BMC Public Health volume 25, Article number: 1262 (2025) Cite this article
Few studies have explored the impact of multimorbidity patterns on premature mortality. This study aimed to assess the associations between multimorbidity patterns and long-term mortality and whether the associations were modified by socioeconomic status (SES) and healthy lifestyles.
Data were from the National Health and Nutrition Examination Survey (NHANES) 1999–2018 in the US. The latent class analysis was used to establish multimorbidity patterns based on 11 chronic conditions. Mortality outcomes were ascertained by linking with the public-use mortality data from the National Death Index through December 31, 2019. Accelerated failure time models were used to estimate time ratios (TRs) and corresponding 95% confidence intervals (CIs) for the associations between multimorbidity patterns and all-cause and CVD mortality and to exmine the extent to which SES and healthy lifestyles modified those associations.
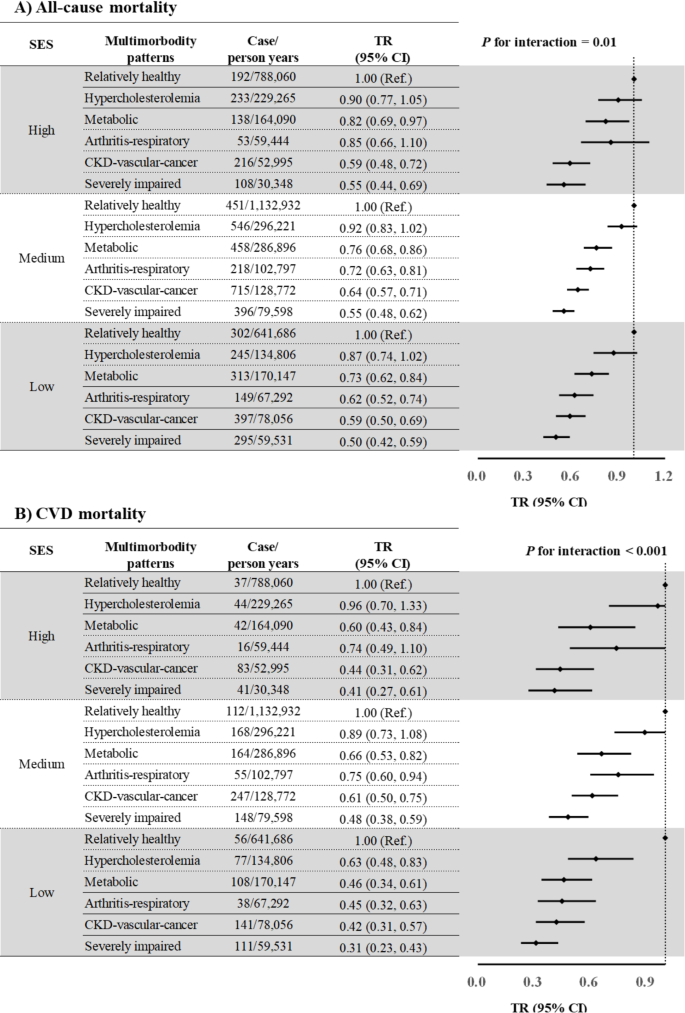
In our study, six multimorbidity patterns were identified, including “relatively healthy”, “hypercholesterolemia”, “metabolic”, “arthritis-respiratory”, “CKD-vascular-cancer”, and “severely impaired” classes. Compared with the “relatively healthy” class, TRs for all-cause and CVD mortality progressively decreased across the multimorbidity classes, with the “severely impaired” class showing the shortest survival time (TR, 0.53; 95% CI: 0.48, 0.58 for all-cause mortality; 0.42; 0.35, 0.50 for CVD mortality). A significant interaction was noted between SES and multimorbidity patterns for survival time, with a stronger positive association in individuals with low SES. Adherence to healthy lifestyles was related to longer survival time across all multimorbidity patterns, especially in those with relatively less severe multimorbidity.
Multiple multimorbidity patterns were identified and associated with mortality. Lower SES was associated with higherexcess multimorbidity-associated mortality, while adopting healthy lifestyles contributed to longer survival regardless of multimorbidity patterns. Efforts should be mobilized to reduce SES gaps and promote healthy lifestyles to alleviate the health burden of multimorbidity.
Multimorbidity, usually defined as the co-occurrence of two or more chronic conditions, has increased worldwide due to population aging and improved medical care [1]. Among US adults, the prevalence of multimorbidity steadily increased from 45.7% in 1988–1994 to 59.6% in 2013–2014 [2]. Multimorbidity has been reported to lead to higher functional limitations [3], increased medical service utilization, and healthcare expenditure [4]. Thus, multimorbidity poses substantial challenges to healthcare systems.
Evidence accumulates that multimorbidity is associated with elevated mortality, with more morbidities being related to a higher risk of death [5]. However, previous studies mainly measured multimorbidity by counting the number of conditions rather than profiling condition patterns. Certain morbidities often cluster as they share similar pathological pathways [6], potentially resulting in synergistic effects on mortality. Prior studies used latent class analysis (LCA) to identify multimorbidity patterns, and reported associations between certain multimorbidity patterns (i.e., cardiometabolic group, and depression/cardiovascular disease/cancer group) and higher mortality risk [7, 8]. However, evidence from the US is limited. A study among 166,126 US older adults identified five multimorbidity groups, with the complex cardiometabolic class showing the strongest effects on mortality [9]. Of note, this study only included older US adults. Although the prevalence of multimorbidity is higher in US middle-aged (50% for 45–60 years) and older adults (81% for 65 years and older), 18% of 18–44 years US adults had multimorbidity in 2016 [10]. Evidence on the associations between multimorbidity patterns and mortality is still needed in a representative US population.
In addition, recent studies suggested that SES and healthy lifestyles might modify the process of progression from a single disease or multimorbidity into mortality [11,12,13,14,15]. Previous studies showed that SES disparities in survival increased [16] and adherence to healthy lifestyles decreased in the US [17]. Thus, whether mortality associated with multimorbidity patterns differed by SES, and whether adherence to healthy lifestyles could differentially reduce mortality by multimorbidity patterns are urgently needed to be examined. These questions have important public health implications for minimizing health inequality caused by SES and improving long-term survival in individuals with multimorbidity.
To address the knowledge gap, we used data from the National Health and Nutrition Examination Survey (NHANES) to assess the associations between multimorbidity patterns and premature mortality and whether the associations were modified by SES. In addition, we examined whether the associations of combined lifestyle factors and mortality differed by multimorbidity patterns.
The NHANES is an ongoing series of cross-sectional, nationally representative surveys conducted by the National Center for Health Statistics (NCHS) of the Centers for Disease Control and Prevention to evaluate the health and nutritional status of the US general population. The study design, sampling procedures, and survey methods were described in detail elsewhere [18]. Briefly, the NHANES used a stratified, multistage probability cluster design to enroll a nationally representative sample. Data were collected from study participants at enrollment using questionnaires, physical examinations, and laboratory tests. All NHANES protocols were approved by the NCHS Research Review Board, and written informed consent was provided by study participants.
For our analyses, we used data from 52,398 participants aged more than 20 years old recruited in ten cycles (NHANES 1999–2000, 2001–2002, 2003–2004, 2005–2006, 2007–2008, 2009–2010, 2011–2012, 2013–2014, 2015–2016, and 2017–2018). We excluded (1) participants without any of the eleven chronic conditions (n = 6,349: see the “identification of chronic conditions and multimorbidity” section); (2) participants without information for SES, including family poverty-to-income ratio, educational level, and insurance status (n = 4,084); (3) participants without information for healthy lifestyles (n = 4,084); and (4) participants without information for healthcare utilizations (n = 33; Supplementary Fig. 1). 38,011 participants were included for final analyses.
Eleven chronic conditions (i.e., stroke, arthritis, cancer, chronic obstructive pulmonary disease [COPD], asthma, cardiovascular disease [CVD], diabetes, hypertension, hypercholesterolemia, obesity, chronic kidney disease [CKD]) were determined based on self-reported doctor diagnosis, physical examinations, and laboratory tests according to previous work [2]. Stroke, arthritis, and cancer were defined as self-reported doctor diagnoses. All study participants were asked three following questions: (1) “has a doctor or other health professional ever told you that you had a stroke?” (2) “has a doctor or other health professional ever told you that you had an arthritis?” (3) “has a doctor or other health professional ever told you that you had a cancer?”. COPD was defined as a composite of self-reported doctor diagnoses of emphysema or chronic bronchitis [19]. All study participants were asked three following questions: (1) “has a doctor or other health professional ever told you that you had emphysema?” (2) “has a doctor or other health professional ever told you that you had chronic bronchitis?” (3) “has a doctor or other health professional ever told you that you had COPD?” for NHANES 2013–2018. Asthma was defined if participants reported ever being told that they had asthma and still had it [20] using the following two questions: (1) “Has a doctor ever told you that you had asthma?” (2) “Do you still have asthma?”. CVD was defined as a composite of self-reported doctor diagnoses of coronary heart disease, myocardial infarction, angina pectoris, and congestive heart failure. All participants were asked the following four questions: (1) “has a doctor or other health professional ever told you that you had a coronary heart disease?” (2) “has a doctor or other health professional ever told you that you had a heart attack (also called myocardial infarction)?” (3) “has a doctor or other health professional ever told you that you had an angina, also called angina pectoris?” (4) “has a doctor or other health professional ever told you that you had a congestive heart failure?”. Diabetes was determined by fasting glucose measures (HbA1c ≥ 6.5%) or self-reported antidiabetic medication taken or self-reported doctor diagnosis of diabetes [21]. Hypertension was determined by blood pressure (systolic blood pressure (SBP) ≥ 140 mm Hg or diastolic blood pressure (DBP) ≥ 90 mm Hg) or self-reported antihypertension medication taken or self-reported doctor diagnosis of hypertension via questionnaires. Hypercholesterolemia [2] was defined as total cholesterol ≥ 200 mg/dL or self-reported anti-hypercholesterolemia medication taken [22]. Obesity was defined as body mass index (BMI) ≥ 30 kg/m2 at enrollment. CKD was determined by eGFR < 60 ml/min/1.73m2 or a one-time urine albumin-to-creatinine ratio (ACR) ≥ 30 mg/g. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula [23]: eGFR CKD-EPI = 141 × min (SCr/κ,1)α × max (SCr/κ,1)−1.209 × 0.993Age × 1.018 (if female), where SCr is serum creatinine, κ is 0.7 for females and 0.9 for males, α is − 0.329 for females and − 0.411 for males, min indicates the minimum of SCr/κ or 1 and max indicates the maximum of SCr/κ or 1.
LCA is a statistical procedure used to identify qualitatively different subgroups (also known as latent classes) within populations who share similar characteristics [24]. It was used to define multimorbidity patterns based on these 11 chronic conditions (Supplementary methods).
Self-reported family poverty-to-income ratio, educational level, and insurance status were used to measure SES according to previous work [14, 25]. The family poverty-to-income ratio was calculated as the total family income divided by the poverty threshold, and a higher poverty-to-income ratio indicated a higher family income status. The family poverty-to-income ratio was grouped into three groups: ≤1, 1–4, and ≥ 4 [14]. Educational level was classified into three groups: less than high school, high school, and college or above. Health insurance status was categorized into no insurance, public health insurance only (including Medicare, Medicaid, military healthcare, Indian Health Service, State Sponsored Health Plan, or other government program), and private health insurance (including any private health insurance, Medi-Gap, or single-service plan). LCA was used to define the overall SES based on the three components according to the item-response probabilities (Supplementary methods).
Based on previous work [14] and evidence on modifiable lifestyle factors for the prevention of major noncommunicable diseases recommended by the World Health Organization [26], a combined healthy lifestyle score was assessed using four major modifiable lifestyle factors, including smoking, alcohol drinking, diet, and physical activity. A healthy level for smoking was defined as never smoking (participants who smoked fewer than 100 cigarettes in a life-long time). A healthy level of alcohol drinking was defined as daily consumption of one drink or fewer for women and two drinks or fewer for men. Diet quality was assessed in terms of the healthy eating index-2015 (HEI-2015) using 24-hour dietary recalls [27]. A total score of diet quality ranged from 0 to 100, with a higher total diet quality score indicating better diet quality. A healthy diet was defined as a HEI-2015 score in the top 40% of distributions. A healthy physical activity was defined as more than 150 min of moderate-to-vigorous leisure-time physical activity per week. We assigned 1 point for the healthy level and 0 point otherwise for each lifestyle factor. The total healthy lifestyle score was calculated as a sum of four factors and ranged from 0 to 4, with a higher score indicating a healthier lifestyle. The healthy lifestyle score was categorized into three groups: 0–1 (low), 2 (medium), and 3–4 (high).
Healthcare utilization was determined by self-reported use of outpatient and inpatient services in line with previous studies [28, 29]. The outpatient utilization was defined according to whether the participants ever had at least one visit to a doctor or other health professional in the past year. The inpatient utilization was defined according to whether participants had at least one overnight hospital stay in the past year, excluding an overnight stay in the emergency room.
Several potential covariates were included in our analyses, including NHANES cycles, age, sex, and race/ethnicity. The NHANES cycles were treated as an ordinal variable (0–9 from 1999–2000 to 2017–2018). Age was treated as a continuous variable in the analyses. Sex was categorized as male and female. Race/ethnicity was grouped as Hispanic (including Mexican and non-Mexican Hispanic), non-Hispanic white, non-Hispanic black, and others.
Mortality outcomes were ascertained using the public-use mortality data from the National Death Index through December 31, 2019, to which the NHANES 1999–2018 eligible participants were matched using a probabilistic matching algorithm. The International Classification of Diseases Tenth Revision (ICD-10) codes were used to identify causes of death, and codes I00-I09, I11, I13, and I20-51 were used to ascertain deaths from CVD. Participants not matched with a death record were regarded as alive throughout the entire follow-up period.
Complex survey design and sampling weights were considered in our analyses according to the NHANES analytic guidelines. Baseline characteristics were compared across six multimorbidity patterns using Rao-Scott Chi-square (for categorical variables) and Wald F-tests (for continuous variables). Logistic regression models were conducted to generate odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for the associations between multimorbidity patterns and inpatient and outpatient healthcare utilization, respectively. We added product terms of multimorbidity patterns and healthcare utilization in the model to examine potential modification using the Wald test. The Poisson regression model was conducted to assess incident rate ratios (IRRs) and 95% CIs for the association between SES and the number of chronic conditions.
As the proportion hazards assumption was not met, we did not use the Cox proportional hazard regression models to assess the associations between multimorbidity patterns and all-cause and CVD mortality. We fitted the accelerated failure time with Weibull distribution for the baseline hazard function to assess the time ratios (TRs) and 95% CIs for our analyses [30]. Covariates were adjusted in a stepwise manner: Model 1 included NHANES cycles, age, sex, and race/ethnicity, and Model 2 additionally included SES and healthy lifestyle score.
We further assessed the associations between multimorbidity patterns and all-cause and CVD mortality in subgroups stratified by SES. We included a product term of multimorbidity patterns and SES to examine the potential effect modification using the Wald test. We also cross-classified participants according to categories of multimorbidity patterns and SES, and explored the combined associations of multimorbidity patterns and SES with all-cause and CVD mortality.
In addition, we conducted analyses stratified by multimorbidity patterns to assess the associations between healthy lifestyles and all-cause and CVD mortality in different multimorbidity pattern subgroups.We evaluated the effect modification of multimorbidity patterns for the association between healthy lifestyles and mortality using the Wald test. We also cross-classified participants according to categories of multimorbidity patterns and healthy lifestyles and explored the combined associations of multimorbidity patterns and healthy lifestyle with all-cause and CVD mortality.
We conducted subgroup analyses by stratifying participants by age (< 40, 40–60, and ≥ 60 years), sex (male and female), and years of enrollment (1999–2008 and 2009–2018). We added a product term of the stratifying variables and multimorbidity patterns to the final models to examine potential effect modifications by the stratifying variables using the Wald test. We conducted two sensitivity analyses. First, we adjusted for three individual SES components and individual lifestyle factors to assess the associations between multimorbidity patterns and mortality. Second, instead of using LCA, we classified the SES based on the levels of three SES components. We assigned 0, 1, and 2 to represent the following categories respectively: PIR ≤ 1, PIR 1–4, and PIR ≥ 4; less than high school, high school, and college or above; no insurance, public insurance only, and private insurance. SES score was calculated as a sum of three SES indicators and ranged from 0 to 6, with a higher score indicating a higher SES. The SES score was categorized into three groups: low (0–2), medium (3–4), and high SES (5–6).
The LCA for the complex survey was conducted with Mplus 8 following the Mplus User Guide Version 8, and diet quality was calculated in SAS 9.4. All other statistical analyses were conducted with R 4.2.0 and STATA/SE 17.0, and all significance tests were two-sided with P < 0.05 indicating statistical significance.
Of 38,011 participants, the mean age was 46.90 (SE, 0.20) and 49.01% were males. Six multimorbidity patterns were identified through the LCA according to the item-response probabilities, including “relatively healthy”, “hypercholesterolemia”, “metabolic”, “arthritis-respiratory”, “CKD-vascular-cancer”, and “severely impaired” classes(Supplementary Methods, Supplementary Tables 1–2, and Supplementary Fig. 2). Three SES classes were also established, including the low, medium, and high SES classes (Supplementary Tables 3 and Supplementary Fig. 3). 38.30% had high SES and 16.39% had low SES; 49.72% had a high lifestyle score and 16.18% had a low lifestyle score.
Participants from the “CKD-vascular-cancer” class had the oldest age (P < 0.001), while those from the “severely impaired” class were more likely to have low SES (P < 0.001), and to have a low lifestyle score (P < 0.001; Table 1). The aged- and sex-standardized prevalence of “relatively healthy” class and “CKD-vascular-cancer” class decreased from 56.98% and 5.53% in 1999–2000 to 54.81% and 4.21% in 2017–2018 (P = 0.01 and < 0.001; Supplementary Fig. 4), respectively. In contrast, the age- and sex-standardized prevalence of “metabolic” and “severely impaired” increased from 9.79% and 3.48% in 1999–2000 to 15.48% and 4.73% in 2017–2018 (P = 0.01 and < 0.001), respectively. Meanwhile, the prevalence of “metabolic” class and “severely impaired” class increased from 1999 to 2018 across all SES classes (P ≤ 0.02 for all), while the prevalence of “CKD-vascular-cancer” decreased for participants in the medium and low SES classes (P ≤ 0.003 for all). Low SES was associated with a higher number of chronic conditions (IRR, 1.16; 95% CI: 1.13, 1.19; Supplementary Table 4) after adjusting for covariates.
Compared with study participants in the “relatively healthy” class, the odds of having at least one outpatient visit in the past year and at least one inpatient overnight hospital stay were progressively elevated in “metabolic”, “hypercholesterolemia”, “arthritis-respiratory”, “CKD-vascular-cancer”, and “severely impaired” classes (ranging from 2.49 to 17.43 for the outpatient visit; 1.60 to 6.48 for inpatient admission; Supplementary Table 5). There was no significant interaction between SES and multimorbidity patterns for outpatient visits or inpatient overnight hospital stays (Supplementary Table 6).
During a median follow-up of 9.58 years, 5,425 deaths (1,688 from CVD) were documented. Compared with the “relatively healthy” class, other multimorbidity classes showed shorter survival time after adjustment for the NHANES cycle, age, sex, race/ethnicity, SES, and healthy lifestyles. The “severely impaired” class had the shortest survival time with TR (95% CI) of 0.53 (0.48, 0.58), followed by the “CKD-vascular-cancer” class (0.61; 0.56, 0.67), the “arthritis-respiratory” class (0.70; 0.64, 0.77), the “metabolic” class (0.76; 0.70, 0.83), and the “hypercholesterolemia” class (0.89; 0.82, 0.97). The “severely impaired” class had the shortest survival time due to CVD mortality with TR (95% CI) of 0.42 (0.35, 0.50), followed by the “CKD-vascular-cancer” class (0.52; 0.44, 0.62), the “metabolic” class (0.59; 0.50, 0.70), the “arthritis-respiratory” class (0.65; 0.54, 0.79), and the “hypercholesterolemia” class (0.84; 0.71, 0.99; Table 2). The effect estimates did not change appreciably after further adjustment for SES components and lifestyle factors (Supplementary Table 7) or using an alternative SES measurement (Supplementary Table 8). We found some heterogeneities in the associations between multimorbidity patterns and survival time by age, sex, and years of enrollment (all P for interaction ≤ 0.04; Supplementary Table 9).
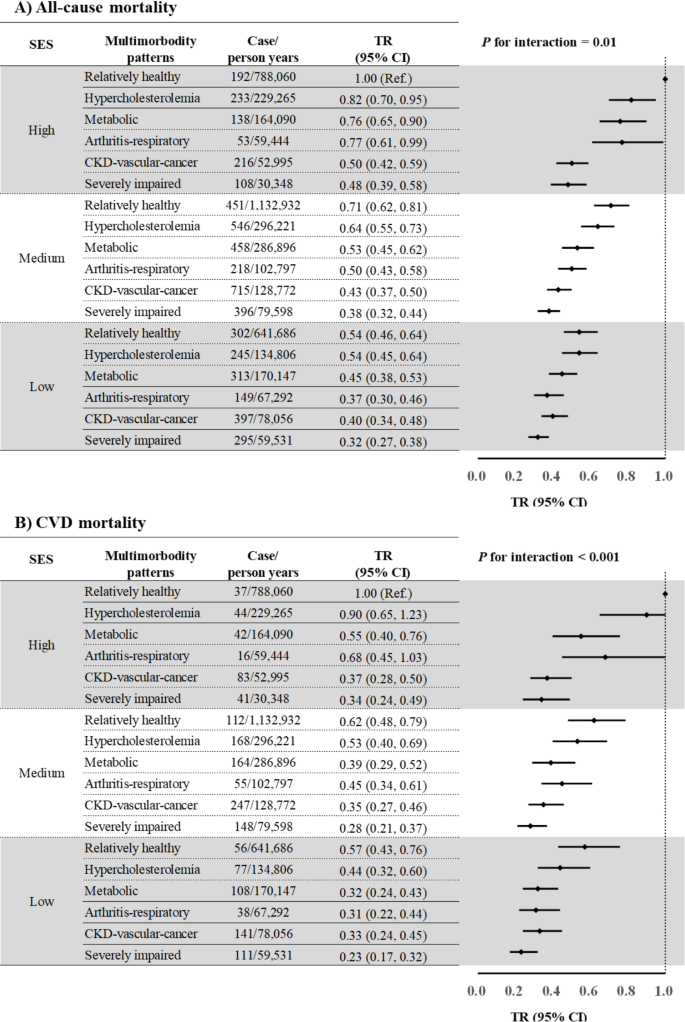
As for SES, study participants with low SES had a shorter survival time, compared to those with high SES (Supplementary Table 10). Significant interactions between SES and multimorbidity were noted for survival time due to all-cause (P for interaction = 0.01) and CVD mortality (P for interaction < 0.001), with stronger negative associations between multimorbidity and survival time in study participants with low SES than those with high SES for all multimorbidity patterns (Fig. 1). There was no noticeable change after further adjustment for SES components and lifestyle factors (Supplementary Fig. 5) or using an alternative SES measurement (Supplementary Fig. 6). In a joint analysis to investigate the combined association of SES and multimorbidity patterns with survival time, TR (95% CI) for individuals of low SES and the “severely impaired” class compared with those with high SES and the “relatively healthy” class were 0.32 (95% CI: 0.27, 0.38) for all-cause mortality and 0.23 (95% CI: 0.17, 0.32; Fig. 2) for CVD mortality.
Associations of multimorbidity patterns with survival years by SES
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; NHANES, National Health and Nutrition Examination Survey; SES, socioeconomic status; TR, time ratio. All models were adjusted for NHANES cycles, age (continuous, years), sex (male and female), race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, and others), and healthy lifestyle score (0–1, 2, and 3–4).
Joint associations of multimorbidity patterns and SES with survival years
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; NHANES, National Health and Nutrition Examination Survey; SES, socioeconomic status; TR, time ratio. All models were adjusted for NHANES cycles, age (continuous, years), sex (male and female), race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, and others), and healthy lifestyle score (0–1, 2, and 3–4)
Individuals with higher lifestyle scores (3–4) had a longer survival time than those with lower lifestyle scores (0–1; Supplementary Table 11). A significant interaction between healthy lifestyles and multimorbidity patterns was noted for survival time (all P for interaction < 0.001). The positive association between healthy lifestyles and survival time due to all-cause mortality was stronger in the less severe multimorbidity class: TRs were from 1.64 to 1.25 for the “relatively healthy”, “hypercholesterolemia”, “metabolic”, “severely impaired”, and “CKD-vascular-cancer” classes, but TR was 1.68 for the “arthritis-respiratory” classes (Fig. 3). Similar associations were found after further adjustment for SES components and individual lifestyle factors (Supplementary Fig. 7).
Associations of healthy lifestyles with survival years by multimorbidity patterns
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; CVD, cardiovascular disease; HL, healthy lifestyle; NHANES, National Health and Nutrition Examination Survey; SES, socioeconomic status; TR, time ratio. All models were adjusted for NHANES cycles, age (continuous, years), sex (male and female), race/ethnicity (Hispanic, non-Hispanic white, non-Hispanic black, and others), and SES (low, medium, and high)
In our large prospective study, six statistically distinct and clinically meaningful classes of multimorbidity were identified in the US population. Compared with study participants in the “relatively healthy” class, those in the “severely impaired” class showed the lowest survival time as well as the highest healthcare utilization (i.e., outpatient visits and inpatient overnight stay). In addition, SES modified the associations between multimorbidity patterns and survival time, with stronger associations in those with lower SES. We also noted significant interactions between multimorbidity patterns and healthy lifestyles for survival time: while adherence to healthy lifestyles seemed protective across multimorbidity patterns, healthy lifestyles showed stronger positive associations with survival time among participants with less severe multimorbidity patterns.
Given that specific clusters of multimorbidity often share common pathophysiological pathways, identifying these clusters and their associations with long-term mortality can provide valuable insights to enhance effective management of multimorbidity and optimize healthcare utilization. Instead of defining multimorbidity by the number of chronic conditions [5, 31], we used LCA to identify six distinctive multimorbidity patterns and showed that these different patterns had differential effects on premature mortality. Study participants in the “severely impaired” class (i.e., high prevalence of most 11 diseases), and the “CKD-vascular-cancer” class characterized by high prevalence of cancer, CKD, stroke, CVD, and hypertension, had the shortest survival time due to all-cause and CVD mortality, which was consistent with the reported dose-response relationship between the number of chronic conditions and mortality [5, 31]. Consistently, a study among 166,126 US adults aged more than 50 years from the National Health Interview Survey 2002–2014 identified five multimorbidity classes, and the “complex cardiometabolic” class showed higher mortality than the “healthy” class [9]. Another study that used data from 512,723 Chinese participants aged 30 to 79 years from the China Kadoorie Biobank identified four multimorbidity patterns based on 15 chronic conditions and showed that the cardiometabolic multimorbidity class had higher mortality, compared with participants without multimorbidity [32]. Individuals in the “metabolic” and “arthritis-respiratory” classes showed similarly higher mortality in our work, compared to the “relatively healthy” class. In addition, the prevalence of the “metabolic” class (from 11.55 to 15.48%) persistently increased in the US, especially in adults with high SES. These findings highlight the importance of monitoring the health of the “metabolic” class to reduce premature mortality. Of note, we found that participants in relatively severe multimorbidity classes were more likely to use healthcare services in both outpatient and inpatient settings, which was consistent with findings from previous studies [33, 34]. Current models of healthcare services mainly focus on the treatments of single diseases, and multimorbidity receives inadequate attention [35]. Since the rising prevalence of multimorbidity poses a huge challenge to healthcare systems worldwide, integrated patient-centered healthcare services are needed for the management of multimorbidity [36].
SES inequalities in morbidity and mortality are widely explored. Consistent with previous studies [37], our study observed that lower SES was associated with a greater burden of multimorbidity. However, very few studies explored whether SES inequalities existed for mortality associated with multimorbidity, especially multimorbidity patterns. Our study showed that SES modified the associations of multimorbidity patterns with all-cause and CVD mortality; in particular, the negative associations of most multimorbidity patterns with survival time were stronger in those with low SES. Consistently, a study among 50,807 Norwegian participants aged 35–75 years showed an interaction between socioeconomic position (measured by occupation) and multimorbidity in relation to mortality in men, with a stronger association between multimorbidity and mortality in the lower occupational group [11]. However, a study among 500,769 adults from the UK Biobank revealed no significant interactions between the number of chronic conditions and socioeconomic status (based on Townsend quintiles) in risk prediction for all-cause mortality [38]. Data for 6,425 participants from the Whitehall II study showed that the SES based on education, occupation, and literacy did not modify the transition to mortality from multimorbidity [12], and a similar finding was observed in 1,768 participants from the Health, Well-being, and Ageing Cohort Study [13]. These inconsistent findings may be due to different population characteristics, and different definitions of SES and multimorbidity. As extant evidence was mainly from European adults, our work added data from a nationally representative US general population. In addition, most studies used SES based on a single measure that could not capture the full domains of SES. Our study addressed this limitation by generating SES using LCA based on PIR, education, and insurance status in US adults. Our findings suggest that SES inequalities may influence not only the development of adverse health conditions but also their clinical trajectories, which could partially account for the documented widening mortality gap and diverging life expectancy trends across SES groups in US adults [39]. As the rising prevalence of multimorbidity might further widen the SES inequalities in health, health policies for the management of multimorbidity should consider sociodemographic factors.
Our previous work showed that adherence to healthy lifestyles could be an effective way to reduce long-term mortality in both the general population [40] and those with chronic diseases [41]. However, these studies did not address the topic with considerations of multimorbidity, although the presence of multimorbidity could complicate treatment and management. Recently, some studies suggested that adherence to a healthy lifestyle could benefit those with multimorbidity. For example, a study among 480,940 middle-aged adults from the UK Biobank showed that adherence to a healthy lifestyle could be associated with lower mortality in individuals with or without multimorbidity [42]. Another study among 290,795 participants with cardiometabolic multimorbidity from the UK Biobank revealed that regular physical activity and non-current smoking can increase life expectancy in patients with specific cardiometabolic multimorbidity patterns [43]. But no studies ever explored the interaction between healthy lifestyles and multimorbidity status for mortality risk. Our study showed that healthy lifestyles were associated with elevated survival time in participants with any of the six identified multimorbidity patterns, and the benefit was particularly stronger in those with less severe multimorbidity (e.g., “relatively healthy”, “hypercholesterolemia”, and “metabolic” classes). Thus, adopting healthy lifestyles should be considered as an effective intervention in the management of multimorbidity, especially in individuals with relatively less severe multimorbidity. It also suggests that public health recommendations involving engagement in healthy lifestyles should also be emphasized in individuals who already have multimorbidity. Although healthy lifestyles were reported to partially explain the SES inequalities in health [44], our previous work suggested that adopting healthy lifestyles could not substantially reduce the SES inequalities in health for general populations [14, 45]. As SES was negatively related to multimorbidity and modified the association between multimorbidity and mortality in our current work, the policies for reducing SES inequalities in mortality for general populations or those with multimorbidity should go beyond adherence to healthy lifestyles.
Our study has major strengths, including prospective study design, a large sample size, and a well-characterized study population. However, several limitations should be acknowledged. First, the definitions of certain diseases (i.e. COPD, asthma, arthritis, and cancer) were based on self-reported doctor diagnoses and mental health conditions were not considered due to lack of accurate information. SES and healthy lifestyle factors were also mainly self-reported, which could lead to measurement errors. Second, certain healthy lifestyle factors, such as sleep duration, were not included as information was not fully available. Third, chronic conditions were assessed at a single time point, and thus could not capture the impact of dynamic changes of multimorbidity patterns on long-term mortality. Future studies with measurements at multiple time points are needed to address the dynamic changes of multimorbidity patterns. Fourth, although our study adjusted for multiple covariates, residual confounding could still be possible as disease-specific treatments and other unascertained factors were not collected. Fifth, the median follow-up time was 9.58 years, which was moderate to examine the associations between multimorbidity patterns and mortality. Large prospective studies with longer follow-ups are needed to confirm our findings. Sixth, the multimorbidity patterns identified by data-driven methods showed high variations due to different populations, methods, and eligible chronic conditions [46, 47], and a consensus is still needed for clustering chronic conditions in future studies.
In conclusion, we identified six multimorbidity patterns in US adults, and their associations with all-cause and CVD mortality were noted across multimorbidity patterns and were strongest in the study participants with low SES. In addition, healthy lifestyles were associated with longer survival time in all multimorbidity patterns, particularly in those with relatively less severe multimorbidity patterns. Our findings highlight that efforts should be made to address SES inequalities in multimorbidity-related deaths and to promote healthy lifestyles for mortality reductions among individuals with multimorbidity.
This research has been conducted using the NHANES1999-2019. The NHANES data are available online (https://www.cdc.gov/nchs/nhanes/index.htm).
The work was supported by the Fundamental Research Funds for the Central Universities (YJ202346) (Xiong-Fei Pan) and the National Natural Science Foundation of China (72204031) (Qingping Xue) and the CMC Excellent-talent Program (No. 2024qnGzn17) (Qingping Xue). The funders were not involved in the study design, collection, analysis, and interpretation of data.
The U.S. National Centre for Health Statistics Ethics Review Board approved the NHANES survey protocols (Protocol #98 − 12, #2005-06 and #2011–2017), and the study complied with the Declaration of Helsinki. All participants gave written informed consent.
Not applicable.
The authors declare no competing interests.
Not applicable.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Xue, Q., Zhang, S., Yang, X. et al. Multimorbidity patterns and premature mortality in a prospective cohort: effect modifications by socioeconomic status and healthy lifestyles. BMC Public Health 25, 1262 (2025). https://doi.org/10.1186/s12889-025-22216-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12889-025-22216-2