Critical Care volume 29, Article number: 241 (2025) Cite this article
The end-expiratory occlusion (EEO) test detects preload responsiveness through changes in cardiac index (ΔCI) during a 15-second respiratory hold at end-expiration. We investigated the diagnostic accuracy of EEO-induced changes in arterial pulse pressure (∆PP), especially when the duration of EEO is reduced to 10’’ and 5’’, and whether adding an end-inspiratory occlusion (EIO) improves this diagnostic accuracy.
In 143 mechanically ventilated patients with sinus rhythm, EEO and EIO were performed while recording ΔCI and ∆PP values. Either a fluid bolus-induced ΔCI ≥ 15% or a passive leg raising-induced ΔCI ≥ 10% defined preload responsiveness. The effects of the EEO and EIO tests on PP and CI were evaluated as the percentage difference between values averaged either over the last five seconds of the 15-sec respiratory holds (ΔPPEEO−15’’ and ΔPPEIO−15’’, ΔCIEEO−15’’ and ΔCIEIO−15’’), or between the 5th and the 10th seconds of the 15-sec respiratory holds (ΔPPEEO−10’’ and ΔPPEIO−10’’, ΔCIEEO−10’’ and ΔCIEIO−10’’), or during the five first seconds of respiratory holds (ΔPPEEO−5’’ and ΔPPEIO−5’’, ΔCIEEO−5’’ and ΔCIEIO−5’’) and baseline.
Sixty-one (43%) patients were preload responders. Both ∆CIEEO−15’’ and ∆CIEEO−10’’ were higher in responders than in non-responders’ (5.8 [4.5–7.3]% vs. 1.1 [0.1–3.4]% and 3.0 [2.4–4.3]% vs. 0.6 [0.1–1.6]%, respectively; p < 0.001), whereas ∆CIEEO−5’’ did not differ between responders and non-responders. ∆PPEEO−5’’, ∆PPEEO−10’’ and ∆PPEEO−15’’ were significantly higher in responders than in non-responders (5.2 [2.8–8.7]% vs. 1.2 [0.3–2.8]%, 7.7 [5.0–12.4]% vs. 1.8 [0.5–3.1]% and 8.1 [5.1–11.8]% vs. 1.5 [0.5–3.0]%, respectively; p < 0.001). For detecting preload responsiveness, compared to the area under the receiver operating characteristic (AUROC) of ∆CIEEO−15’’ (0.935 [0.881–0.969]), the AUROC of ∆CIEEO−10’’ was similar (0.910 [0.851–0.951], p = 0.410), but the AUROC of ∆CIEEO−5’’ was smaller (0.541 [0.456–0.625], p < 0.001); the AUROC of ∆PPEEO−15’’ (0.913 [0.857–0.952], p = 0.346), and ∆PPEEO−10’’ (0.912 [0.860–0.947], p = 0.336) were similar, but the AUROC of ∆PPEEO−5’’ (0.834 (0.763–0.891, p = 0.005) was significantly smaller. Evaluation of ∆CIEEO+EIO and ∆PPEEO+EIO did not enhance reliability of the test at each test duration.
In ventilated patients with sinus rhythm, real-time changes in PP during a 10-second EEO reliably detect preload responsiveness.
No. IDRCB 2010A0095942. Registered 04 January 2010.
In mechanically ventilated patients, the end-expiratory occlusion (EEO) test has been developed to detect preload responsiveness by using heart-lung interactions [1, 2]. It consists in interrupting mechanical ventilation at end-expiration for 12 [3] to 30 [4, 5] sec, which stops the cyclic impediment in venous return created by each insufflation in positive pressure. Cardiac preload increases transiently and, in the case of preload responsiveness of both ventricles, cardiac output significantly increases [6]. Conversely, a respiratory hold performed at end-inspiration (end-inspiratory occlusion, EIO) decreases cardiac output to a larger extent in the case of preload responsiveness than in the case of preload unresponsiveness [7, 8]. When both EEO and EIO tests are combined, the absolute change in cardiac output is larger than during EEO alone. This may allow one to detect preload responsiveness with techniques measuring cardiac output that are not precise enough for detection of cardiac output changes induced by EEO alone [7, 8], even though discrepant results have been reported [9].
The main drawback of the EEO and EIO tests is that the respiratory hold must be rather long, which prevents its use in patients who are awake enough to interrupt ventilator occlusion. Durations ranging from 12 to 30 s have been used in published studies [2]. There are two reasons for this long duration. The first is that the increase in right cardiac preload must be transmitted to the left side after the pulmonary transit time. The second reason is that monitoring devices average cardiac output over several seconds, which delays the occurrence of its changes. For instance, the pulse wave analysis technique used in the first article describing the EEO test [6] uses a moving average of 12 s.
In this regard, monitoring the effects of EEO and EIO through continuous arterial pressure might be interesting, especially pulse pressure (PP), the changes of which are proportional to changes in cardiac output [10, 11]. During respiratory holds, PP values may be stable enough to use a moving average of a few seconds only. First, this would allow the test to be performed without any specific device measuring cardiac output. Second, this may allow the reduction of the duration of respiratory holds, as there is no moving average for PP, and increase the applicability of the test to patients who cannot sustain a 15-sec respiratory occlusion. In the seminal study demonstrating the reliability of the EEO test, changes in PP reliably detected preload responsiveness when measuring its changes during the last 5 s of a 15-sec EEO [6]. Nevertheless, shorter durations of EEO assessed through changes in PP, and the potential advantage of adding a shortened EIO to EEO, have not been tested yet.
Thus, in the present study, we investigated (i) whether an EEO test shorter than 15 s and performed by assessing its effects on PP reliably detects preload responsiveness, and (ii) whether adding an EIO to EEO improves the detection of preload responsiveness when their effects are assessed through changes in PP.
This retrospective analysis was conducted in patients prospectively included in previous studies by our group in whom EEO and EIO had been performed [12,13,14,15,16]. If patients had been included in two studies, only the data of the study published first were retained. All studies had been approved by an Institutional Review Board. All patients or next of kin had been informed about the study and consented to participate.
Inclusion criteria common to all studies were: (i) age ≥ 18 y.o., (ii) mechanical ventilation in volume assist-control mode, (iii) monitoring already in place with a transpulmonary thermodilution device, and (iv) decision of the attending physicians to perform a passive leg raising (PLR) test [12,13,14,15] or to infuse a 500-mL fluid bolus [7, 8]. Exclusion criteria common to all studies were (i) spontaneous breathing activity preventing 15-second respiratory holds, (ii) atrial fibrillation, (iii) venous compression stockings and abdominal hypertension [17], (iv) intracranial hypertension, and (v) refusal of the patient or next of kin to participate in the study.
In all studies, patients were equipped with a jugular central venous catheter and a thermistor-tipped arterial catheter in the femoral artery connected to a pressure sensor to perform transpulmonary thermodilution (PiCCOplus or PiCCO2 device, Pulsion Medical Systems, Getinge, Feldkirchen, Germany). Cardiac index (CI) was measured intermittently by injecting three consecutive 15-mL boluses of cold saline and averaging the obtained CI values [18]. CI was also measured continuously through pulse contour analysis (CIpulse) after flushing the arterial line and checking for the absence of under- and overdamping of the arterial pressure curve [18, 19]. Both the PiCCOplus and PiCCO2 devices average CIpulse values with a moving average of 12 s and refresh the CIpulse value displayed on the screen every second. Variables measured by transpulmonary thermodilution devices were continuously recorded through PiCCO-Win software v.4-x to 6-x (Pulsion Medical Systems, Getinge, Felkirchen, Germany).
Arterial pressure was recorded through the femoral arterial catheter. Central venous pressure was measured at the base of the C wave at end-expiration, and the average of three successive respiratory cycles was calculated. Arterial and central venous pressure, cardiac rhythm and airway pressure were continuously and digitally recorded via HEM 4.2 software (Notocord, Croissy-sur-Seine, France). Respiratory variables such as positive end-expiratory pressure (PEEP), plateau pressure (Pplat), respiratory rate and tidal volume (Vt) were collected.
In all studies, patients were ventilated in the volume assist-control mode (Evita 4 or Infinity V500 (Dräger, Lübeck, Germany) or CARESCAPE R860 (General Electric Healthcare, Chicago, IL, USA)), with a tidal volume of 6 mL/kg of predicted body weight. The fraction of inspired oxygen (FiO2) and respiratory rate were decided by the attending physician. Neuromuscular-blocking agents were used if clinically required. These settings were kept unchanged during the study period.
Before EEO and EIO, pulse contour analysis was calibrated via transpulmonary thermodilution and haemodynamic stability was ensured. The 15-second EEO or EIO manoeuvres were performed in a random order. Between both respiratory holds, CIpulse and PP were allowed to return to baseline. After haemodynamic status had stabilised, a 15-second EIO test was performed (Fig. 1). After haemodynamic variables had stabilised again, a PLR test was performed [12,13,14,15] as previously reported [20] or a 500-mL fluid bolus was infused [7, 8]. No change in sedative drugs or catecholamines was allowed during the study time.
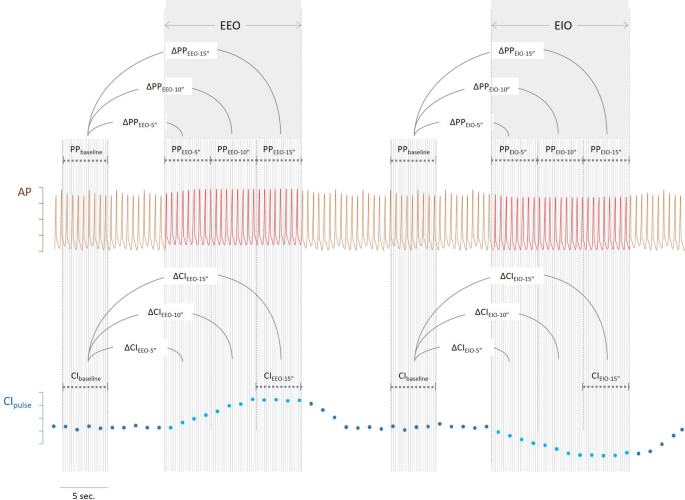
Description of the calculation of changes in arterial pulse pressure (∆PP) and of pulse contour-derived cardiac index (CIpulse) during end-expiratory (EEO) and end-inspiratory (EIO) occlusions using theoretical arterial pressure traces and cardiac index ∆PPEEO−5’’: changes in pulse pressure during the first 5 s of the end-expiratory occlusion, ∆PPEEO−10’’: changes in pulse pressure between the 5 th and 10 th seconds of the end-expiratory occlusion, ∆PPEEO−15’’: changes in pulse pressure during the last 5 s of the end-expiratory occlusion, ∆PPEIO−5’’: changes in pulse pressure during the first 5 s of the end-inspiratory occlusion, ∆PPEIO−10’’: changes in pulse pressure between the 5 th and 10 th seconds of the end-inspiratory occlusion, ∆PPEIO−15’’: changes in pulse pressure during the last 5 s of the end-inspiratory occlusion, ∆CIEEO−15’’: changes in cardiac index during the last 5 s of the end-expiratory occlusion, ∆CIEIO−15’’: changes in cardiac index during the last 5 s of the end-inspiratory occlusion
Effects of EEO and EIO on pulse pressure and cardiac index
The effects of the EEO and EIO tests on PP and CI were evaluated as the percentage difference between, on the one side, values averaged either over the last five seconds of the 15-sec respiratory holds (ΔPPEEO−15’’ and ΔPPEIO−15’’, respectively for PP and ΔCIEEO−15’’ and ΔCIEIO−15’’, respectively for CI), or between the 5th and the 10th seconds of the 15-sec respiratory holds (ΔPPEEO−10’’ and ΔPPEIO−10’’, respectively for PP and ΔCIEEO−10’’ and ΔCIEIO−10’’, respectively for CI) or during the five first seconds of respiratory holds (ΔPPEEO−5’’ and ΔPPEIO−5’’, respectively for PP and ΔCIEEO−5’’ and ΔCIEIO−5’’, respectively for CI) and, on the other side, values averaged over five seconds at baseline (Fig. 1). The effects of combined EEO and EIO tests (ΔPPEEO+EIO−5’’, ΔPPEEO+EIO−10’’ and ΔPPEEO+EIO−15’’ for PP and ΔCIEEO+EIO−5’’, ΔCIEEO+EIO−10’’ and ΔCIEEO+EIO−15’’ for CI) were calculated as the sum of the relative changes in PP and CI observed during the corresponding EEO and EIO.
Definition of preload responsiveness
Preload responsiveness was defined as a PLR-induced increase in CIpulse ≥10% [12,13,14,15] or a fluid-induced increase in CIpulse ≥15% [7, 8] when fluid challenge had been performed.
Statistical analysis
Data are expressed as mean ± SD or median [IQR], depending on data distribution. The normality of data distribution was evaluated visually. Pairwise comparisons of data were done with the paired Student’s t test or Wilcoxon test, depending on data distribution. The two-tailed Student t test or Mann-Whitney-U test, depending on data distribution, was used to compare preload responders and preload non-responders. The area under the receiver operating characteristic curve (AUROC) was calculated for the ability of changes in PP and CI during the various respiratory holds to detect preload responsiveness. The best diagnostic threshold was determined as the one providing the best Youden index. AUROCs were compared using the Hanley-McNeil test for repeated comparisons, and a Bonferroni correction was used for multiple comparisons. Also, we calculated the least significant change (LSC) of PP in 10 patients chosen at random in our population, as previously explained [21]. Briefly, the coefficient of error – the ratio of the coefficient of variation over √(number of replicates) – was multiplied by 1.96 and √2, in order to identify the minimum change measured that can be trusted as a real change of measurement.
Statistical significance was set at a p value < 0.05. Statistical analysis was performed with MedCalc software 19.6 (Mariakerke, Belgium).
We retrieved data from 143 patients enrolled between May 2016 and December 2019, including 61 preload responders and 82 preload non-responders. Twenty-one patients (12%) were excluded from the analysis for inability of sustaining a respiratory hold. Preload responsiveness was defined most frequently according to PLR test results (121 patients). Seventy-nine patients (55%) had acute respiratory distress syndrome (ARDS) and no patient was in prone position during the study period. Forty-two patients had a past medical history of chronic obstructive pulmonary disease and ten patients of asthma. Patients were enrolled after 1.0 [1.0–3.0] days from shock onset, with no difference between responders and non-responders (1.0 [1.0–3.0] days vs. 1.0 [1.0–3.0] days, respectively, p > 0.05). The remaining patient characteristics are shown in Table 1.
The changes in haemodynamic variables are shown in Table 2. ∆CIEEO−5’’ did not differ between responders and non-responders (0.7 [0.4–1.4]% vs. 0.3 [0.1–1.7]%, respectively, p = 0.22). ∆CIEEO−10’’ was significantly higher in responders than in non-responders’ (3.0 [2.4–4.3]% vs. 0.6 [0.1–1.6]%, respectively, p < 0.001). This was also the case for ∆CIEEO−15’’ (5.8 [4.5–7.3]% vs. 1.1 [0.1–3.4]%, respectively, p < 0.001).
∆CIEIO−5’’ was significantly higher in responders than in non-responders (−1.4 [−2.8 – −0.7]% vs. −0.0 [−0.3 – −3.3]%, respectively, p = 0.02). This was also the case for ∆CIEIO−10’’ (−4.8 [−7.3 – −2.8]% vs. −0.6 [−1.3–0.0]%, respectively, p = 0.002) and for ∆CIEIO−15’’ (−7.9 [−11.2 – −6.5]% vs. −3.5 [−4.3 – −1.4]%, respectively, p < 0.001).
∆CIEEO+EIO−5’’ was significantly higher in responders than in non-responders (1.9 [1.0–6.3]% vs. 0.4 [0.1–1.6]%, respectively, p = 0.03). This was also the case for ∆CIEEO+EIO−10’’ (8.9 [5.4–12.4]% vs. 1.5 [0.9–2.3]%, respectively, p < 0.001) and ∆CIEEO+EIO−15’’ (15.0 [12.7–18.5]% vs. 4.5 [2.8–7.1]%, respectively, p < 0.001).
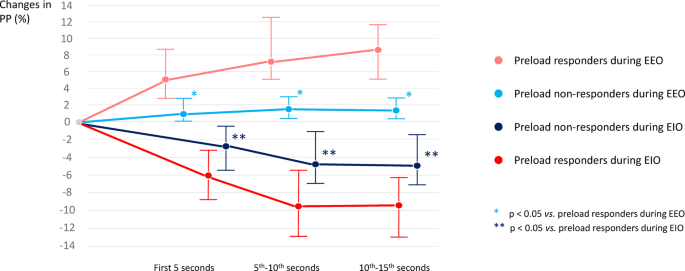
The LSC of PP among the 10 randomly chosen patients was 2.0%. ∆PPEEO−5’’ was significantly higher in responders than in non-responders (5.2 [2.8–8.7]% vs. 1.2 [0.3–2.8]%, respectively, p < 0.001). This was also the case for ∆PPEEO−10’’ (7.7 [5.0–12.4]% vs. 1.8 [0.5–3.1]%, respectively, p < 0.001 – Supplementary material S1) and for ∆PPEEO−15’’ (8.1 [5.1–11.8]% vs. 1.5 [0.5–3.0]%, respectively, p < 0.001) (Fig. 2).
Percentage changes in arterial pulse pressure (∆PP) during end-expiratory (EEO) and end-inspiratory (EIO) occlusion in preload responders and preload non-responders depending on the time of measurements
∆PPEIO−5’’ was significantly higher in responders than in non-responders (−6.0 [−8.8 – −3.2]% vs. −2.6 [−5.4 – −0.5]%, respectively, p = 0.031). This was also the case for ∆PPEIO−10’’ (−9.5 [−12.7 – −5.7]% vs. −4.7 [−7.0 – −1.4]%, respectively, p < 0.001) and for ∆PPEIO−15’’ (−9.4 [−12.8 – −6.2]% vs. −4.8 [−7.1 – −1.7]%, respectively, p < 0.001) (Fig. 2).
∆PPEEO+EIO−5’’ was significantly higher in responders than in non-responders (11.3 [8.2–15.4]% vs. 3.9 [1.2–7.7]%, respectively, p < 0.001). This was also the case for ∆PPEEO+EIO−10’’ (17.2 [12.9–24.3]% vs. 6.8 [2.1–10.7]%, respectively, p < 0.001) and ∆PPEEO+EIO−15’’ (17.7 [12.3–24.1]% vs. 6.3 [12.7–9.8]%, respectively, p < 0.001).
With a cut-off value of 2.1%, the AUROC of ∆CIEEO−10’’ was not significantly different from the one of ∆CIEEO−15’’ in the ability of detecting preload responsiveness (0.910 [0.851–0.951] vs. 0.935 [0.881–0.969], respectively; p = 0.801). On the contrary, ∆CIEEO−5’’ could not detect preload responsiveness (Supplementary material S2). Also, there was no difference between the AUROC of ∆CIEEO−15’’ and that of ∆PPEEO−15’’.
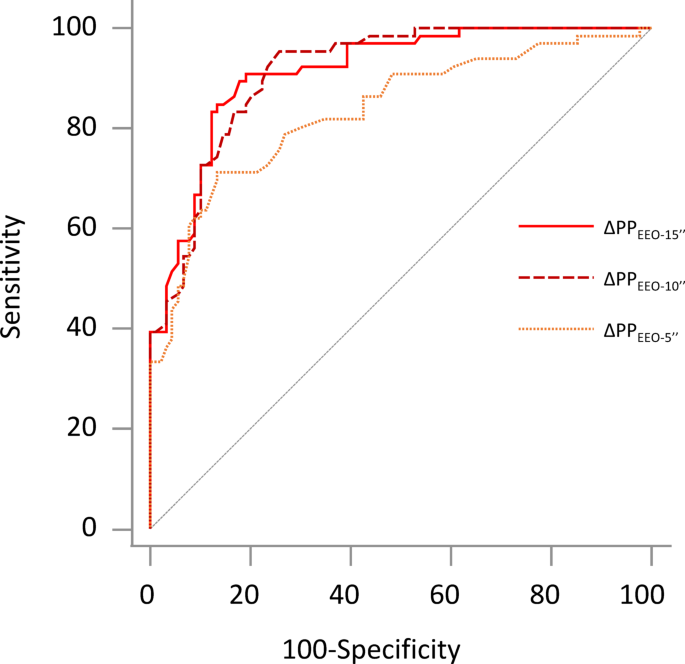
As for PP, the AUROC of ∆PPEEO−10’’ was 0.912 [0.860–0.947], with sensitivity of 86.9 [75.8–94.2]%, specificity of 80.5 [70.3–88.4]% and a cut-off value of 3.7%. It did not differ from the AUROC of ∆PPEEO−15’’ (0.913 [0.857–0.952], p = 0.983) but was significantly higher than the one of ∆PPEEO−5’’ (0.834 [0.763–0.891], p = 0.001) (Fig. 3).
Area under the receiver operating characteristic curves for changes in arterial pulse pressure (∆PP) during end-expiratory occlusions (EEO) to detect preload responsiveness. ∆PPEEO-5’’: changes in pulse pressure during the first 5 seconds of the end-expiratory occlusion, ∆PPEEO-10’’: changes in pulse pressure between the 5th and 10th seconds of the end-expiratory occlusion, ∆PPEEO-15’’: changes in pulse pressure during the last 5 seconds of the end-expiratory occlusion.
When EEO and EIO were combined, analogous results were observed, with no differences between the AUROCs of ∆PPEEO+EIO−15’’ and ∆PPEEO+EIO−10’’ (0.899 [0.837–0.943] and 0.882 (0.818–0.930), respectively) but with the one of ∆PPEEO+EIO−5’’ (0.832 [0.761–0.889]) that was significantly lower.
When patients were stratified according to the PEEP level, similar results were observed among patients in the lowest and in the highest quartile of PEEP level (Supplementary material S3).
This study demonstrates that, in mechanically ventilated patients with sinus rhythm, real-time changes in PP during a 10-second EEO reliably detect preload responsiveness.
The EEO test is one of the methods that use heart-lung interactions to test preload responsiveness in mechanically ventilated patients [22]. Specifically, it uses the transient increase in cardiac preload created by stopping positive pressure ventilation for a few seconds. The present study confirms previous ones showing that changes in CIpulse during a 15-sec EEO detect preload responsiveness with high accuracy [2].
Our goal was to investigate ways to circumvent the two main limitations of the EEO test, i.e., its relatively long duration and the need for a device monitoring cardiac output [1]. In particular, we investigated the advantage of combining EIO and EEO, as we previously demonstrated that it reliably predicts fluid responsiveness when using ultrasound techniques [7, 8], which are less precise than pulse contour analysis [21]. We also investigated the ability of PP changes to reflect changes in cardiac output over a shorter period of time.
First, the present study confirms that a 15-second EEO detects preload responsiveness when PP, averaged over the last 5 s of the hold, is used to assess its effects. This had been shown in the first study describing the EEO test [6], but it has not been re-evaluated since then. Second, the EEO-induced changes in PP still reliably detect preload responsiveness when assessed between 5 and 10 s, with an AUROC larger than 0.90. This raises the possibility of shortening the duration of the EEO test. This would likely increase the number of patients in whom the test can be performed without any spontaneous respiratory effort.
Third, combining EEO and EIO did not allow reduction in the time over which PP changes were assessed. However, the thresholds for tests’ positivity were larger than those of EEO alone. Then, a reason for choosing to perform EIO + EEO may be that the EEO alone has induced changes in PP or CI that are close to the diagnostic thresholds. The haemodynamic effects of EIO alone, on either CI or PP and whatever the length of measurement, were less reliable in detection of preload responsiveness than the effects of EEO.
For clinical practice, our study shows that, provided that an arterial line is present, no cardiac output monitor is necessary to assess the effects of EEO. However, the current bedside monitors do not display the PP value, which must be calculated from systolic and diastolic arterial pressure values. Moreover, our study shows that the duration of EEO could be reduced to 10 s when its effects are assessed on PP rather than on CI. Nevertheless, we averaged PP values over 5 s, which again is not automatically performed by bedside monitors. Our results suggest that bedside monitors could be improved by directly displaying PP values. This would allow automatic and averaged measurements of PP changes during interventions such as EEO, and improve the precision for assessing PP changes. The bedside applicability of the method may be improved in this way. Regarding the effects of EEO on CI, it must be noted that the thresholds indicating tests’ positivity were small, but higher than the least significant change reported for pulse contour analysis [23], which is a very precise technique. These results are not applicable to less precise monitoring techniques. Finally, it must be emphasized that our results are only applicable to patients without cardiac arrhythmia and any triggering inspiration during expiratory and inspiratory pauses.
The first limitation of this research is that the current analysis was retrospectively performed on the data of previous studies, albeit prospectively acquired. Second, in most studies, preload responsiveness was defined according to the effects of PLR, not of a fluid bolus. However, the evidence is nowadays strong that PLR detects preload responsiveness. Also, we could not retrieve the total amount of fluids administered to the patients before study inclusion. Third, we did not evaluate whether a sequence of shortened EIO + EEO with no interruption would improve the reliability of the combined tests. This manoeuvre may have induced larger changes in cardiac output and PP, and limited some false positives, especially in those patients for whom a titrated vascular filling is particularly important (severe ARDS patients, cardiogenic shock). Fourth, we did not calculate our sample size a priori, but rather included as many patients as allowed by the previous studies of our group. Finally, we assessed the absence of spontaneous breathing by observing the pressure curve of the ventilator. Some mild respiratory efforts may have been missed, while the monitoring of oesphageal pressure would have allowed us to detect them. However, their hemodynamic impact would likely be neglectable.
In ventilated patients with sinus rhythm, real-time changes in PP during a 10-second EEO detect preload responsiveness.
Individual, de-identified participant data are available from the corresponding author on reasonable request.
- AUROC:
-
area under the receiver operating characteristics
- CI:
-
cardiac index
- EEO:
-
end-expiratory occlusion
- EIO:
-
end-inspiratory occlusion
- PLR:
-
passive leg raising
- PEEP:
-
positive end-expiratory pressure
- PP:
-
pulse pressure
- Pplat:
-
plateau pressure
- Vt:
-
tidal volume
- ∆PP’ :
-
changes in pulse pressure
- ∆CI:
-
changes in cardiac index
Data collection of each study was approved by an independent ethics committee (Comité pour la Protection des Personnes, Ile-de-France VII). Informed consent was obtained from each patient or from the patient’s legally authorized representative if the patient was unable to provide consent. The study was conducted in accordance with the Declaration of Helsinki.
All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript. The data were used anonymously.
FG has no conflicts of interest to disclose.NDV has no conflict of interest to discloseCL has received fees for lectures from Sedana MedicalDA has no conflict of interest to discloseAP has no conflict of interest to discloseMJ has no conflict of interest to discloseSR has no conflict of interest to discloseIA has no conflict of interest to discloseAB has no conflict of interest to discloseJLT has received fees for lectures from Masimo and Edwards Lifesciences. He has received fees for consulting from Pulsion Medical Systems (Getinge).XM has received fees for lectures from Baxter Healthcare, Philips, Masimo, AOP and Pulsion Medical Systems (Getinge). He has received fees for consulting from Pulsion Medical Systems (Getinge).
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Gavelli, F., De Vita, N., Lai, C. et al. Real-time changes in pulse pressure during a 10-second end-expiratory occlusion test reliably detect preload responsiveness. Crit Care 29, 241 (2025). https://doi.org/10.1186/s13054-025-05483-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13054-025-05483-8