Journal of Nanobiotechnology volume 23, Article number: 483 (2025) Cite this article
The emergence of SARS-CoV-2 variants has underscored the urgent need for innovative vaccine strategies that provide robust and enduring protection against diverse strains. Our study introduces the FP-HR5 nanoparticle vaccine, designed to target the highly conserved S2 subunit of the spike protein, including the fusion peptide (FP) and heptad repeats (HR1 and HR2), using a 24-mer Helicobacter pylori ferritin platform. Administered intranasally, the FP-HR5-NP vaccine elicits robust systemic and mucosal immune responses in vivo, generating high titers of FP- and HR5-specific IgG antibodies. Notably, intranasal immunization resulted in elevated levels of secretory IgA and IgG in bronchoalveolar lavage fluid (BALF) and stimulated T-cell immune responses, significantly increasing resident memory B cells (BRM) and resident memory T cells (TRM) in the lungs. In hACE2 transgenic mice, three doses of FP-HR5-NP conferred substantial protection against Delta and Omicron variant challenges, with undetectable viral RNA levels in the lungs and no pathological changes observed. Overall, the FP-HR5-NP vaccine triggers comprehensive humoral and cellular immune responses at the mucosa, providing broad defense against SARS-CoV-2 variants and positioning it as a promising candidate for a universal COVID-19 vaccine solution.
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has imposed a significant public health burden and economic loss globally [1]. SARS-CoV-2 infection begins with the entry of the virus, which depends on the intact spike (S) protein located on the surface of the virion. The S protein is functionally divided into S1 and S2 subunits, responsible for receptor binding and membrane fusion, respectively. Specifically, the S1 subunit relies on the receptor-binding domain (RBD) to interact with human angiotensin-converting enzyme 2 (ACE2) [2]. Thus, the RBD is the primary target for neutralizing antibodies (nAbs) and vaccine designs [3,4,5,6]. However, the RBD is highly prone to mutation, and new Omicron sublineages continue to emerge due to the progressive accumulation of mutations and increasing immune selective pressure [1]. Consequently, vaccine efficacy is markedly reduced in the wake of emerging variants [7, 8]. The development of variant-proof SARS-CoV-2 or Sarbecovirus vaccines is warranted.
In contrast to the massive accumulation of mutations within the RBD, the S2 subunit—including the fusion peptide (FP), two heptad repeats (HR1 and HR2), a transmembrane (TM) domain, and a cytoplasmic tail (CT) domain—has remained highly conserved among SARS-CoV-2 variants [9]. The membrane fusion mechanism mediated by the coiled-coil motif is common among viruses, such as influenza, HIV-1, and coronaviruses. After the RBD binds to human hACE2, host proteases (such as TMPRSS2 and cathepsin L) cleave at the S2 site, exposing the FP [2, 10, 11]. The FP is then inserted into the cell membrane, guiding the formation of a three-stranded coiled-coil HR1 and a helical-conformed HR2 to form a six-helix bundle (6HB). This structure stabilizes the post-fusion state of the S protein and allows the viral genome to enter the cytoplasm [12]. Recently, monoclonal nAbs targeting FP, HR1, or HR2 have been isolated from COVID-19 convalescent individuals and have shown efficacy in inhibiting viral infection [10, 11, 13,14,15,16]. These nAbs can interfere with the conformational transition of the HR domains from the fusion intermediate to the post-fusion structure of 6HB, demonstrating broad-spectrum antiviral activities against multiple SARS-CoV-2 variants, other coronaviruses [17,18,19]. Therefore, the HR1, HR2, and FP domains are promising targets for vaccine development. It’s noteworthy that previous reports suggested the challenges of targeting the fusion intermediate stage for vaccine development due to the transient and unstable nature as well as the low immunogenicity of the HR1, HR2, and FP [10, 11, 20]making it difficult to design an immunogen capable of mimicking its conformation and evoking an immune response in vivo. Thus, further investigation is warranted.
There is a growing appreciation of mobilizing mucosal immunity for prophylactic respiratory virus vaccine development [21,22,23,24]. Vaccination at mucosal surfaces can take advantage of mucosa-associated lymphoid tissues to initiate immunity programming of mucosa-specific resident lymphocytes. At these barrier tissues, antigens elicit specific resident memory T cells (TRM) and resident memory B cells (BRM) [21, 24]which produce a fast and efficient immune response when the virus enters the tissue and reduces tissue damage. Despite the advantages of mucosal immunity, delivering vaccines across mucosal barriers has been a significant challenge, including rapid antigen loss due to enzymatic degradation and acidic conditions, and lack of proper release and uptake by the antigen presenting cells across the junction of the epithelial layer [25, 26]. Most COVID-19 vaccines currently in clinical trials or use are administered intramuscularly, primarily activating systemic immunity but not adequately inducing mucosal immune response [27]. Developing technologies to overcome barriers to mucosal delivery while meeting the safety and efficacy requirements of prophylactic COVID-19 vaccines remains an unmet need.
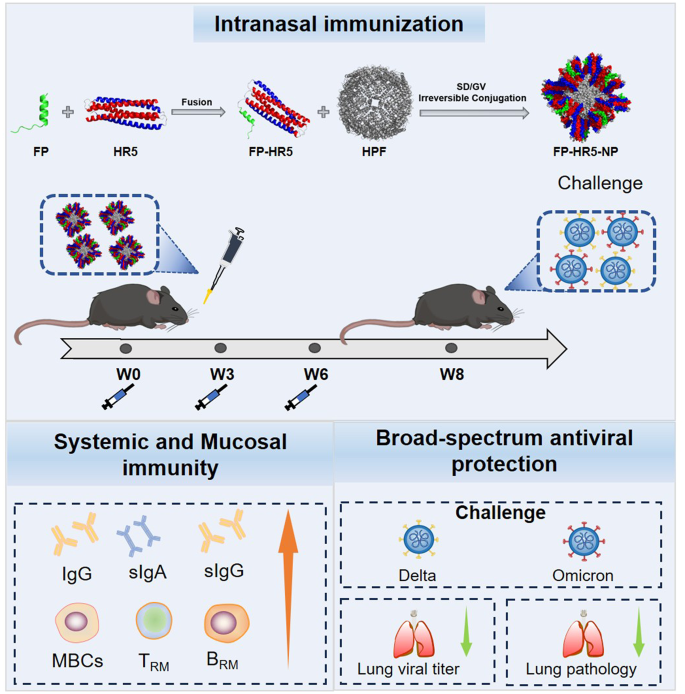
We previously developed a vaccine platform utilizing the multi-antigen displaying capability of self-assembled 24-mer Helicobacter pylori ferritin (HPF) through an antigen conjugation system [4]. Building on this platform and leveraging the properties of the 6HB structure of HR1HR2, we combined the FP with self-assembled 24-mer Helicobacter pylori ferritin to form the FP-HR5 nanoparticle vaccine. Administered intranasally in three doses, FP-HR5-NP elicited strong humoral and cellular responses and enhanced mucosal immunity in the lungs in vivo. Additionally, intranasal vaccination of the FP-HR5 nanoparticle provided significant protection against the replication of authentic Delta and Omicron strains, supporting the notion of a universal SARS-CoV-2 vaccine candidate.
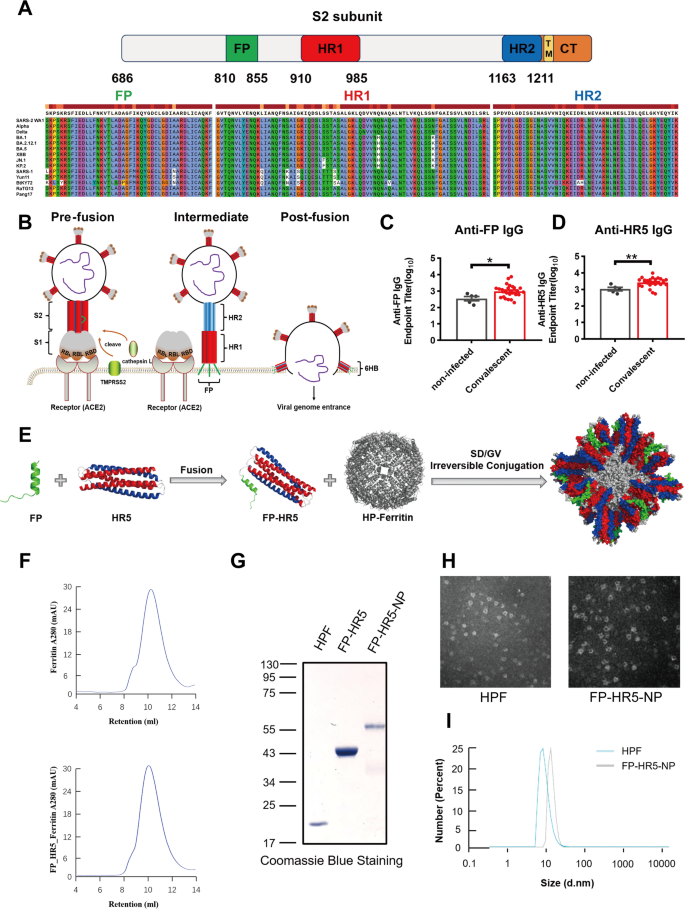
To develop variant-proof vaccines, we targeted the S2 subunit, focusing on the highly conserved FP, HR1, and HR2 across SARS-CoV-2 variants and Sarbecoviruses (Fig. 1A) [4, 11]. The sequences of FP and HR2 are conserved across all SARS-CoV-2 variants, and HR1 maintains over 96% similarity in different variants (Fig. 1A). Structural dynamics of FP and HR1 HR2 in the pre- and post-fusion states of spike trimer are illustrated in Fig. 1B [28,29,30]. In the pre-fusion stage, only a few FP peptides are spatially exposed; however, during the intermediate and post-fusion stages, FP fully protrudes and inserts into the host cell membrane. Most regions of HR1 are exposed throughout the fusion, while HR2 is gradually exposed as fusion progresses, collectively forming a 6HB structure in the post-fusion stage (Supplementary Fig. 1A and B). Neutralizing antibodies against FP could prevent viral invasion before membrane fusion is completed, and inhibiting the formation of the 6HB structure—primarily composed of HR1 and HR2—may further impede viral replication post-initial contact with host cells [16, 31]. Targeting the highly conserved and functionally crucial FP, HR1, and HR2 regions may provide broad protection against various SARS-CoV-2 variants.
Design and characteristics of the FP-HR5 nanoparticle vaccines. (A) Sequence alignment of different SARS-CoV-2 variants and Sarbecoviruses. (B) Schematic of the binding and membrane fusion process mediated by the SARS-CoV-2 spike protein. (C-D) Levels of FP- or HR5-specific IgG were measured by enzyme-linked immunosorbent assay (ELISA) in non-infected individuals (n = 5) and Omicron convalescent patients (n = 26). (E) Schematic representation of the FP-HR5 nanoparticle vaccine. (F) Size-exclusion chromatography (SEC) profiles of FP-HR5-NP and Ferritin, showing UV absorption at 280 nm. The retention volume corresponds to the nanoparticle peak. (G) Coomassie blue staining of HPF, FP-HR5, and FP-HR5-NP proteins. (H) Transmission electron microscopy (TEM) images of HPF and FP-HR5-NP. (I) Dynamic light scattering (DLS) analysis of HPF and FP-HR5-NP
Data are presented as mean (SD). Adjusted p-values were calculated using Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = not significant
FP: fusion peptide. HR: heptad repeat. SD: SdCatcher. GV: GVTag. TM: transmembrane domain. CT: cytoplasmic tail domain. HPF: Helicobacter pylori ferritin. NP: nanoparticle. TEM: negative-staining transmission electron microscopy
To mimic the fusion intermediate 6HB structure, we designed a helical pentamer (HR5) antigen by fusing three coiled-coil fragments of HR1 (residues 910–950) with two monomers of HR2 (residues 1168–1208), linked by Gly-Ser-Gly (GSG) linkers. The exposed interspace of the HR5 antigen served as an epitope in its spatial conformation. Initial assessments revealed FP and HR5-specific IgG in sera from Omicron convalescent individuals, compared with non-infected controls (Fig. 1C-D). Importantly, antibodies induced by HR1HR2 recognized the HR5 pentamer, suggesting that the HR5 antigen may elicit IgG responses similar to those from natural infection [15].
To harness the potential of both FP and HR5, we developed a nanoparticle vaccine by employing the 24-mer Helicobacter pylori ferritin vehicle via GVTag/SdCatcher covalent conjugation (Fig. 1E) [4, 6, 32, 33]. The pre-fusion segment (20 amino acids) of FP was fused to the N-terminus of HR5 (FP-HR5) to simulate a trimmed post-fusion stem of S2. An Sd fragment was ligated at the C-terminus of FP-HR5 (FP-HR5-Sd), facilitating the conjugation of these protomers onto the surface of the 24-mer ferritin via a short GV tag (GV-Ferritin) (Fig. 1E). The components were incubated in a conventional buffer to construct the FP-HR5-nanoparticle (FP-HR5-NP), which was then purified using size-exclusion chromatography (SEC) (Fig. 1F). SDS-PAGE analysis indicated successful conjugation, showing a major band between 55 and 75 kDa (Fig. 1G). The complete formation of the FP-HR5-NP was further confirmed using negative-staining transmission electron microscopy (TEM) and Zeta-potential distribution analysis (DLS) (Fig. 1H-I). Thus, we developed a new nanoparticle vaccine candidate aiming for broader protection against SARS-CoV-2 variants by displaying the S2 post-fusion structure mimicking antigens in multimers.
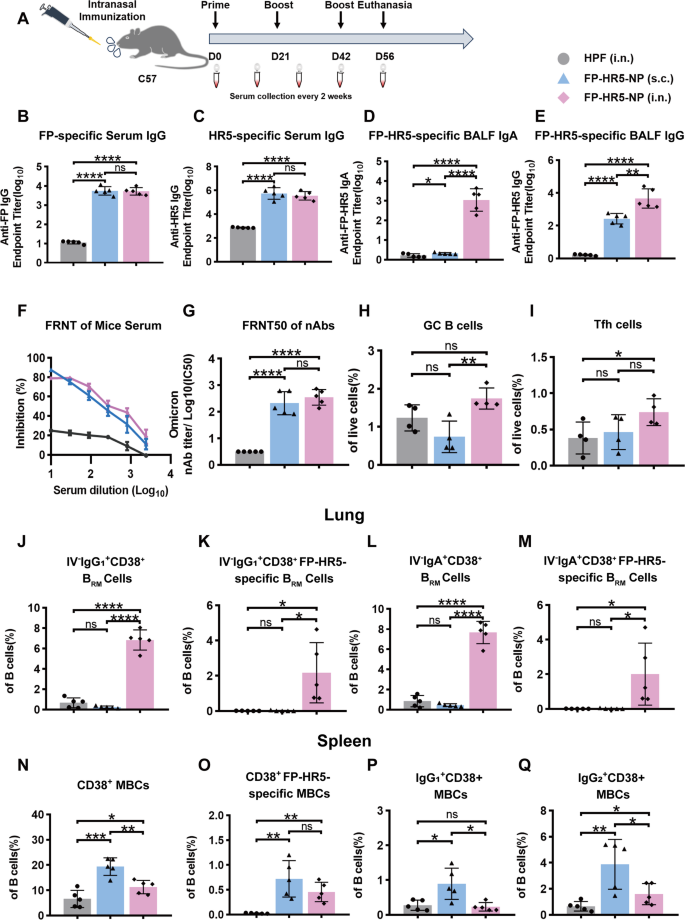
Mucosal immunity provides the first line of defense against respiratory pathogens [21]. However, the current COVID-19 vaccination strategy often induces suboptimal mucosal immunity [27]. Aiming to elicit both robust systemic and local mucosal immune responses, we first conducted intranasal immunization with FP-HR5-NP in C57BL/6 mice. Mice were intranasally (i.n.) primed with 10 µg of FP-HR5-NP at week 0, followed by booster vaccinations at weeks 3 and 6 (Fig. 2A). The control group received equivalent dosages of FP-HR5 via subcutaneous immunization to elicit systemic response or Ferritin vehicle via intranasal vaccination.
Humoral immune responses induced by FP-HR5 nanoparticle vaccines following intranasal immunization in mice. (A) Experimental schema of immunization. C57BL/6 mice were immunized with either 10 µg FP-HR5-NP intranasally or subcutaneously, while the control group received an intranasal immunization with a molar equivalent of HPF. Five mice per group were prime-boost vaccinated at weeks 0, 3, and 6. Serum was collected every two weeks, and all mice were euthanized at week 8. (B-C) FP- or HR5-specific IgG levels in serum from different vaccine groups were measured by ELISA. (D-E) FP-HR5-specific IgG and IgA levels in bronchoalveolar lavage fluid (BALF) were measured by ELISA. (F) Inhibition of viral replication by serum from different vaccine groups, with dilution factors expressed as Log10 values. (G) FRNT50 of neutralizing antibodies (nAbs) for each vaccine group was determined by Focus Reduction Neutralizing Test (FRNT) and is represented as half-maximal inhibitory concentration (IC50), which is the reciprocal of the half-maximal neutralizing dilution. (H) Percentages of germinal center B (GC B) cells (CD19+CD95+GL7+) in the mediastinal lymph nodes were measured by flow cytometry. (I) Percentages of T follicular helper (Tfh) cells (CD4+CXCR5+PD-1+) in the mediastinal lymph nodes were determined by flow cytometry. (J-M) Peripheral circulating lymphocytes were prelabeled by intravenously injected CD45 antibodies. Tissue-resident (IV labeling antibody negative) B cell subsets were measured, including IgG1+CD38+ BRM cells, IgG1+CD38+ FP-HR5-specific BRM cells, IgA+CD38+ BRM cells, and IgA+CD38+ FP-HR5-specific BRM cells in lung tissues from subcutaneous or intranasal immunization mice. (N-Q) B cell subsets were measured by flow cytometry, including CD38+ memory B cells (MBCs), CD38+ FP-HR5-specific MBCs, IgG1+CD38+ MBCs, and IgG2+CD38+ MBCs in spleen from subcutaneous or intranasal immunization mice
Data are represented as mean (SD). Adjusted p values were calculated by one-way ANOVA with Tukey’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = not significant
Humoral immunity was assessed by collecting serum every two weeks, with all mice euthanized for final analysis at week 8. Both intranasal and subcutaneous vaccinations with FP-HR5-NP induced FP- and HR5-specific IgG antibodies, which were further enhanced by subsequent boosts (Fig. 2B-C and Supplementary Fig. 2A-B). Additionally, analysis of bronchoalveolar lavage fluid (BALF) revealed high levels of FP-HR5-specific secretory IgA and IgG antibodies in the intranasal group (Fig. 2D-E). Functional assessment via the focus reduction neutralizing test (FRNT50) indicated that the antibodies elicited by intranasal and subcutaneous vaccination had comparable neutralizing capacities against authentic Omicron strains, with FRNT50 titers exceeding 2.5 × 10² and 2.3 × 10², respectively (Fig. 2F-G). Collectively, our data suggested that, regardless of immunization route, a potent systemic immune response was elicited by FP-HR5-NP, while intranasal vaccination drove stronger mucosal humoral protection.
B cell responses were evaluated following vaccination. In the mediastinal lymph nodes, the primary draining lymph nodes for lungs, intranasal immunization induced a significant increase in the proportion of germinal center B (GC B) cells and T follicular helper (Tfh) cells (Fig. 2H-I). To assess lung BRM, we administered an anti-CD45 antibody intravenously to exclude circulating lymphocytes [34,35,36]. Notably, intranasal immunization led to a significant increase in overall and antigen-specific FP-HR5- IgG1+ and IgA+ BRM in lung tissues compared to the systemic subcutaneous route (Fig. 2J-M). The intranasal immunization was able to elicit splenic overall and antigen-specific FP-HR5- memory B cell response, albeit weaker than the systemic subcutaneous route (Fig. 2N-Q). Our B cell characterization further supported the notion that intranasal immunization with FP-HR5 nanoparticle vaccines can induce potent humoral and memory B cell responses, particularly within lung mucosal tissues.
There is a growing appreciation of vaccine-induced cellular immune response to convey durable prophylactic protection [4, 10, 11]. We asked if our ferritin-based FP-HR5-NP is capable of inducing T-cell immune responses.
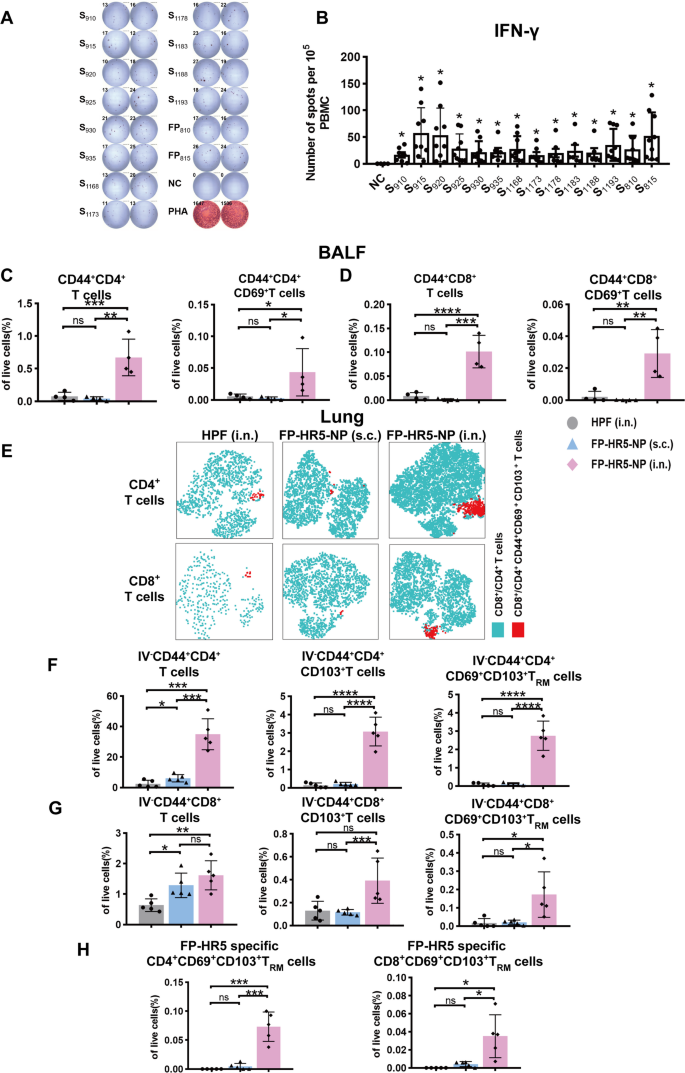
To this end, we first characterized the T-cell response against HR1, HR2, and FP in Omicron convalescent individuals to evaluate the immunogenicity of these conserved regions. A 15-mer peptide pool covering the entire HR1, HR2, and FP regions of SARS-CoV-2, with 10-amino acid overlaps each other, was synthesized for T-cell epitope screening (Supplementary Table 1). This peptide pool was designed to comprehensively cover potential T-cell epitopes within the conserved HR1, HR2, and FP regions, without restriction to specific HLA alleles, thereby allowing the detection of T-cell responses across diverse HLA types in the human population. Peripheral Blood Mononuclear Cells (PBMC) from Omicron convalescent individuals were pulsed with peptides, and IFN-γ secretion was measured to assess the T-cell response. Strong T-cell responses were observed (Fig. 3A-B), affirming the immunogenicity of the conserved HR1, HR2, and FP regions, and also suggesting the capacity of the FP-HR5-NP to stimulate cellular immune responses.
Cellular Immune Responses by FP-HR5-NP vaccines following intranasal immunization in mice. (A-B) ELISpot assays measuring IFN-γ secretion from PBMCs of Omicron convalescent individuals were performed with the addition of SARS-CoV-2 S2 peptide pools as a stimulus. (C-D) CD4+ T cell and CD8+ T cell responses from the cells collected from Bronchoalveolar Lavage Fluid (BALF). Shown are the proportion of CD44+CD4+ T cells, CD44+CD4+CD69+ T cells, CD44+CD8+ T cells, and CD44+CD8+CD69+ T cells in BALF live cells (n = 4). (E-H) Lung-tissue-resident CD8+ T cell and CD4+ T cell responses from subcutaneous or intranasal immunization with FP-HR5-NP (n = 5) were determined by Flow Cytometry. Peripheral circulating lymphocytes were prelabeled by intravenously injected CD45 antibodies. (E) tSNE plot of CD69+CD103+ TRM cells in CD4+ T cells and CD8+ T cells. (F) The proportion of IV-CD44+CD4+ T cells, IV−CD44+CD4+CD103+ T cells, and IV−CD44+CD4+CD69+CD103+ TRM cells in lung tissue. (G) The proportion of IV−CD44+CD8+ T cells, IV−CD44+CD8+CD103+ T cells, and IV−CD44+CD8+CD69+CD103+ TRM cells in lung tissue. (H) The proportion of FP-HR5-specific CD4+CD69+CD103+ TRM cells and FP-HR5 specific CD8+CD69+CD103+ TRM cells in lung tissue
Data are represented as mean (SD). Adjusted p values were calculated by one-way ANOVA with Tukey’s multiple comparison test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = not significant
Next, to evaluate if cellular immune responses in the mouse model can be stimulated by the conserved HR1, HR2, and FP regions, splenic T cells from FP-HR5-NP immunized mice were stimulated with the peptide pool, where robust IFN-γ secretion was also observed (Supplementary Fig. 3). Our data suggested that the conserved HR1, HR2, and FP regions are immunogenic in both human and mouse, despite the drastic differences between human HLA and murine MHC molecules, confirming the applicability of the mouse vaccination platform as a surrogate to evaluate the cellular immune responses elicited by the FP-HR5-NP.
Taking advantage of the mouse immunization platform, we assessed the cellular immune responses in BALF and lung tissues following either the intranasal or systemic route. In BALF, intranasal immunization led to significant increases in CD4+CD44+ and CD8+CD44+ effector T cells (Fig. 3C-D). Additionally, in lungs, higher proportion of TRM cells in lung infiltrating lymphocytes were detected in intranasal immunized mice (Fig. 3E), exhibiting notable increases in the proportion of CD69+ and/or CD103+ expressing CD4+ T and CD8+ T cells (Fig. 3F-G). Furthermore, after pulsing with the SARS-CoV-2-spike peptide library, mice immunized intranasally exhibited a significantly higher IFN-γ secretion in CD4+CD69+CD103+ TRM cells and CD8+CD69+CD103+ TRM cells in the lungs, indicating the induction of antigen-specific response in lung-resident T cells (Fig. 3H).
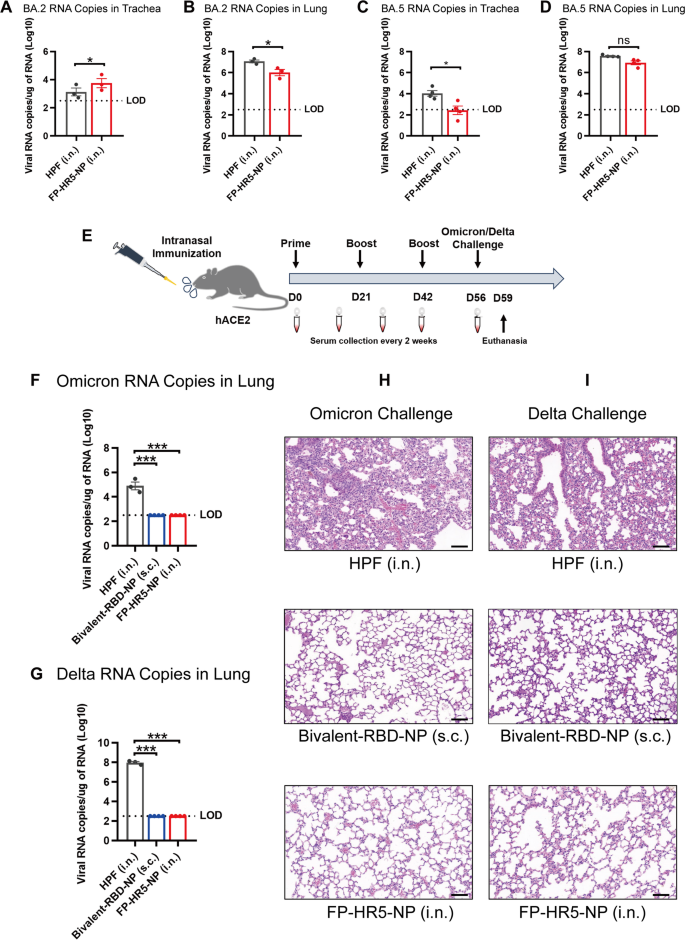
Lastly, we sought to determine the optimal vaccination regimen. We first evaluated the two-dose schedule: hACE2 transgenic mice received intranasal immunization with 10 µg FP-HR5-NP at weeks 0 and 3. Ferritin via intranasal immunization served as a control. 2 weeks after the second immunization, mice were challenged with sublethal dose (2 × 10⁴ FFU) of authentic Omicron BA.2 or BA.5 variants. The two-dose regimen fell short in effectively suppressing viral replication in both the trachea and lungs when challenged by BA.2- and BA.5 (Figs. 4A-D).
Intranasal immunization with the FP-HR5-NP vaccines protected against Delta or Omicron variant infections in hACE2 mice. (A-D) Administering two doses of the vaccine. BA.2 RNA copies in the trachea (A) and lung (B) were quantified by qRT-PCR. BA.5 RNA copies in the trachea (C) and lung (D) were quantified by qRT-PCR. (E) Immunization schedule. hACE2 transgenic mice were immunized with either 10 µg FP-HR5-NP vaccine (n = 4) or the molar equivalent of HPF (n = 3) through the intranasal route at week 0, week 3, and week 6; the bivalent BA.5(SR)/Delta-RBD nanoparticle vaccine (Bivalent-RBD-NP) was included as systemic vaccination control [32]. The vaccinated mice were challenged with authentic Delta or Omicron variants at 2 weeks after the last immunization and euthanized three days post-challenge for analysis. (F-G) Omicron (F) and Delta (G) RNA copies in the lungs were determined by qRT-PCR and plotted as log10 copies per microgram. The dotted line indicates the limit of detection (LOD). Each dot represents a tissue sample from one animal. (H-I) HE staining of Omicron (H) and Delta (I) challenge was evaluated in the lungs of mice
Data are presented as mean (SD). Adjusted p-values were calculated using Student’s t-test. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns = not significant
We proceeded with a three-dose regimen (immunizations at weeks 0, 3, and 6).
A previously reported bivalent BA.5(SR)/Delta-RBD nanoparticle vaccine (Bivalent-RBD-NP) [32]capable of eliciting potent and broad neutralizing antibodies targeting a wide range of variants, were included as systemic vaccination control. 2 weeks after the final immunization, mice were challenged with sublethal dose (2 × 10⁴ FFU) of authentic Omicron or Delta variants and assessed for immunization-mediated protection (Fig. 4E, Supplementary Fig. 4).
Unsurprisingly, Bivalent-RBD-NP immunization mediated potent protection against both Omicron and Delta challenge while ferritin vehicle immunization failed (Figs. 4F-I), in line with our previous report [32]. Notably, three-dose intranasal vaccination with FP-HR-NP provided protection comparable to systemic Bivalent-RBD-NP immunization, as both FP-HR-NP and Bivalent-RBD-NP exhibited undetectable viral RNA levels (Figs. 4F-G) and no pathological changes in the lung (Figs. 4H-I). In contrast, ferritin control group showed high viral loads and notable histopathological damage, including alveolar collapse and immune cell infiltration (Figs. 4F-I). These results highlight the FP-HR5-NP potential as an intranasal broad-spectrum defense.
The emergence of SARS-CoV-2 variants has highlighted the urgent need for innovative vaccine strategies that provide robust and lasting protection against diverse strains. Current vaccines primarily targeting the RBD or the full spike protein have shown diminished efficacy over time, particularly in light of increasing immune escape and breakthrough infections [7, 8]. Additionally, existing vaccines, which are typically administered intramuscularly, are often unable to adequately stimulate mucosal immunity—the first line of defense against respiratory pathogens [21, 27]. This underscores the necessity for a universal vaccine capable of eliciting strong systemic and mucosal immune responses to effectively combat various SARS-CoV-2 strains. Previous studies have identified conserved sites of the spike protein, specifically FP and HR1HR2, as promising targets [4, 10, 11, 13, 15, 16]. However, these regions generally exhibit low immunogenicity, making it challenging to elicit a robust immune response. To address this, we developed the universal vaccine candidate, FP-HR5-NP, which targets the conserved pre-fusion segment FP and the post-fusion structure HR5 using our ferritin nanoparticle platform [4, 6, 32].
Our study demonstrates the distinct advantages of intranasal immunization in eliciting both systemic and localized mucosal immunity against respiratory pathogens. In C57BL/6 mice, intranasal immunization generated humoral immunity comparable to subcutaneous vaccination, with similar specific IgG titers in serum and memory B cells in the spleen. The increased proportion of GC B and TFH cells in mediastinal lymph nodes suggested that intranasal immunization enhances antigen presentation and antibody production. Importantly, this route also elicited a strong local mucosal response, with higher levels of FP-HR5-specific secretory antibodies in BALF. The FRNT demonstrated that the serum antibodies effectively inhibited authentic Omicron strains, while lung tissue analysis showed that intranasal vaccination with FP-HR5-NP was superior in inducing specific IgG1+ and IgA+ resident memory B cells compared to both ferritin and subcutaneous vaccination. These findings align with emerging evidence of mucosal immunity’s pivotal role in respiratory diseases like SARS-CoV-2 infection, where both localized defense mechanisms [37, 38] and serum neutralizing capacity prove essential for comprehensive protection.
The establishment of cellular immunity against SARS-CoV-2 also plays a central role in the control of SARS-CoV-2 infection and the containment of COVID-19 severity [39, 40, 41]. Here, we demonstrated that the convalescent patients’ T cells are reactive to the conserved FP/HR1/HR2 regions, highlighting the immunogenicity of those conserved regions and justifying the rationale of adapting them into vaccine design. Of note, the intranasal vaccination elicited strong cellular immune responses in the murine model, providing a surrogate platform to evaluate the cellular immune responses. Taking advantage of this mouse model, we characterized cellular immune responses in BALF and lung via intranasal and systemic vaccination routes. Flow cytometry analysis of the cells from BALF revealed that intranasal vaccination induced a large proportion of activated CD4+ and CD8+ T cells, and stimulation with the SARS-CoV-2 spike peptide library in lung tissue resulted in prominent IFN-γ secretion levels in resident memory T cells, suggesting the formation of specific TRM against SARS-CoV-2. We demonstrated that intranasal vaccination induced potent mucosal cellular immune responses, which could synergize with the mucosal humoral immune responses to mediate potent protection.
Finally, we evaluated the protective efficacy of this vaccine by challenging hACE2 transgenic mice with the Delta and Omicron variants. Our results demonstrated that the FP-HR5-NP vaccine significantly prevented virus replication, highlighting its potential to confer immunity against different viral infections. Notably, the three-dose intranasal regimen is required to achieve adequate protection. This finding aligns with recent research that establishes durable mucosal memory requires repetitive antigen exposure at respiratory surfaces, allowing sufficient time for tissue-resident lymphocyte differentiation [42, 43].
In summary, the FP-HR5-NP intranasal vaccine represents a promising candidate for a universal COVID-19 vaccine, effectively inducing comprehensive humoral, cellular, and mucosal immune responses. By addressing both systemic and mucosal immunity, our study contributes valuable insights toward developing a more effective vaccine strategy against SARS-CoV-2 and its variants.
Given the difference between human HLA and murine MHC molecules, human HLA transgenic mice or non-human primates are warranted in future studies to more precisely map the T cell reactive epitopes in the FP-HR5-NP, and the vaccine’s efficacy in human populations. Furthermore, to comprehensively evaluate the protective efficacy of the FP-HR5-NP, additional variants of concerns should be tested. Vaccinated mice challenged with varying ranges of SARS-CoV-2 virus dosage are also warranted to better evaluate the efficacy in clinical-relevant settings.
Vero E6 cells were acquired from ATCC and cultured in DMEM supplemented with 1% penicillin-streptomycin (ThermoFisher) and 10% fetal bovine serum (ThermoFisher). FreeStyle 293 F suspension cells were cultured in Union 293 F (Union) containing 8 mM glutamine (ThermoFisher), and 1% penicillin-streptomycin (ThermoFisher) and placed on a polycarbonate vented conical flask shaker at 37 °C, 8% CO2, and a shaker at 120 rpm. All cells underwent PCR regular mycoplasma DNA testing, confirming they were mycoplasma-negative. The authentic GDPCC-nCoV-Delta-, GDPCC-nCoV-Omicron BA.2- and GDPCC-nCoV-Omicron BA.5- related experiments, which were isolated and authorized by the Guangdong Center for Disease Control, were carried out in the BSL-3 facility at Sun Yat-sen University.
hACE2 Transgenic mice (C57BL/6 background, Strain ID: T037659) were purchased from GemPharmatech Co, Ltd. Specific-pathogen-free (SPF) female C57BL/6 mice, aged 5–6 weeks, were purchased from Guangdong Medical Laboratory Animal Center. All mice were accommodated and immunized within SPF facilities at the Laboratory Animal Center of Sun Yat-sen University.
HR5, FP-HR5-Sd, and GV-Ferritin proteins were expressed and purified from Escherichia coli (E.coli). DNA sequences of 6×His-tagged HR5, FP-HR5-Sd, and GV-Ferritin were cloned into the pET28a vector. The cloned construct was transformed into BL21 (Takara), and the single clone was then amplified in LB with kanamycin at 37 °C, 220 rpm. When the bacterial solution’s OD reached 0.6, isopropyl β-D-1thiogalactopyranoside (IPTG) (Takara) was added to induce protein expression. After 18 h of induction, the protein-expressing bacteria were harvested, lysed using sonication, and then subjected to protein enrichment using Ni-NTA agarose (GE Healthcare). The His-tagged protein was eluted using Imidazole-containing Tris buffer (20 mM Tris, 50 mM NaCl, pH 7.5). The proteins were purified and concentrated, and their buffer was replaced with Tris-HCl-NaCl buffer. The concentration of HR5, FP-HR5-Sd, and GV-Ferritin proteins was determined by BCA assay. FP-HR5-Sd was genetically fused to the C-terminus of GVTag, enabling covalent conjugation to GV-Ferritin via an isopeptide bond mediated by the SdCatcher/GVTag system. Following the conjugated processes, the supernatants were applied to a Superose 6 Increase (GE Healthcare) size exclusion column and subsequently eluted using a Tris buffer at a flow rate of 0.5 mL per minute. The bacterial endotoxins in the nanoparticle were quantified by the tachypleus amebocyte lysate test (less than 10 EU/dose). All purified proteins were confirmed using SDS-PAGE gel electrophoresis, along with Coomassie blue staining.
FP-HR5-Sd and GV-Ferritin were incubated in Tris buffer at room temperature. After seventy hours, the Sd/GV conjugated proteins (FP-HR5-NP) were subjected to size-exclusion chromatography (SEC) at a rate of 0.5 mL per minute. The proteins were eluted in retention of 8 mL to 12 mL.
Transmission electron microscopy (TEM) grids of FP-HR5-NP and GV-Ferritin were used for negative-stain electron microscopy. 5 µL of each sample (0.1 µg/µL) were applied to glow-discharged TEM grids covered by a thin layer of continuous film and stained with 2% uranyl acetate. The samples were then imaged using a Talos microscope (ThermoFisher) operating at an acceleration voltage of 120 kV. Images were recorded at a magnification of 73,000× and a defocus of 1.5 μm.
For the intranasal vaccination route, each mouse was intranasally immunized with a 10 µg dose of FP-HR5-NP or the molar equivalent of Gv-Ferritin formulated with c-di-GMP (InvivoGen) on days 0, 21, and 42. For the subcutaneous vaccination route, each mouse was subcutaneously immunized with a 10 µg dose of FP-HR5-NP with Sigma Adjuvant System (SAS) adjuvant on days 0, 21, and 42. Serum samples were collected every two weeks since the initial vaccination, and the mice were euthanized at week 8. After the complete three-dose internasal or subcutaneous vaccination routes, the transgenic hACE2 mice (C57BL/6) underwent the challenge with authentic SARS-CoV-2 Delta, Omicron strains in the BSL-3 facility. Littermates of the same sex were randomly assigned to un-infection or infection groups. Mice were anesthetized with isoflurane and intranasally with 2 × 104 FFU of SARS-CoV-2 viruses. The lungs were collected at 3 days post-infection (d.p.i.).
Recombinant HR5 protein or FP (Synthesized by GenScript) was coated on high-binding 96-well plates (Corning) at 2 ug/mL overnight at 4 °C. After washing with PBS, the plates were blocked for 1 h using 5% non-fat milk/PBS for HR5 plates and 2% BSA/PBS for FP plates. The serum or BALF was serially diluted (PBS for HR5 plates and 2% BSA/PBS for FP plates) and added into each well in duplicate, followed by incubating at 37 °C for 1 h. After washing with PBS/T (containing 0.05% Tween-20), the detection of antigen-specific IgG antibody in serum or BALF was conducted by adding HRP-conjugated goat anti-mouse or goat anti-human IgG secondary antibody (abcam) incubating for another 1 h at 37 °C. Meanwhile, the antigen-specific IgA antibody in BALF was detected by adding HRP-conjugated goat anti-mouse IgA secondary antibody(abcam). After washing with PBS/T, HRP substrate TMB solution (eBioscience) was added, followed by stopping the reaction with stop solution (Solarbio) after sufficient reaction. Absorption was measured at 450 nm. The data was analyzed using GraphPad Prism 8.0 software for non-linear regression to calculate endpoint titers.
Vero E6 cells were seeded in 96-well plates at a density of 2 × 104 cells per well and cultured until reaching 90–100% confluence. Serum from C57BL/6 mice was diluted in a 3-fold serial manner. Then, five hundred FFU of authentic Omicron BA.5 viruses were mixed with the diluted serum at a 1:1 ratio and incubated at 37 °C for 1 h. After removing the cell culture medium from each well, the serum/virus mixture was added, and the plates were incubated again for 1 h at 37 °C. Following this, the supernatant was discarded, and cells were covered with DMEM medium containing 1.6% carboxymethyl cellulose (CMC). The plates were then incubated for another 24 h. On the next day, cells were initially fixed with 200 µL of 4% paraformaldehyde for 12 h at 4°C, then the fixative was discarded, and cells were rinsed three times with 200 µL PBS. After fixation, a permeabilization step was carried out by treating cells with 100 µL of a solution containing 0.2% Triton X-100 and 1% BSA in PBS for 30 min. Post-washing three times with 200 µL PBS, cells were exposed to 50 µL of a primary antibody against SARS-CoV-2 nucleocapsid (N) (Sino Biological), diluted 1:1000 in PBS supplemented with 1% BSA, and incubated at 37 °C for one hour. Following the primary antibody binding, cells were thrice washed with 200 µL of PBS/Tween-20 (0.1%), then treated with an HRP-conjugated secondary antibody against rabbit IgG (Sino Biological), diluted 1:2000 in PBS with 1% BSA, at 37 °C for an hour. After removing the secondary antibody, cells were washed three more times with 200 µL PBS/T. 50 µL of TrueBlue (KPL) was added to each well for 5 min under agitation for visualization. Following the removal of TrueBlue, wells were washed twice with ddH2O, which was then completely removed prior to imaging with an ImmunoSpot microanalyzer.
The FRNT50 titer was defined as the reciprocal of serum dilution at which nAbs caused 50% inhibition of infection of cells. The inhibition was represented by the decrease in the number of SARS-CoV-2-infected cells in the sample wells compared to virus control wells. Reduction rates of the serial dilution assay were analyzed by GraphPad Prism 8.0 using non-linear regression to measure the FRNT50 titer.
The lung tissue of challenged hACE2 mice was procured, followed by homogenization using gentle-MACS M dissociator (Miltenyi Biotec, 130-093-235). Subsequently, RNA was extracted through the RNeasy Mini Kit (QIAGEN, 4104) according to the manufacturer’s instruction, then followed by the qRT-PCR to analyze for viral RNA copies of lung tissue utilizing a one-step SARS-CoV-2 RNA detection kit (PCR-Fluorescence Probing) (DaAn Gene Co., DA0931). For calibration, a construct of the SARS-CoV-2 N gene was integrated into a pcDNA3.1 vector to generate a standard curve. Indicated copies of N standards were 10-fold serially diluted and proceeded to qRT-PCR utilizing the same one-step SARS-CoV-2 RNA detection kit to obtain standard curves.
Mice were intravenously (IV) administered anti-CD45 antibody (2 µg per mouse) 5 min before euthanasia to exclude circulating immune cells. The lung tissue of mice was procured, followed by dissociating lung tissue using the gentle-MACS dissociator (Miltenyi Biotec, 130-093-235) and homogenized through a 70 μm filter. The spleen was collected and ground within PBS and homogenized through a 70 μm strainer. Subsequently, red blood cells (RBCs) were eliminated using an ACK lysis buffer, and the single-cell suspension was prepared for further applications.
To analyze surface markers, the relevant fluorochrome-conjugated monoclonal antibodies and the LIVE/DEAD Fixable Viability Dyes (Thermo Scientific) were employed to stain surface proteins for a duration of 30 min at room temperature, within a PBS solution containing 0.5% bovine serum albumin (BSA). The following antibodies were used: CD19-BV510 (Biolegend 115546), IgD-PE (Biolegend 405706), CD38-R718 (BD 752576), IgG1-PE-CY7 (Invitrogen M1-14D12), IgG2a-FITC (BD 562028), Streptavidin-BV421 (BD 563259), IgA-FITC (Invitrogen mA-6E1), CD4-R718 (BD 566939), CD8-BV510 (BD 563068), CD69-PE-CY7 (BD 552879), CD103-BV421 (Biolegend 121422), CD44-FITC (Biolegend 103006), CD95-PE-CY7 (BD 557653), GL7-FITC (Biolegend 144604), CXCR5-Biotin (Biolegend 145510), PD-1-APC (Invitrogen RMP1-30). The gating strategies for splenocytes are described in Supplementary Fig. 5.
Isolated cells were stimulated with a pool of SARS-CoV-2 spike (subunit 1 + subunit 2) peptide pools (GenScript, RP30223CN) for 1 h, in the presence of anti-CD28 (Biolegend, 302934) at a concentration of 1 µg/mL. Following stimulation, cells were co-incubated with Brefeldin A (MCE, HY-16592) at a concentration of 5 µg/mL and monensin (MCE, HY-N0150) at a concentration of 2 µM, at a temperature of 37℃ with 5% CO2 for a duration of 5 h. DMSO was utilized as the negative control, while Cell Stimulation Cocktail plus protein transport inhibitors (Invitrogen, 00-4975-93) served as the positive control. After 6 h of stimulation and the staining of T cell surface markers, the cells were immobilized and made permeable using IC Fixation Buffer (eBioscience, 00-8222-49) and Permeabilization Buffer (eBioscience, 00-8333-56). Subsequently, the cells were additionally stained with IFN-γ-APC (BD Biosciences, 551385) for a period of 2.5 h on ice. The flow cytometry technique was employed to analyze the proportions of T cells exhibiting cytokine specificity. All flow cytometry data were analyzed by FlowJo.
In order to detect the T cell response in both human and murine models, we imitated the method of establishing the SARS-CoV-2 S2 peptide pools [4, 6, 44] to construct peptide pools targeting the conserved regions of the SARS-CoV-2 S2 region, specifically FP, HR1, and HR2. The peptide pool was designed as a 15-mer with 10-amino acid overlaps with each adjacent peptide, covering the entire FP, HR1, and HR2 regions. Human PBMCs or murine splenic T cells were cultured with T cell peptides (GenScript) at a concentration of 2 µg per well at 37 °C for 18–24 h. Activated lymphocytes stimulated by given peptide were detected by the Human or murine IFN-γ ELISpot kit (Dakewe) according to the manufacturer’s protocol. The antigen-specific spots, indicating IFN-γ-positive T cells, were then counted using an S6 ultra immunoscan reader (Cellular Technology Ltd.), and the number of IFN-γ positive T cells was calculated by ImmunoSpot 5.1.34 software (Cellular Technology Ltd.). The number of spots was converted into the number of spots per million cells and plotted as mean (SD).
The hACE2 mice challenged with SARS-CoV-2 were euthanized in the BSL-3 facility on day 3. Lungs were then collected and fixed in 4% paraformaldehyde buffer for 48 h at 4 ˚C, and subsequently embedded in paraffin. Longitudinal slices of these tissues were then prepared, with Sects. (3–4 μm thick) undergoing Hematoxylin and Eosin (H&E) staining, and pathological injury was evaluated.
The statistical details of the specific experiment, including the statistical test used, number of samples, mean (SD), and p-values, were described in the figure legends. A student’s t-test was used to compare the two groups. For comparison between each group with the mean of every other group within a dataset containing more than two groups, one-way ANOVA with Tukey’s multiple comparison test was used. Statistical analyses were conducted utilizing GraphPad Prism 8.0 or Microsoft Excel. A value of p < 0.05 was considered to be statistically significant and represented as an asterisk (*). Value of p < 0.01 was considered to be more statistically significant and represented as double asterisks (**). A value of p < 0.001 was considered to be the most statistically significant and represented as triple asterisks (***). A value of p < 0.0001 was considered to be extremely statistically significant and represented as quadruple asterisks (****).
Data is provided within the manuscript or supplementary information files.
Not applicable.
This work was supported by the Natural Science Foundation of China (32470981), Guangdong Natural Science Funds for Distinguished Young Scholar (2023B1515020005) to Y.Z. Important Key Program of Natural Science Foundation of China (NSFC) (92169201,9236205), the Key R&D Program of Department of Science and Technology of Guangdong (2022B1111020004), Major Project of Guangzhou National Laboratory(GZNL2024A01017) to H.Z. Guangzhou Basic and Applied Basic Research Program (2024A04J4927) to R.C. Technology innovation group project of Foshan 2019 (FS0AA-KJ919-4402-0027)to T.W.
The Ethics Review Board of Sun Yat-sen University approved this study. All participants provided written informed consent with the approval of the Ethics Committees. Animal experiments were conducted in strict compliance with the guidelines and regulations of the Laboratory Monitoring Committee of Guangdong Province of China and were approved by the Ethics Committee of Zhongshan School of Medicine (ZSSOM) of Sun Yat-sen University on Laboratory Animal Care (Assurance Number: 2017-061). Authentic SARS-CoV-2 challenge studies were approved by the Ethics Committee of ZSSOM of Sun Yat-sen University on Laboratory Animal Care (Assurance Number: 2017-061) as well.
All authors agree to publication.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Liang, C., Li, R., Pu, Z. et al. Targeting heptad repeats and fusion peptide: nanoparticle vaccine elicits mucosal immune response against SARS-CoV-2 variants. J Nanobiotechnol 23, 483 (2025). https://doi.org/10.1186/s12951-025-03582-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-025-03582-w