized into intervention (MDT and dry cupping therapy) or sham group (MDT and sham dry cupping therapy). Dry cupping therapy will be applied bilaterally to the vertebrae (L1 to L5). Interventions will be performed twice a week for eight weeks, and participants will be assessed before treatment (T0), immediately after the first intervention (T1), and at four (T4) and eight weeks of intervention (T8). Primary outcomes will be disability (Oswestry Disability Index). Secondary outcomes will be functionality (Timed Up and Go Test), pain (Numeric Pain Rating Scale), trunk range of motion (Toe-touch Test) and participant expectation and perception (Global Perceived Effect Scale).
Despite recent research, there is no consensus in the literature regarding the effectiveness of dry cupping therapy in the treatment of people with non-specific CLBP. Also, no study with high methodological rigor has evaluated the combination of dry cupping therapy with active exercise in this population and whether this combination potentiates any clinical effect. Thus, this protocol may guide further research to support the prescription of exercise combined with dry cupping therapy in people with non-specific CLBP. The results of the study will be disseminated to participants through social networks and will be submitted to a peer-reviewed journal and scientific meetings.
ClinicalTrials.gov, October, 2023 (report number: NCT05459376).
• Study will evaluate the clinical effects of dry cupping therapy with exercise in people with CLBP compared with a sham group.
• Study will evaluate short-term and medium-term effects of dry cupping therapy with exercise in people with CLBP.
• Blinding physiotherapists is difficult due to the nature of the interventions.
Low back pain (LBP) is one of the most prevalent health conditions worldwide [1] and a leading cause of disability and absenteeism [2, 3]. Given the high socioeconomic and health service impact, [3, 4] clinical practice guidelines recommend active non-pharmacological modalities, such as pain education and exercise, as a first-line approach to chronic LBP (CLBP) [2, 5,6,7].
The McKenzie method of Mechanical Diagnosis and Therapy (MDT) assesses symptomatic and mechanical responses to repeated movements and sustained positions [8, 9]. In MDT, people are categorized into subgroups (syndromes), such as derangement, dysfunction, and postural [10, 11]. MDT also features the prescription of repeated exercises in a specific direction with an educational approach [8, 9, 12]. According to guidelines [13, 14], MDT is an effective technique, being within a set of recommended interventions for people with CLBP, which in turn are present better results than passive therapies in CLBP [15]. Moreover, Lam et al. [16] found moderate to high-quality evidence that MDT is better than other interventions to reduce pain and disability in people with CLBP.
Traditional Chinese medicine is a passive treatment emerging as a non-pharmacological alternative to reduce CLBP symptoms [17]. It includes mental and body practices, such as acupuncture, tai chi, and cupping therapy [18, 19], aiming to promote homeostatic, energetic, and organic integrality [20]. Cupping therapy treats health conditions [21,22,23,24], such as LBP [25, 26], and has many variations (e.g., dry, wet, massage, and flash suction cup), using different suction forces (e.g., light, medium, strong, and pulsating) and durations (e.g., from 5 to 10 min) [27, 28].
Some studies investigated the isolated short-term effects of cupping therapy on CLBP [29, 30], but little is known about its additional effects on exercise-based therapy. A study [31] evaluating the effects of cupping therapy combined with exercise on CLBP showed improved pain, function, and quality of life. However, there were important flaws in the methods of this study such as lack of blinding of participants and therapists, lack of intention-to-treat analysis and inadequate follow-up. In another study, pain and functionality improved equally in people with CLBP who received real or sham cupping therapy only without exercise [32]. The methods of this study were more robust and the authors'conclusion was that real cupping is no more effective than sham cupping for chronic low back pain. However, the additional effects of cupping in individuals who perform specific exercises for non-specific chronic low back pain are not fully known. Although some theories might explain its mechanism (e.g., gate control theory of pain, diffuse noxious inhibitory controls, and reflex zone), little is known about cupping therapy and its applicability in clinical practice [33, 34].
Currently, cupping therapy is constantly used only or combined with active exercises, however, there are still important gaps in the literature about the real effectiveness of additional effects of cupping therapy on low back pain symptoms. Therefore, this clinical trial protocol aims to evaluate the additional effects of dry cupping therapy with MDT exercises on disability, pain, trunk range of motion, and physical function of people with CLBP classified according to MDT. The non-inferiority hypothesis is that after an 8-week intervention, people with CLBP who will perform exercises with the addition of real dry cupping, showing better clinical results compared to the sham group.
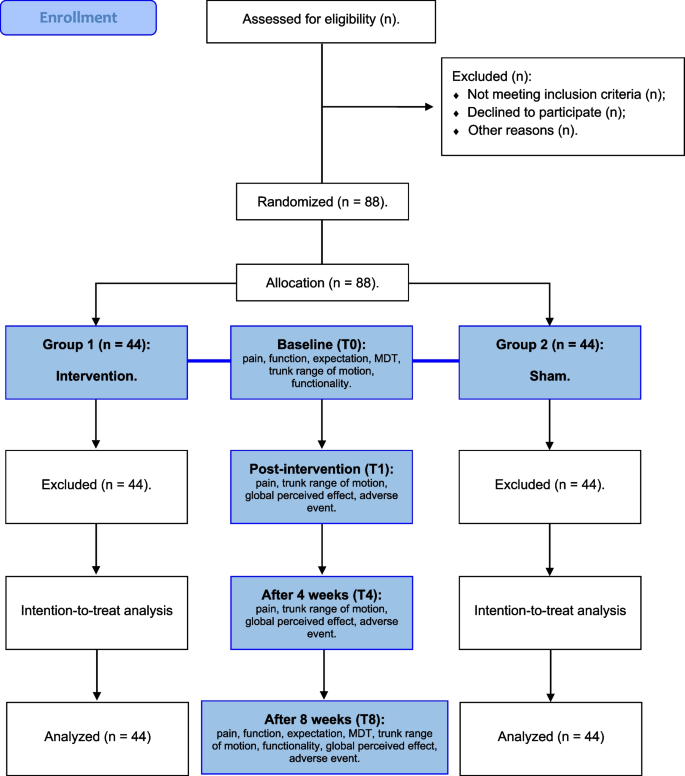
This protocol described a randomized and blinded sham-controlled clinical trial approved by the ethics committee of Onofre Lopes University Hospital of the Federal University of Rio Grande do Norte (report number: 5.345.554) and follow the checklist from the Standard Protocol Items (Fig. 1): Recommendations for International Trials (SPIRIT) [35]. The trial will be registered prospectively in Clinical Trials (ClinicalTrials.gov identifier: report number NCT05459376), and reports will follow the Consolidated Standards of Reporting Trials guidelines (CONSORT) [36], Template for Intervention Description and Replication (TIDieR) [37], and the Standards for Reporting Interventions in Clinical Trials of Cupping (STRICTOC) [38].
Participants will be recruited via social media, the communication sector, and the waiting list of the Physical Therapy Outpatient Clinic. A single evaluator will schedule first contact with participants after a phone call with the service secretary. If eligible, participants will be informed about study objectives and procedures and must sign an informed consent form.
Six researchers will be involved in the study with the following roles: (A) screening and blind assessment of participants; (B) randomization; (C) assessment, classification, and treatment with MDT; (D) application of dry cupping therapy (E) data input; and (F) statistical analysis. Supplementary file represents the research team flowchart.
After screening and baseline assessments (T0) performed by researcher A, participants will be randomly distributed into two groups: intervention (MDT and dry cupping therapy) or sham group (MDT and sham dry cupping therapy [without negative pressure on cups]). The randomization sequence will be generated by researcher B, who is not involved in the other procedures, using the sealed envelope website (https://www.sealedenvelope.com) and a simple randomization (1:1) was adopted.
Opaque, sealed, and sequentially numbered envelopes will be used to avoid allocation bias.
Researcher A will perform all assessments blinded to randomization and not involved with the interventions. Participants will be identified by numbers and informed about study objectives. Participants will undergo the same assessments and perform the interventions at different schedules to avoid contact and information exchange between groups and to assure study blinding. Assessment data will not be disclosed to the researchers responsible for the interventions (Fig. 1).
Participants will remain clothed during exercise interventions given by researcher C, who will be responsible for the classifications and interventions with MDT, possibly reducing a confirmation bias. Each participant will be asked which intervention they received to verify the blinding effectiveness. After the last assessment, researcher E will input the data delivered to researcher F, who will be responsible for statistical analysis and will not participate in the previous stages (Supplementary file).
The program will last eight weeks with 16 treatment sessions (twice a week; 30 to 40 min) of two combined interventions (exercise and dry cupping therapy). The Mckenzie method exercises are shown in Supplementary file. The interventions with the use of dry cupping are shown in Fig. 2.
Assessments and interventions will occur in the rehabilitation unit in the Laboratory of Insoles and Rheumatology of the Onofre Lopes University Hospital, starting in the January 2023 to December 2023.
Baseline assessments (T0) comprise clinical and functional aspects of the LBP: pain, functionality, participant expectations with the treatment, physical function, and trunk range of motion. Randomization will occur after T0.
Researcher C will be a physical therapist with 13 years of clinical experience and certification in levels A, B, C, D, and advanced, granted by the McKenzie Institute of Brazil. Researcher C will be responsible for assessment, classification, and treatment with MDT. Next, researcher D, a physical therapist with 18 years of clinical practice and training in integrative and complementary health practices, will be responsible for delimiting and applying the suction cups to the lower back region.
Intervention group: |
The intervention group will be assessed and classified as to the MDT syndrome and indicate the directional preference of movement (flexion, extension, or lateral displacement of the spine) (Supplementary figure). They will also receive basic pain education, guidance to perform the exercises at home, and information on how and why to exercise and possible responses to the exercise program. This group will perform MDT exercises followed by dry cupping therapy with two suctions for 10 min, twice a week, for eight weeks. Dry cupping therapy will be applied with 6 acrylic cups size 1 (4.5 cm internal diameter) with a distance of 3 cm between them, parallel to the vertebrae L1 to L5, bilaterally according to acupoint delimitations (B23, B24, and B25) [45] (Supplementary figure) |
Sham group: |
The sham group will follow the same principles of assessment, classification, and treatment with the MDT (described above). In this group, sham dry cupping therapy will be applied for 10 min, twice a week, for eight weeks. Sham dry cupping will be applied using 6 acrylic cups size 1 (4.5 cm internal diameter) with a distance of 3 cm between them, parallel to the vertebrae L1 to L5, bilaterally according to acupoint delimitations (B23, B24, and B25) [45]. However, cups will be prepared with small holes of < 2 mm [46] diameter to release the negative pressure in seconds (Supplementary figure) |
Participants will be assessed and treated following the MDT [8, 9], in which exercise protocol will be built according to medical history, physical examination, and classification into derangement, dysfunction, postural syndrome, or others [8, 9]:
Directional preference may be modified during the intervention period. Therefore, researcher C may use force alternatives or force progression in response to possible worsening or non-altered symptoms [8, 9]. Participants will register the frequency of home exercises using a control form checked by researcher C at each assessment. Participants will receive a digital book,'Treat Your Spine Yourself,'[12] with a lumbar roll if needed. All exercises and variations are described in Supplementary figure.
The application of dry cupping therapy will occur after MDT exercises. Participants will be in prone position on a comfortable stretcher and informed about the initial suction sensation, and the application site will be trichotomized to avoid interference. Also, the researcher will record any adverse effects or ecchymosis on the application site and, if needed, refer the participant to a specialized professional. The dry cupping therapy will require a manual suction pump and six Dong Yang® acrylic cups.
Despite the lack of consensus, suggestions are a suction force of 300 millibars, two suctions for musculoskeletal pain [46], and application time of 5 to 15 min [28]. Studies suggest using 10 min and suction with a 1.6 cm elevation of the subcutaneous tissue to avoid discomfort [29, 47]. Two suctions with force for 10 min are widely used in clinical practice. Therefore, this application parameter will be applied to the intervention group.
Participants will be assessed before treatment (T0), immediately after the first intervention (T1), at four weeks of intervention (T4), and eight weeks of intervention (T8) (Table 1).
Disability
The Brazilian version of the Oswestry Disability Index questionnaire will be used to evaluate disability [48, 49]. It contains ten items assessing the impact of LBP on functional activities. Values range from zero to five, with high values indicating more disability. The final result is the sum of all items. The disability will be evaluated at times T0 and T8. The Worthwhile effect is 10 to 17 points decrease in the score [50].
Functionality
Timed Up and Go Test quantifies mobility in seconds using the time to perform the task. It was primarily designed to classify fall risk in older adults as low (< 20 s), medium (20 to 29 s), and high (> 30 s) [51]. Participants are instructed to get up from a chair, walk 10 feet, turn around, and return to the chair. Participants will perform the test three times, and the average between attempts will be registered [52]. The Worthwhile effect is 3.4 s reduction in total time to complete the test [53].
Pain
Pain intensity will be measured by the Numeric Pain Rating Scale [54]. Participants will rate their pain from 0 (no pain) to 10 (the worst possible pain). The score will be collected during rest, trunk range of motion assessment, and Timed Up and Go Test. The Worthwhile effect is 2.4 points reduction in pain [50].
Trunk range of motion
The trunk range of motion will be measured using the Toe-touch Test, which has high reliability and applicability for clinical practice and scientific studies [54, 55]. Participants will be standing upright with feet together and asked to lean forward as far as possible, keeping knees, arms, and fingers fully extended. The vertical distance between the tip of the middle finger and the floor will be measured with a measuring tape. The Worthwhile effect is 4.5 cm improvement in finger-to-floor distance [56].
Participant perception
The Brazilian version of the Global Perceived Effect Scale will assess participant self-perception of interventions. It uses a scale of 11 points ranging from a negative five (extremely worse) to a positive five (completely recovered) compared with baseline [57]. The Worthwhile effect is 2.5 points improvement in the scale [58].
Blinding
Blinding of suction sensation will be assessed by five questions about what treatment participants think they received and group allocation. Namely: 1 st, ‘Which treatment do you think you received?’ with the following three choices: real cupping treatment, sham cupping treatment or don’t know; 2nd, ‘Did you have any sensations during dry cupping?’ with a yes or no response; 3rd, ‘What sensations did you experience?’ with the following options: pressing, infl ating, painful, squeezing, relaxing, refreshing, burning, pulling or hot tingling sensations; 4 th, ‘Where was the feeling of cupping located?’ with the answers being the cupping area, a broad area around the cupping jar or the whole lower back area; 5 th, ‘How much sensation did you feel?’ using a 100-mm visual analogue scale (0 mm: not at all; 100 mm: very strong) [59].
Participant expectation
A Likert-type scale will be used to assess the expectation of participants regarding the treatment. The following question will be used:"Do you think that with the application of dry cupping therapy, you will: (1) get much worse, (2) get a little worse, (3) get neither better nor worse, (4) get a little better, or (5) get a lot better"[60].
Participants will not be involved in study design, the establishment of research questions, or recruitment. At the end of the study, results may be reported to the participants using a lecture. If the intervention shows effectiveness, it will be offered and guaranteed to the sham group.
Training will be provided for evaluation and interventions before the study to promote standardized actions. Techniques and treatment measures will be used to reach a consensus among the researchers during training.
All adverse events will be measured from the reports of the participants.
The sample size was calculated based on a non-inferiority relationship between groups. We adopted disability assessed by the Oswestry Disability Index as the primary outcome, using data from the study by Silva et al. (30). Therefore, considering a statistical power of 80%, a significance level of 0.05, an average difference of 3 points between groups, a lower limit of 10 points and an upper limit of 17 points for minimal clinically important difference and standard deviation equal to 12, we reached a sample of 38 patients per group. Considering possible sample losses, we added 15% in each group, a total sample of 44 patients per group will be required.
Statistical analyses will be performed by a blinded statistician using commercial software. Independent variables of group (intervention and sham) and time (T0, T1, T4, and T8) will be considered for each dependent variable: pain, trunk range of motion, and physical function. Kolmogorov–Smirnov test will verify data normality, and the Levene test will analyze the homogeneity of variance.
In the case of a normal distribution, an analysis of variance with a mixed design will be conducted for primary and secondary outcomes, with time as an intragroup factor and group as an intergroup factor. If data are not normally distributed, the Friedman test will be used. Interaction of time by group and intergroup and intragroup differences will be analyzed for all variables. Finally, the Bonferroni test will be used post hoc.
An intention-to-treat analysis will be applied to ensure the effects of randomization so that prognostic factors are evenly distributed among groups. A significance level of 5% and 95% confidence interval will be adopted.
This study described a protocol for a blinded and randomized sham-controlled trial to evaluate the additional effects of dry cupping therapy with the MDT on physical function, pain, and functionality of people with non-specific CLBP. Interventions will occur twice a week for eight weeks, divided into intervention and sham groups to reach the objective.
Only one study has investigated the combination between cupping therapy and active exercises in people with CLBP. However, the lack of a sham group and the non-blinding of participants hampered the analysis of real effects. Moreover, uncertainty about doses and application requires further studies. Although the combination of dry cupping therapy and exercise is widely used in clinical practice, we do not know its real impact on people with CLBP. This randomized sham-controlled trial will be the first to evaluate the effectiveness of combining dry cupping therapy with an active exercise (MDT) in people with non-specific CLBP during eight weeks of intervention.
High-quality clinical practice guidelines [6, 61] recommend education, self-management, and supervised therapeutic exercise as first-line treatment for CLBP, without specifying which exercise and following patient and therapist preferences. MDT comprises the prescription of active exercises and education and self-management promotion. Also, it focuses on repeated exercises in a specific direction with an educational component [8, 9, 12], helping people with CLBP in the short term [13, 14].
Cupping therapy is frequently used as an alternative to treat LBP. However, a recent review showed many methodological flaws in studies using dry cupping therapy, hindering the evaluation of its effectiveness on CLBP symptoms [62]. Similarly, a recent clinical trial [32] of high methodological rigor showed that the dry cupping therapy was equal to its sham regarding pain, physical function, participant perception, quality of life, and psychological symptoms of 90 people with CLBP. However, dry cupping therapy was applied alone, not representing the common clinical practice. Therefore, this protocol design aimed to evaluate the possible additional effects of dry cupping therapy combined with a widely used and recommended active exercise [14].
The dry cupping therapy will be performed with MDT [8, 9, 12] using cups with holes, according to Silva et al. [63] Cups will be positioned according to Moura et al. [64], who standardized the application of dry cupping therapy in people with CLBP. Participants will not have previous experience with dry cupping therapy or MDT exercises, and different groups will not be treated on the same day, minimizing possible risks of bias.
Previous studies had difficulties blinding participants treated with dry cupping therapy, mainly because they had previous experience with it [21, 26]. Thus, we will include participants without previous treatment with MDT or dry cupping therapy to avoid a confirmation bias. During MDT, participants of both groups will remain dressed to blind the researcher conducting the exercises.
This protocol favors a study of high methodological quality. Procedures and outcome measures were well established, with adequate randomization, previously calculated sample size, and blinding of assessors and researchers. Also, pain self-management (prescribed exercises) will be recorded during interventions with an intention-to-treat approach. Nonetheless, this study may have limitations, such as the lack of blinded researchers applying the interventions due to their nature and absence of follow-up.
Despite recent research, the literature lacks a consensus on the effectiveness of dry cupping therapy in treating people with non-specific CLBP. Also, no study with high methodological rigor has evaluated the combination of dry cupping therapy with active exercise in this population and whether this combination potentiates any clinical effect. Thus, this protocol may guide further research to help prescription exercises combined with dry cupping therapy in people with non-specific CLBP.
No datasets were generated or analysed during the current study.
- LBP:
-
Low back pain
- CLBP:
-
Chronic low back pain
- TIDieR:
-
Template for Intervention Description and Replication
- SPIRIT:
-
Standard Protocol Items: Recommendations for International Trials
- STRICTOC:
-
Standards for Reporting Interventions in Clinical Trials of Cupping
- T0:
-
Baseline evaluation
- T1:
-
Post-intervention evaluation
- T4:
-
4 Weeks follow-up
- T8:
-
8 Weeks follow-up
- MDT:
-
The Mckenzie method of Mechanical Diagnosis and Therapy
The authors thank Probatus Academic Services for providing scientific language translation, revision, and editing.
Participants were not recruited at the time of manuscript submission.
This study was partially supported by the Coordination for the Improvement of Higher Education Personnel (CAPES, code 001). Role of funding sources: The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
This study was approved by the ethics committee of Onofre Lopes University Hospital of the Federal University of Rio Grande do Norte (report number: 5.345.554). An informed consent form will be explained and signed by each individual. Procedures will be conducted according to the Declaration of Helsinki. Respect for individuals will be ensured, and autonomy will be maintained. Individuals will be informed about the aims of the study, risks and benefits, and the right to withdraw from the study at any time without explanations.
Informed consent was obtained from all subjects and/or their legal guardians for the publication of identifying information/images in an open-access online publication.
Mariana Arias Avila and André Pontes-Silva are BMC Musculoskeletal Disorders’ Editors and Reviewers. All other authors do not have any Competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Cite this article
da Silva, K.M.P., Almeida Silva, H.J., Pontes-Silva, A. et al. What are the effects of dry cupping therapy combined with the McKenzie method on clinical outcomes in chronic low back pain? A protocol for a randomized, sham-controlled trial. BMC Complement Med Ther 25, 219 (2025). https://doi.org/10.1186/s12906-025-04940-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12906-025-04940-9