Reproductive Biology and Endocrinology volume 23, Article number: 92 (2025) Cite this article
Tirzepatide (TZP), a dual agonist of glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors, has recently been introduced in Italy for the treatment of obesity. Obesity is frequently associated with metabolic hypogonadism, which is characterized by low testosterone levels and normal low levels of gonadotropin. This condition exacerbates metabolic dysfunction and increases the risk of type 2 diabetes mellitus (DM). This study aims to evaluate the effects of TZP on metabolic hypogonadism in patients with obesity.
Male patients with obesity and metabolic hypogonadism were enrolled. Exclusion criteria included recent use of medications for hypertension, dyslipidemia, DM, anti-androgens, or hyperprolactinemia. All participants followed a hypocaloric diet and engaged in 20 min of daily brisk walking. Patients were allocated to one of the following treatment groups: Group A received 2.5 mg of TZP weekly for the first month, with the dose increased to 5 mg from the second month; Group B received no pharmacological treatment: Group C received transdermal testosterone. Clinical evaluations were conducted at 2 months including assessment of body composition, the Binge Eating Scale (BES), 5-item International Index of Erectile Function (IIEF-5) questionnaire to evaluate erectile dysfunction (ED), and serum levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), sex hormone-binding globulin (SHBG), total testosterone (TT), and 17β-estradiol (E2). Free (fT) and bioavailable testosterone (bioT) were calculated using the Vermeulen formula.
A total of 83 patients with obesity (mean age 55.3 ± 5.5 years) were included in the study, divided into three groups: Group A (28 patients, mean age 56.3 ± 4.7 years), Group B (30 patients, mean age 55.1 ± 5.2 years), and Group C (25 patients, mean age 54.0 ± 6.5 years). At baseline, significant differences were observed in waist circumference (WC), which was higher in Group B, as well as in the BES score, lean mass (LM), and serum LH levels, all of which were higher in Group A. After 2 months, Group A showed significantly greater reductions in body weight, WC, BES score, and fat mass, along with a notable increase in LM and IIEF-5 score compared to Groups B and C. Additionally, Group A exhibited significantly higher serum levels of LH, FSH, SHBG, TT, fT, and bioT, while E2 levels were significantly lower than both Groups B and C.
The results of this study suggest that TZP is effective in improving both metabolic parameters, ED, and gonadal hormone levels in patients with obesity and metabolic hypogonadism. These findings position TZP as a promising treatment for obese patients with functional hypogonadism arising from metabolic-related alterations.
Obesity, characterized by excessive fat accumulation, has become a global epidemic, with an increasing recognition of its association with a wide range of metabolic disturbances [16]. The health risks associated with obesity are profound and extend beyond the traditional complications of cardiovascular disease and diabetes mellitus (DM) [7]. One often-overlooked consequence of obesity is metabolic hypogonadism, which is defined by low testosterone levels in men [19]. Specifically, metabolic hypogonadism refers to a condition in which low testosterone levels are associated with metabolic disorders, commonly observed in obese or insulin-resistant individuals [19]. It is characterized by a disruption of normal testosterone production and secretion due to underlying metabolic factors. In metabolic hypogonadism, excessive adiposity, insulin resistance, and altered levels of sex hormone-binding globulin (SHBG contribute to reduced testosterone levels [29, 33] . Obesity, particularly abdominal fat, leads to increased aromatization of testosterone to 17ß-estradiol (E2), further decreasing the availability of free testosterone (fT) [14, 26]. Additionally, inflammatory cytokines and altered hypothalamic-pituitary–gonadal (HPG) axis signaling may impair testosterone secretion from the testes [25]. These disruptions contribute to the symptoms and metabolic dysfunction associated with the condition. Testosterone plays a crucial role in regulating metabolic functions, including the maintenance of muscle mass, fat distribution, and insulin sensitivity [13]. Metabolic hypogonadism exacerbates these dysfunctions, contributing to insulin resistance and increasing the risk of developing type 2 DM, a condition that has a bidirectional relationship with obesity [13].
Research has demonstrated that testosterone deficiency in patients with obesity significantly impacts metabolic health, leading to further weight gain, increased fat mass (FM), and impaired glucose metabolism [31]. Low testosterone levels are associated with reduced muscle mass and increased adiposity, both of which contribute to the metabolic syndrome frequently observed in patents with obesity [20]. Additionally, testosterone deficiency can promote an increase in visceral fat, which is particularly harmful as it contributes to insulin resistance and inflammation, two key drivers of type 2 DM [22].
Traditionally, the treatment of metabolic hypogonadism in patients with obesity has relied on testosterone replacement therapy (TRT), primarily due to the challenges of achieving substantial weight loss through lifestyle changes alone in real-world settings [27]. However, TRT alone may not adequately address the underlying metabolic dysfunction in these patients, as it does not specifically target obesity or insulin resistance [8]. Therefore, a more holistic, pathophysiologically-driven approach is needed, one that simultaneously addresses both the hormonal imbalance and the obesity. Recent advancements in pharmacological treatments for obesity have introduced new possibilities for managing these interconnected conditions. Among the most promising of these drugs is tirzepatide (TZP), a dual agonist of the glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1) receptors (https://www.fda.gov/news-events/press-announcements/fda-approves-new-medication-chronic-weight-management).
TZP has demonstrated remarkable efficacy in promoting weight loss and improving metabolic parameters, including glucose control, insulin sensitivity, and lipid profiles [5]. Its dual action on both GIP and GLP-1 receptors provides a unique mechanism to regulate appetite, increase satiety, and reduce fat accumulation. Given its significant impact on weight and metabolism, TZP represents a novel approach for managing obesity and its associated complications [5]. However, integrating treatment with TZP into practical guidelines presents several challenges. One of the primary obstacles is the cost, as TZP is relatively expensive and may not be accessible to all patients, particularly in healthcare systems with limited coverage for newer medications. Furthermore, although TZP has shown effectiveness in managing obesity and metabolic disorders, its side effects, including gastrointestinal disturbances, such as nausea, vomiting, and diarrhea [12], can affect patient adherence to the treatment. These factors, in addition to the necessity for regular monitoring and individualized adjustments, make it difficult to incorporate TZP into routine clinical practice, particularly for patients with limited financial resources or those experiencing adverse reactions.
Despite the advantages of TZP in improving metabolic parameters, its potential benefits in the treatment of metabolic hypogonadism remain underexplored. TZP may offer promise in improving sexual hormones in patients with metabolic hypogonadism due to its multifaceted impact on metabolic health. By promoting significant weight reduction, TZP helps alleviate the metabolic disturbances that suppress testosterone production, such as the influence of adipokines, inflammatory cytokines, hyperinsulinemia, and hyperestrogenism. This reduction in metabolic dysfunction can lead to increased testosterone secretion by reducing HPG axis suppression. Additionally, TZP has been shown to improve liver function, particularly by resolving metabolic dysfunction-associated steatohepatitis without exacerbating liver fibrosis [17]. GLP-1 receptor agonists have been found to increase SHBG levels in patients with polycystic ovary syndrome [32]. Overall, these molecules have proven effective in improving the gonadal function in patients with metabolic hypogonadism, particularly those affected by obesity [15] or diabetes mellitus [28]. As a dual agonist targeting both GIP and GLP-1 receptors, TZP may also produce similar effects, further supporting hormonal balance in these patients.
This study aims to evaluate the effects of TZP in patients with obesity and metabolic hypogonadism. The primary objective is to assess the impact of TZP on both metabolic parameters and gonadal hormone levels, particularly testosterone, in this patient population. By investigating the effects of TZP on body composition, hormone levels, and other metabolic markers, this study seeks to determine whether TZP offers a more comprehensive treatment approach that addresses both obesity and metabolic hypogonadism. The findings of this study may have significant implications in clinical practice, providing evidence to support the use of TZP as a promising therapeutic option for patients with obesity and metabolic hypogonadism. If effective, TZP could provide an innovative strategy for managing both obesity and its associated hormonal imbalances, ultimately improving patient outcomes and quality of life.
Eligible participants were required to meet the criteria previously outlined by La Vignera et al. (2023) [15] for the diagnosis of metabolic hypogonadism. These criteria included moderate to severe obesity (BMI between 30 and 39.99), a waist circumference greater than 94 cm, total testosterone (TT) levels below 12 nmol/L, presence of sexual symptoms, normal-to-low gonadotropin levels (within the lowest quartiles of the reference range), SHBG levels within the lower quartile of the reference range, and a Homeostasis Model Assessment Index (HOMA) greater than 2.5. Eligible patients were assigned to one of three treatment groups between October 7 and December 7, 2024: Group A: TZP and lifestyle changes (n = 28); Group B: lifestyle changes only; (n = 30); or Group C: TRT and lifestyle changes (n = 25). The treatment protocol involved an initial dose of 2.5 mg of TZP weekly during the first month, which was increased to 5 mg weekly from the second month onward, in accordance with the TZP technical data sheet. All participants followed a hypocaloric dietary regimen, reducing their daily caloric intake by 20%, based on age, along with a daily physical activity regimen consisting of a 20-min brisk walk. For Group C, TRT was administered as transdermal testosterone [(23 mg from day 1 to day 14, followed by dose titration based on serum TT levels at day 14 according to the manufacturer's guideline].
Clinical evaluations were conducted during the first follow-up visit, which took place between December 7 and 21, 2024. These evaluations were performed at baseline and after two months of treatment and included the following measures: a) anthropometric parameters [body weight and waist circumference (WC)]; b) the Binge Eating Scale (BES) questionnaire to assess eating behavior; c) body composition analysis via bioimpedance analysis (BIA); d) the 5-item International Index of Erectile Function (IIEF-5) questionnaire to evaluate the presence and severity of erectile dysfunction (ED); and e) measurement of serum levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), TT, 17β-estradiol (E2), and SHBG using electrochemiluminescence immunoassay. A before-after analysis compared baseline changes, while an intergroup analysis assessed both within- and between-group differences in the distribution of these variables.
The study enrolled patients diagnosed with functional hypogonadism [4], naïve to GLP-1 receptor agonist therapy. Inclusion criteria were as follows: moderate to severe obesity, defined as a body mass index (BMI) ranging from 30.0 and 39.90 kg/m2, and a WC greater than 94 cm. Eligible patients also had TT levels below 3.46 ng/dL, consistent with the criteria of the Italian Society of Endocrinology [10], along with low or normal gonadotropin levels, typically within the lower quartiles of the reference range. Additionally, participants were required to have low levels of SHBG compared to the reference values. A Homeostasis Model Assessment Index (HOMA) score greater than 2.5, indicating insulin resistance [21], was another inclusion criterion.
Exclusion criteria included the use of medications for comorbid conditions such as hypertension, dyslipidemia, or DM in the six months before enrolment, prior treatment with anti-androgenic drugs, or a diagnosis of hyperprolactinemia.
The treatment allocation was guided by patient counseling and their clinical conditions, and individual preference. Each therapeutic option was thoroughly explained, and patients were assigned to Groups A, B, or C based on several factors such as economic situation (e.g., TZP was not prescribed to those could not afford its costs), the desire to delay pharmacological treatment, and the presence of contraindications. TRT was not prescribed to patients with contraindication, such as prostate or breast cancer, severe obstructive low urinary tract symptoms, polycythemia and/or elevated hematocrit, or personal or family venous thromboembolism [4]. Similarly, TZP was avoided in patients with gallstone disease. The decision-making process aimed the allocation process aimed to consider each patient’s preferences, health status, and overall circumstances.
All participants followed a hypocaloric dietary regimen, reducing their daily caloric intake by 20% based on their age and individual nutritional needs. The dietary plan was designed to create a caloric deficit, promoting weight loss while ensuring adequate nutrient intake for overall health. The dietary regimen adhered to the published guidelines for Mediterranean-based meals, consisting of 40–65% carbohydrates, 28–40% fats, and 10–35% proteins [1]. In addition to the dietary changes, each participant engaged in a daily physical activity routine, which included a 20-min brisk walk. This walk aimed to enhance cardiovascular health, increase energy expenditure, and support weight loss efforts. The combination of dietary restriction and regular physical activity was designed to achieve gradual, sustainable weight reduction while fostering a healthier lifestyle. Both interventions were tailored to the participants’ specific needs, considering their age, fitness levels, and health goals, ensuring the plan was both effective and achievable over time.
Body composition was evaluated using BIA, a non-invasive method that measures the resistance of body tissues to a small electrical current. It was performed using the Tanita BC 418 MA body composition analyzer for segmental measurement (Tanita Corporation, Tokyo, Japan). Measurements were performed under standardized conditions, ensuring that patients were fasting and had refrained from intense physical activity for at least 24 h prior to testing. The BES questionnaire was used to assess the severity of disordered eating behaviors. This validated 16-items self-report questionnaire evaluates emotional and behavioral aspects of binge eating. Both assessments were conducted at baseline and after two months from the beginning of the treatment to monitor changes in body composition and eating behavior.
The IIEF-5 questionnaire, a validated questionnaire for assessing the presence and severity of ED, was administered to all patients. Scores range from 5 to 25, with lower scores indicating greater severity. Specifically, scores between 5 and 7 suggest severe ED, 8 to 11 indicate moderate ED, 12 to 16 represent mild to moderate ED, and 17 to 21 signify mild ED [24].
Blood samples were collected in the morning, between 8:00 and 9:00 am after an overnight fast, and two hours after testosterone administration for patients on TRT. Serum levels of LH, FSH, TT, E2, plasma glucose, insulin, SHBG, and albumin were measured using electrochemiluminescence immunoassay (Hitachi-Roche equipment, Cobas 6000, Roche Diagnostics, Indianapolis, IN, USA). The reference ranges for the assays were as follows: LH: 1.14–8.75 mIU/mL, FSH: 0.95–11.95 mIU/mL, TT: 3.46–8.77 ng/dL, E2: 11–44 pg/mL, SHBG: 17–52 nmol/L; albumin: 3.4 to 5.4 g/dL. These levels were assessed at baseline and after two months to track any changes over the study period. fT and bioavailable testosterone (bioT) were calculated using the Vermeulen formula [30].
The homeostasis model assessment (HOMA) index was evaluated using the formula: [glycaemia (mg/dl) × insulin (µIU/ml)]/405, and those values falling into the 0.23–2.5 range were considered as normal.
Data are presented as the mean ± standard deviation (SD) for normally distributed variables and as the median with interquartile range (IQR) for non-normally distributed variables. The delta (Δ) change from baseline was calculated using the formula: [value at two months − baseline value)/baseline value] × 100]. Data distribution was assessed using the Shapiro–Wilk test. For within-group comparisons, differences from baseline were analyzed using the Student’s t-test for paired data or the Wilcoxon signed-rank test, depending on normality of data distribution. Between-group comparisons were performed using the one-way analysis of variance (ANOVA) for normally distributed variables or the Kruskal–Wallis test followed by Dunn’s post hoc analysis for non-normally distributed variables, as appropriate.
All statistical analyses were conducted using MedCalc Software Ltd. (Ostend, Belgium), version 19.6–64-bit. A p-value of less than 0.05 was considered statistically significant.
The study was carried out at the Division of Endocrinology, Metabolic Diseases and Nutrition, University-Teaching Hospital Policlinico “G. Rodolico-San Marco”, University of Catania (Catania, Italy) in accordance with the ethical principles of the Declaration of Helsinki and its subsequent amendments. All participants provided informed consent for the use of their personal and clinical data, which were obtained from the electronic medical records maintained by our Division. This consent followed a thorough explanation of the study’s objectives and the nature of the procedures involved, all of which are routinely used in clinical practice.
A total of 83 patients with a mean age of 55.3 ± 5.5 years (range: 44–63 years) and a mean BMI of 34.9 ± 3.5 kg/m2, met the eligibility criteria and were included in the study. The patients were divided into three groups: Group A (28 patients, mean age 56.3 ± 4.7 years, mean BMI 35.3 ± 3.0 kg/m2), Group B (30 patients, mean age 55.1 ± 5.2 years, mean BMI 34.6 ± 3.4 kg/m2), and Group C (25 patients, mean age 54.0 ± 6.5 years, mean BMI 35.0 ± 4.3 kg/m2).
At baseline, the groups showed significant differences in WC, with Group B having the highest WC [Group A: 109.0 cm (103.0–113.0); Group B: 116.0 cm (108–120.0); and Group C: 109.0 cm (105.3–115.0); p = 0.002]. Group A exhibited the higher BES score [Group A: 24.0 (22.0–25.5); Group B: 21.0 (20.0–22.0); and Group C: 22.0 (19.8–25.0); p < 0.001], while lean mass (LM) also varied significantly across the groups [Group A: 35.0% (27.5–44.5); Group B: 42.0% (41.0–44.0); and Group C: 38.0% (33.5–46.0); p = 0.032]. However, no significant intergroup differences were observed in Dunn’s post hoc analysis. Additionally, LH levels were significantly higher in Group A [Group A: 2.6 mIU/mL (2.2–2.9); Group B: 2.2 mIU/mL (1.8–2.4); and Group C: 2.3 mIU/mL (2.1–2.5); p = 0.008]. There were no significant differences between the groups in terms of body weight, BMI, FM, HOMA index, IIEF-5 scores, and serum levels of FSH, SHBG, TT, fT, bioT, and E2 (Table 1).
All patients suffered from ED, with IIEF-5 scores ranging from 5 to 12. Based on these scores, ED was classified as severe in 18 (64.3%) out of 28 patients in Group A, 15 (50%) out of 30 in Group B, and 13 (52%) out of 25 in Group C. Moderate ED was observed in 10 (35.7%) patients from Group A, 13 (43.4%) from Group B, and 9 (36%) from Group C. Mild to moderate ED was present in none of the patients from Group A, in 2 (6.7%) patients from Group B, and in 3 (12%) patients from Group C.
None of the patients in the pharmacological therapy groups (Groups A and C) experienced side effects that were severe enough to necessitate discontinuation of treatment.
Significant changes were observed across the groups in various outcomes. Specifically, when comparing the differences from baseline, Group A showed significant improvements in anthropometric parameters, BES scores, body composition (including both FM and LM), and hormonal values. Group B exhibited improvements in body weight and hormonal data, while Group C showed improvements in all the parameters except for LM and SHBG (Table 2).
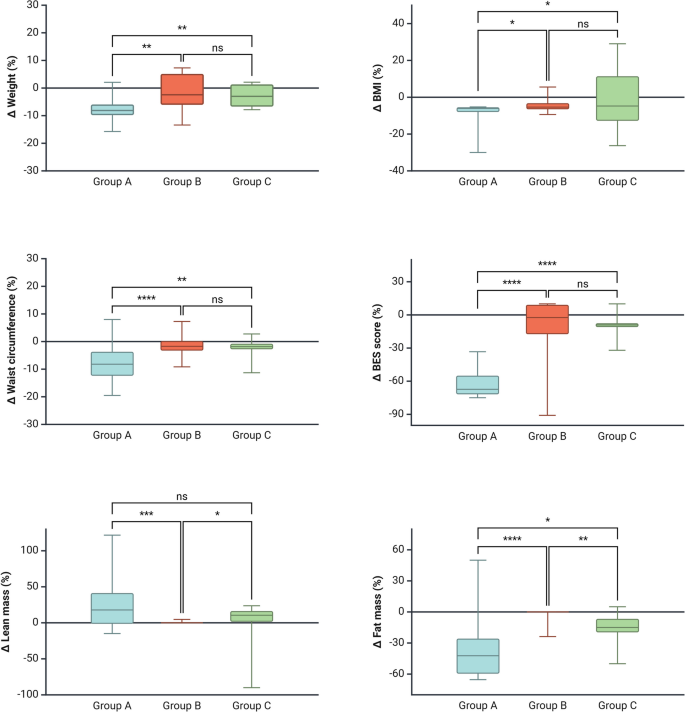
When comparing the percentage of change from baseline across the three groups, Group A exhibited the most substantial reductions, including notable decreases in body weight, BMI, WC, and FM, along with a significant decrease in the BES score. Additionally, the increase in LM was higher in Group A compared to Group B but not compared to Group C (Fig. 1). All groups experienced a decrease in HOMA index; the decrease in Group C was higher than in Group B, but not than Group A. No difference between Group A and Group B was found (Table 3).
Intergroup comparison: Delta chance in Anthropometric parameters, Binge Eating Scale (BES) score and body composition. Group A showed a greater reduction in body weight, body mass index (BMI), waist circumference, BES score, and fat mass percentage compared to Groups B and C. The increase in lean mass was higher in Group A than in Group B, but not significantly different from Group C. The one-way analysis of variance or the Kruskal-Wallis test followed by Dunn’s post hoc analysis were used to evaluate intergroup differences, depending on whether the data were normally or non-normally distributed, respectively. A p-value of <0.05 was considered statistically significant
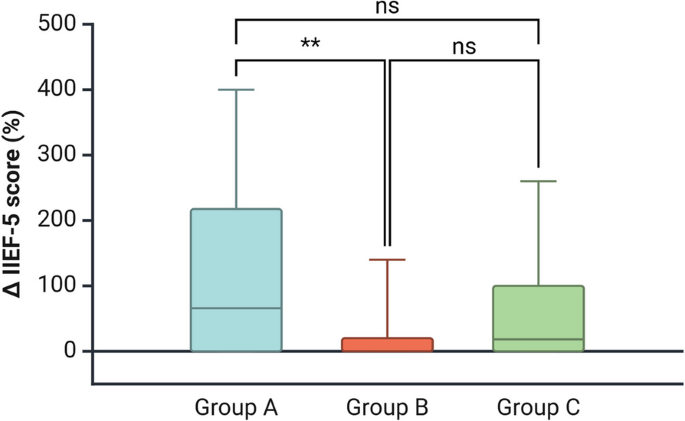
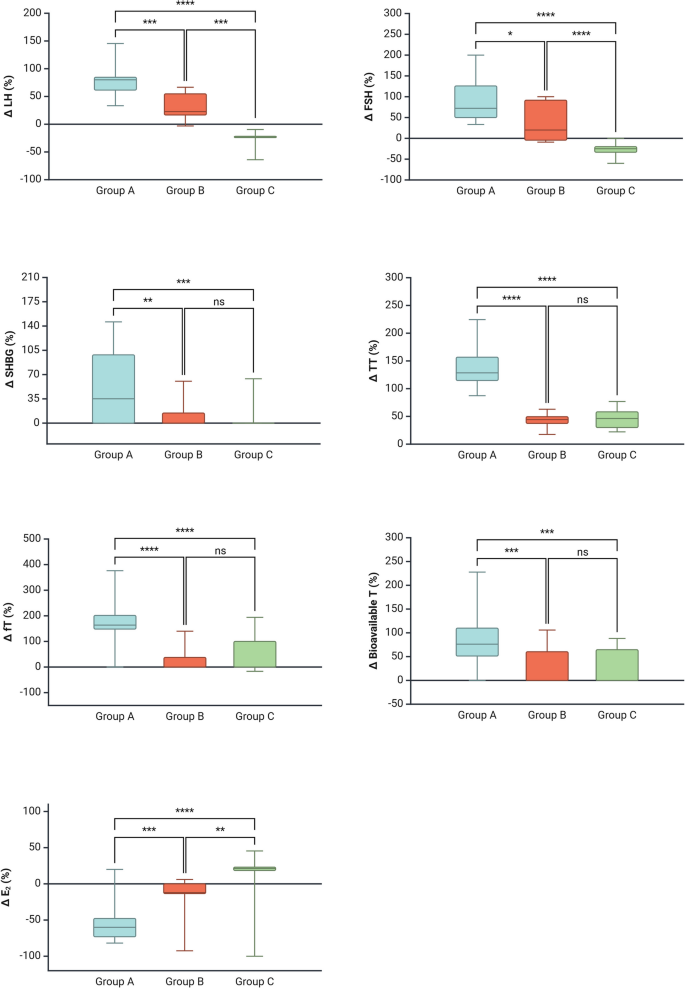
The increase in IIEF-5 score was higher in Group A vs Group B, but not versus Group C (Fig. 2). Hormonal changes in Group A included considerable increments in LH, FSH, and TT, accompanied by a significant decrease in E2 (Fig. 3). Group B demonstrated moderate changes in body weight, WC, and FM. Hormonal changes in this group included increases in LH, FSH, and TT, with only a modest reduction in E2. Group C showed smaller changes in most parameters, including a slight increase in E2 levels (Table 3).
Intergroup comparison: Delta chance in 5-item International Index of Erectile Function (IIEF-5) questionnaire score. The increase in the score was higher in Group A than in Group B, but not significantly different from Group C. The one-way analysis of variance or the Kruskal-Wallis test followed by Dunn’s post hoc analysis were used to evaluate intergroup differences, depending on whether the data were normally or non-normally distributed, respectively. A p-value of <0.05 was considered statistically significant
Intergroup comparison: Delta chance in serum hormone levels. Group A showed a greater increase in serum levels of luteinizing hormone (LH), follicle-stimulating hormone (FSH), sexual hormone-binding globulin (SHBG), total testosterone (TT), free testosterone (fT) and bioavailable testosterone (bioT), along with significantly lower E2 levels compared to both Groups B and C. The one-way analysis of variance or the Kruskal-Wallis test followed by Dunn’s post hoc analysis were used to evaluate intergroup differences, depending on whether the data were normally or non-normally distributed, respectively. A p-value of <0.05 was considered statistically significant
The results of this study highlight the promising role of TZP in improving both metabolic parameters and gonadal hormone levels in patients with obesity and metabolic hypogonadism. Our findings demonstrate that TZP not only effectively reduces body weight, WC, and FM percentage, but also significantly improving levels of LH, FSH, and TT, and decreasing E2 levels. These results suggest that TZP may offer a dual benefit by addressing both obesity and hormonal imbalances commonly observed in patients with metabolic hypogonadism.
Metabolic hypogonadism, characterized by low testosterone levels, is prevalent among patients with obesity and is associated with an increased risk of developing metabolic syndrome and type 2 DM [6]. Although we followed the Italian guidelines for the definition of hypogonadism [10], which align with our clinical practice and previous work [15], it is worth noting that all patients also met the diagnostic criteria proposed by the Endocrine Society [3]. Specifically, no patient had TT or fT levels above the respective thresholds of 264 ng/dL and 64 pg/mL. In addition, consistent with the guidelines, the diagnosis of hypogonadism was not based solely on biochemical parameters but also included the presence of symptoms. All patients presented with sexual symptoms, specifically erectile dysfunction, as indicated by their IIEF-5 scores.
Testosterone plays a crucial role in regulating metabolism, and its deficiency is linked to adverse outcomes such as increased FM, reduced muscle mass, and impaired insulin sensitivity [13, 31]. Given these associations, TRT has traditionally been used to manage this condition [27]; however, TRT alone does not address the underlying obesity or improve insulin resistance [8]. In this context, TZP offers a novel approach, targeting both obesity and metabolic dysfunction simultaneously.
By activating both GIP and GLP-1 receptors, TZP enhances insulin secretion, increase satiety, and reduce appetite, leading to significant weight loss [5]. In our study, TZP treatment was associated with significant reductions in body weight, WC, and FM, consistent with previous studies showing its effectiveness in promoting weight loss. Notably, these effects were more pronounced in Group A, which received TZP, compared to Groups B and C, where patients either received no treatment or transdermal testosterone, respectively.
Notably, although the enrolled population suffered from obesity, their FM percentages (around 20%) fell below the commonly used thresholds for excess adiposity in males, which are often defined as > 25–30% [2]. This likely reflects the relatively moderate obesity profile of our cohort, as suggested by the mean BMI values and the inclusion criteria, which did not specifically select for individuals with high FM. This finding also highlights the importance of evaluating body composition beyond BMI alone, particularly in the context of metabolic disorders such as hypogonadism.
Regarding the observed increase in LM following TZP treatment, this is a particularly interesting finding. While TZP is primarily known for its robust effects on weight and FM reduction, its impact on LM preservation remains controversial [23], with some studies suggesting a modest reduction in LM percentage [11, 18]. However, the potential benefit on LM could be especially relevant for patients with hypogonadism, who often present with reduced muscle mass and strength. Our findings support the potential role of TZP not only in improving metabolic parameters but also in promoting recovery of a healthier body composition profile.
All groups exhibited a reduction in HOMA index over the two-month follow-up, suggesting a general improvement in insulin sensitivity. Notably, the greater decrease observed in Group C (transdermal testosterone) compared to Group B (no treatment) may indicate a potential metabolic benefit of testosterone in this context. However, the absence of a significant difference between Group C and Group A (Tirzepatide) implies comparable efficacy between these interventions over the short term. The lack of difference between Group A and Group B could reflect the limited duration of follow-up, suggesting that the metabolic effects of Tirzepatide may require a longer timeframe to become fully apparent.
One of the most striking findings in our study was the improvement in gonadal hormone levels observed in Group A. Testosterone levels increased significantly following TZP treatment, while E2 levels decreased, indicating a positive shift in the hormonal profile towards a more favorable balance. The increased serum testosterone levels are particularly noteworthy, as hypotestosteronemia in patients with obesity has been linked to exacerbates metabolic dysfunction and increased risk of developing DM [31]. This improvement in testosterone levels following TZP treatment is likely due to the drug’s ability to enhance insulin sensitivity and reduce visceral fat, both of which are known to influence testosterone production.
In addition to its effects on body composition and hormone levels, TZP also had a significant impact on the BES score, a measure of disordered eating behavior. Group A demonstrated a marked reduction in BES scores, suggesting that TZP may help address both the physiological and behavioral aspects of obesity. This reduction in binge eating tendencies is consistent with the appetite-suppressing effects of TZP [9], which may help patients in adhering to a calorie-restricted diet and achieving long-term body weight loss. It is important to note that Group B, which received no pharmacological treatment, showed no significant changes in body weight or BES scores, further emphasizing the potential benefits of TZP in addressing both the metabolic and behavioral aspects of obesity.
Comparing the results of the three treatment groups, TZP provided superior outcomes in terms of weight reduction, body composition, and hormonal balance. Group A demonstrated the greatest reductions in body weight, WC, and FM, along with the highest increases in LM. Furthermore, the improvements in hormonal profiles, including the substantial rise in testosterone levels and the decrease in E2, were most pronounced in this group. These findings support the hypothesis that TZP can improve both metabolic and hormonal dysfunction in patients with obesity and metabolic hypogonadism, providing a more comprehensive treatment approach than traditional TRT alone.
Unexpectedly, serum E2 levels increased by 21.2% in Group C (Table 3), despite improvements in body weight, BMI, and waist circumference in this group. We speculate that this increase may be due to the peripheral aromatization of the administered testosterone. It is important to note that TRT was administered transdermally at doses insufficient to suppress the HPG axis. Additionally, despite the rise in E2, most patients did not experience symptoms/signs of hyperestrogenism.
While the results of this study are promising, several limitations should be considered. First, the sample size was relatively small, which limits the generalizability of the findings. Additionally, the study duration was only two months, and longer follow-up is necessary to assess the long-term effects and sustainability of TZP treatment. Another limitation is the lack of a direct comparison with other pharmacological treatments for obesity, such as semaglutide or liraglutide, which have also shown efficacy in improving metabolic parameters and weight loss. Finally, the lack of randomization in treatment allocation further limits the strength of the study. Future studies could address these limitations by including larger sample sizes, longer follow-up periods, and comparisons with other obesity treatments.
Despite these limitations, the results of this study provide, for the first time in literature, evidence supporting the use of TZP in patients with obesity and metabolic hypogonadism. The improvement in serum testosterone levels observed after just two months of therapy is particularly noteworthy. The dual mechanism of action of TZP, targeting both obesity and metabolic dysfunction, makes it a promising therapeutic option for this patient population. By improving both metabolic parameters and gonadal hormone levels, TZP could help reduce the risk of developing type 2 DM and other obesity-related complications, ultimately improving patient outcomes and quality of life.
In conclusion, TZP represents a novel and effective treatment for patients with obesity and metabolic hypogonadism. The improvements in body composition, ED, hormonal balance, and disordered eating behavior observed in this study suggest that TZP may offer a more comprehensive approach to managing both obesity and its associated metabolic dysfunction. Further studies with larger sample sizes and longer follow-up periods are needed to confirm these findings and determine the long-term benefits of TZP in this patient population. If further supported, the results of this study suggest that TZP could become an essential tool in the treatment of metabolic hypogonadism in patients with obesity, offering an innovative strategy to address the complex interplay between obesity, metabolic dysfunction, and hormonal imbalances.
The data will be accessible upon request to the corresponding author
Not applicable.
None.
Not applicable.
The subjects in this study have not concomitantly been involved in other randomized trials. Data regarding any of the subjects in the study has not been previously published unless specified. Data will be made available to the editors of the journal for review or query upon request.
Data will be made available upon request.
This paper was not funded.
The study protocol was carried out in accordance with the ethical principles of the Declaration of Helsinki and its subsequent amendments. Ethical approval was waived as the procedures that patients underwent are standard components of routine clinical practice, and no participants were subjected to any experimental interventions. All participants provided informed consent for the use of their personal and clinical data.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
La Vignera, S., Cannarella, R., Garofalo, V. et al. Short-term impact of tirzepatide on metabolic hypogonadism and body composition in patients with obesity: a controlled pilot study. Reprod Biol Endocrinol 23, 92 (2025). https://doi.org/10.1186/s12958-025-01425-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12958-025-01425-9