BMC Pulmonary Medicine volume 25, Article number: 159 (2025) Cite this article
Lung cancer surgery has evolved significantly, with minimally invasive video-assisted thoracic surgery (VATS) procedures being compared with traditional open thoracotomies. The incidence of postoperative wound infections is a significant factor influencing the choice of surgical technique. This systematic review and meta-analysis aim to evaluate the impact of thoracoscopic versus open thoracotomy procedures on postoperative wound infections in lung cancer patients.
Following PRISMA guidelines, a comprehensive search across PubMed, Embase, Web of Science, and the Cochrane Library was conducted on September 19, 2023, without time or language restrictions. Peer-reviewed randomized controlled trials, cohort studies, and case-control studies reporting on postoperative wound infections were included. Studies not differentiating between surgical techniques or focusing on irrelevant populations were excluded. Data extraction and quality assessment were independently carried out by two reviewers, using a fixed-effect model for meta-analysis due to the absence of significant heterogeneity (I2 = 0.0%, P = 0.766).
A total of six articles were included. The quality assessment indicated a low risk of bias in most domains. The pooled results showed that open thoracotomy procedures had a twofold increased risk of postoperative wound infections (OR = 2.00, 95% CI: 1.04–3.85) compared to VATS procedures. Publication bias assessment using funnel plots and Egger’s test revealed no significant biases (P > 0.05).
The findings suggest that VATS is associated with a lower risk of postoperative wound infections compared to open thoracotomy, which has implications for surgical decision-making in lung cancer treatment.
Not applicable.
Lung cancer remains a significant global health challenge, consistently ranking as one of the deadliest forms of cancer. This malignancy not only has a high incidence rate but also a notably poor prognosis, primarily due to its aggressive nature and potential for early metastasis [1, 2]. As such, developing effective management strategies that can improve patient survival and quality of life is imperative. Surgery plays a central role in the treatment of non-metastatic lung cancer, often providing the best chance for a curative outcome when the disease is localized [3, 4]. It is within this context that the evolution of surgical techniques, particularly the shift towards minimally invasive approaches, has become a focal point of clinical research and practice.
Traditional open thoracotomy, once the gold standard for lung cancer resection, offers the advantage of direct visual and physical access to the lung, allowing for comprehensive removal of tumor tissue. However, this method is also associated with considerable drawbacks, including significant trauma to the chest wall, prolonged postoperative pain, extensive recovery periods, and a heightened risk of complications such as infections and prolonged hospital stays [5, 6]. In contrast, the introduction of video-assisted thoracic surgery (VATS)—marks a significant shift towards reducing surgical invasiveness. This technique utilizes small incisions and special instruments to perform the surgery, which is guided by a camera [7, 8]. The benefits of VATS include reduced operative trauma, less postoperative pain, shorter hospitalization duration, and quicker return to normal activities [9, 10]. The impact of these surgical approaches on postoperative outcomes, especially wound infections, is a critical area of investigation.
The primary focus of this systematic review and meta-analysis is to evaluate whether the benefits of VATS extend to a lower risk of postoperative wound infections compared to open thoracotomy in lung cancer patients. This question is essential for refining surgical protocols and improving patient care. Current literature provides mixed outcomes, with some studies [11,12,13] favoring VATS procedures in terms of reduced infection rates, while others [14, 15] report negligible differences between the two approaches. These discrepancies underscore the need for a comprehensive meta-analysis to derive more definitive conclusions. Furthermore, assessing the incidence of postoperative wound infections following lung cancer surgeries is crucial due to its implications for patient quality of life, overall treatment success, and cost-efficiency in healthcare management. Infections not only exacerbate patient suffering but also strain hospital resources and can lead to severe complications that jeopardize recovery and survival. In synthesizing data from diverse studies, this meta-analysis aims to offer a robust comparison of VATS and open thoracotomy procedures, providing clear insights that could potentially guide clinical practice.
During the conduct of our systematic review, the search strategy adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16]. We conducted a comprehensive search on September 19, 2023, across four electronic databases: PubMed, Embase, Web of Science, and the Cochrane Library. Our search did not impose any time constraints, allowing for the inclusion of all relevant studies regardless of their publication date. The search terms employed included “Lung cancer,” “Thoraco* OR Minimally invasive,” “Infection,” and “Wound complications.” These terms were carefully chosen to align with the PICO (Patient, Intervention, Comparison, Outcome) framework, thus ensuring a broad and thorough capture of applicable studies. Additionally, we did not apply any language restrictions, enabling the inclusion of studies published in any language. To further enhance the comprehensiveness of our search, we manually screened the reference lists of all relevant articles to identify additional studies that might not have been captured through the database searches.
To achieve a comprehensive analysis of the available evidence, we included randomized controlled trials (RCTs), cohort studies, and case-control studies that provided specific data on postoperative wound infections in lung cancer surgeries. These studies were required to be peer-reviewed and to report original data. Eligible studies exclusively involved adult patients (aged 18 and over) diagnosed with lung cancer.
Studies excluded from our analysis were those that did not clearly distinguish between VATS and open thoracotomy surgical techniques or that combined data from VATS procedures with other minimally invasive surgeries without adequate data separation. Additionally, we excluded non-peer-reviewed literature such as reviews, editorials, commentaries, and conference abstracts due to their potential biases and the incomplete nature of the data typically presented. We also omitted studies focusing on pediatric populations, animal studies, or studies concerning patients with benign conditions, to maintain a focus on relevant human adult oncological outcomes.
During our meta-analysis, the literature screening and data extraction were conducted independently by two evaluators to ensure accuracy and reliability. Each piece of literature was cross-checked between the evaluators to confirm consistency in the data extracted. In cases where discrepancies arose, the reviewers engaged in discussions to resolve the issues, and if consensus could not be reached, a third-party reviewer was consulted as necessary. The specific data extracted from each study included the author(s), the publication year, the number of thoracotomy and thoracoscopy procedures performed, and the country in which the study was conducted. If pertinent data were not available in the published reports, we made efforts to contact the original study’s investigators via email to request any relevant unpublished data.
The quality of the studies incorporated into our meta-analysis was evaluated using the Cochrane Collaboration’s risk of bias tool [17]. This assessment was performed independently by two reviewers who examined several key domains, including random sequence generation, allocation concealment, blinding of participants and personnel, handling of incomplete outcome data, selective reporting, and other sources of potential bias. Each domain within the studies was classified as low, unclear, or high risk of bias based on established criteria. Any disagreements between the reviewers regarding the risk of bias were addressed through discussion or, where resolution was not achievable, by consulting a third reviewer.
In our meta-analysis, we assessed heterogeneity among included studies using chi-square statistics, quantified by the I2 value. An I2 value of less than 50% and a corresponding P-value of 0.10 or greater indicated no significant heterogeneity, prompting the use of a fixed-effect model to compute the combined effect size. Conversely, an I2 value of 50% or more, or a corresponding P-value of less than 0.10, suggested significant heterogeneity, necessitating the use of a random-effects model to account for both within- and between-study variation. To ensure the robustness of our findings, we conducted sensitivity analysis by sequentially removing each study from the analysis and recalculating the overall effect size. This approach helped identify and potentially mitigate the influence of individual studies on the meta-analysis results. Additionally, we assessed publication bias by analyzing the symmetry of the funnel plot. An equitable distribution of studies around the funnel plot’s apex indicated a lower likelihood of results being influenced by publication bias. Additionally, the Egger’s linear regression test was employed as a quantitative measure to detect the presence of any publication bias. All statistical tests were two-sided, with a P-value of less than 0.05 considered statistically significant. Data analysis was performed using Stata version 17 (StataCorp, College Station, TX, USA), ensuring rigorous and precise statistical evaluation.
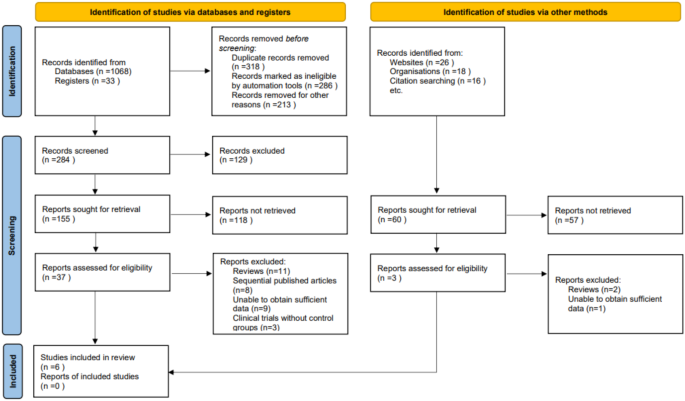
At the initiation of our systematic review and meta-analysis, a comprehensive search across several electronic databases yielded 1,161 potentially relevant articles. To streamline this initial pool, duplicates were removed using a specialized algorithm, ensuring each study was represented uniquely. Subsequently, titles and abstracts were meticulously screened based on strict inclusion and exclusion criteria that encompassed study methodology, demographic characteristics of the participants, clinical outcomes measured, and the quality of research methods. This preliminary evaluation narrowed the selection to 40 articles. Further detailed scrutiny involved multiple investigators independently reviewing the full texts of these articles to ensure an unbiased and thorough assessment. During this phase, 34 articles were excluded for various reasons: 13 were review articles, 8 were sequentially published works, 10 contained insufficient data for analysis, and 3 were clinical trials without control groups. Ultimately, only 6 articles met al.l the rigorous criteria established in our research protocol and were included in the final meta-analysis [18,19,20,21,22,23] (Table 1; Fig. 1).
Study Inclusion Flowchart. A detailed flowchart outlining the selection methodology for studies included in the meta-analysis, from initial identification through to final inclusion
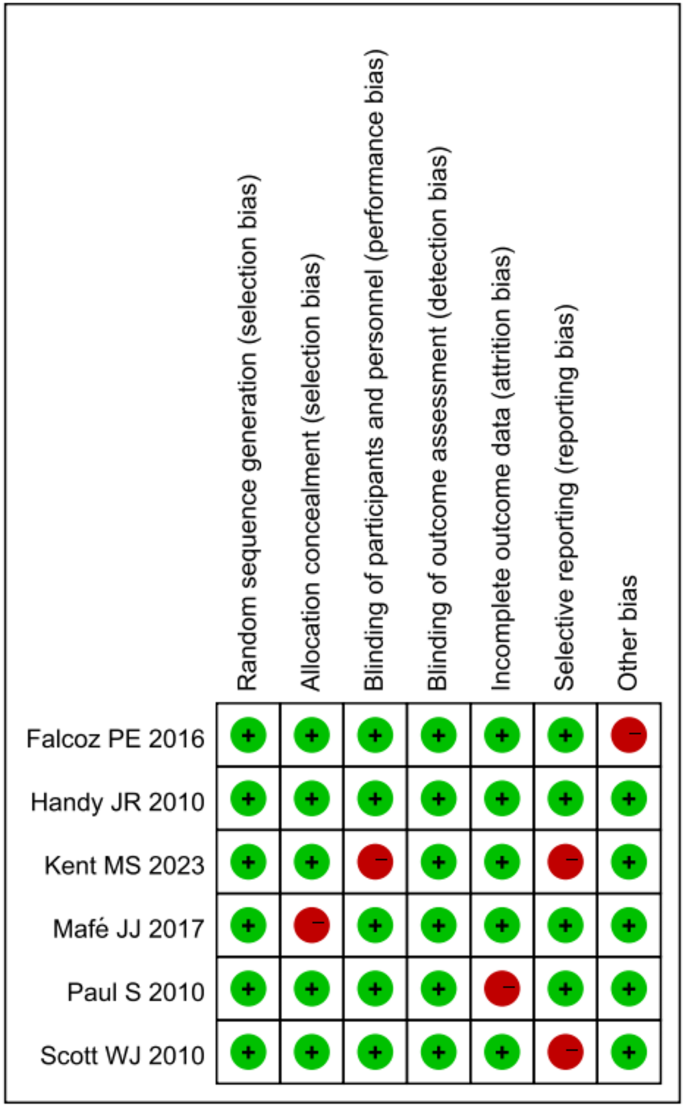
In the quality assessment of the studies included in our meta-analysis, the majority demonstrated robust research methods, with most studies showing a low risk of bias across several key domains. Specifically, random sequence generation, allocation concealment, and blinding of participants and personnel were areas where studies frequently achieved a low risk of bias. However, a few studies exhibited concerns in specific areas, notably in blinding of outcome assessment and other potential sources of bias, where some studies were marked with a high risk of bias. The domain of selective reporting bias was generally well addressed across the majority of studies. The evaluations showed consistent methodological strength in the majority of the assessed criteria, with a minority displaying significant concerns that could influence the meta-analysis outcomes. Overall, the high level of methodological quality in most domains suggests a reliable foundation for the synthesis of findings in our meta-analysis (Fig. 2).
Risk of Bias Assessment. Graphical representation of the risk of bias across included studies, with green indicating a low risk and red signifying a high risk, based on the Cochrane Collaboration’s tool
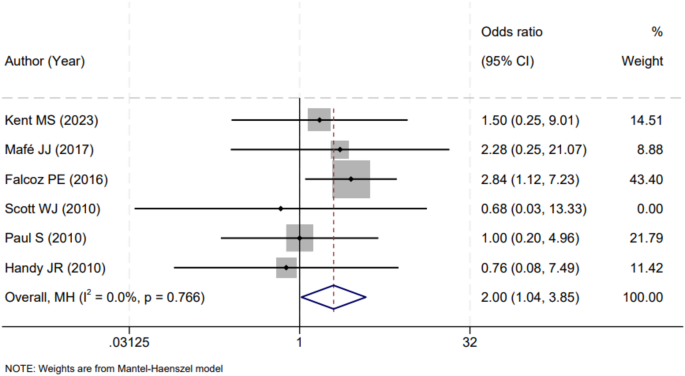
In our meta-analysis, a total of six studies reported on the impact of VATS versus open thoracotomy procedures on the incidence of postoperative wound infections. An examination of these studies revealed no significant heterogeneity (I2 = 0.0%, P = 0.766), permitting the amalgamation of their results using a fixed-effects model. The consolidated findings from these studies indicated that open thoracotomy procedures were associated with a significantly higher rate of postoperative wound infections compared to VATS procedures. The odds ratio (OR) calculated from the meta-analysis was 2.00, with a 95% confidence interval (CI) ranging from 1.04 to 3.85, pointing towards a doubling of the risk when traditional open methods were employed over VATS approaches (Fig. 3). This result underscores the clinical implication that VATS, with its minimally invasive nature, may confer a protective effect against the development of postoperative wound infections.
Forest Plot of Surgical Technique Versus Wound Infection Rates. A forest plot depicting the comparative odds of postoperative wound infections between thoracoscopic and open thoracotomy procedures in lung cancer surgeries
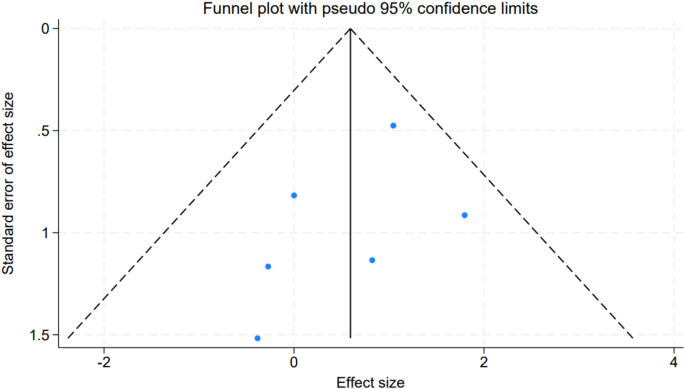
To evaluate potential publication bias within our meta-analysis, we utilized funnel plots corresponding to the included studies. The symmetrical nature of the plots suggested an absence of bias, and the visual inspection did not reveal any significant asymmetry (Fig. 4). This finding was supported by the results of Egger’s linear regression test, which indicated no significant publication bias across the different variables considered (all P-values > 0.05). These results collectively reinforce the credibility of our meta-analysis, suggesting that the findings are not unduly influenced by publication bias and thus, provide a reliable synthesis of the available evidence.
Publication Bias Funnel Plot. A funnel plot assessing the symmetry of study distribution to investigate potential publication bias in the meta-analysis
The surgical management of lung cancer has witnessed significant evolution over the years, with VATS procedures gaining prominence due to their minimally invasive nature. The paradigm shift from open thoracotomy to VATS has been driven by the pursuit of reducing postoperative morbidity and improving patient outcomes. The differential impact of these surgical techniques on the incidence of postoperative wound infections is a critical aspect that merits detailed examination [24, 25]. Postoperative wound infections are a serious complication that can lead to increased morbidity, extended hospital stays, and additional healthcare costs. They pose a substantial burden on patients, often complicating recovery and potentially affecting long-term survival. Within the context of lung cancer surgery, the meticulous comparison between VATS and open thoracotomy procedures is of paramount importance given the fragile health status of patients who typically present with a myriad of comorbidities, which may predispose them to a higher risk of complications [26].
The results of our meta-analysis, encompassing six studies on VATS versus open thoracotomy procedures, have illuminated a critical aspect of postoperative care in lung cancer surgery. Notably, the analysis revealed that patients undergoing open thoracotomy procedures exhibited a twofold increased risk of postoperative wound infections when compared to those receiving VATS. This finding is both statistically significant and clinically relevant, suggesting a substantive disparity in the safety profile of these two surgical modalities, specifically concerning wound-related outcomes. The inherent mechanisms contributing to this discrepancy are multifaceted, rooted in both the physiological impact of the surgical technique and the patient recovery trajectory [27]. Open thoracotomy procedures are typically more invasive than VATS ones, involving larger incisions that breach the integrity of the chest wall and potentially expose a greater surface area to infectious agents. This invasiveness can beget a cascade of systemic inflammatory responses, which may undermine the body’s ability to fend off infections [28]. The larger wounds associated with open thoracotomies require more extensive tissue manipulation and longer operative times, both of which have been correlated with higher infection rates in various types of surgeries.
Furthermore, the minimally invasive nature of VATS procedures typically results in less postoperative pain, allowing for earlier mobilization and lung re-expansion. Pain and prolonged immobility are risk factors for infection, as they can lead to reduced respiratory effort and consequent atelectasis, which may foster bacterial proliferation and subsequent infection. VATS, with smaller incisions and less tissue dissection, are likely to mitigate this risk by enabling quicker patient mobilization and thus enhancing pulmonary hygiene postoperatively. The length of hospital stay, which is generally longer following open surgeries, provides another window into understanding the increased infection risk [29, 30]. A prolonged hospital stay inherently increases the patient’s exposure to nosocomial pathogens, thereby raising the probability of wound contamination. Additionally, the pain and immobilization consequent to an open thoracotomy may lead to a longer reliance on invasive devices such as urinary catheters or central lines, which are known to be potential sources of infection [31, 32].
From an immunological perspective, the systemic stress response triggered by more extensive surgical trauma may impair the immune system’s efficiency. Surgical stress has been shown to temporarily reduce the function of natural killer cells and other immune components, potentially offering a period of increased vulnerability to infections. VATS procedures, inducing less physiological stress, may preserve immune function to a greater degree than open thoracotomy, thereby providing an immunological rationale for the observed differences in infection rates [33]. It is also essential to consider the role of intraoperative and postoperative management strategies. Practices such as antibiotic prophylaxis, aseptic technique, and postoperative wound care are universally critical, but their implementation may be even more pivotal in the context of open thoracotomies due to the increased infection risk. Whether discrepancies in these practices between VATS and open thoracotomy cases contributed to our findings is a question that merits further investigation.
In light of these results, it is clear that VATS presents a preferable option in terms of postoperative wound infection risk. However, the choice of surgical approach should also be contingent on other factors, including tumor location and stage, patient comorbidities, and the surgeon’s expertise. Moreover, while the lower risk of infection is a compelling advantage of VATS, the broader implications for patient survival, recovery quality, and overall cost-effectiveness warrant comprehensive evaluation.
While our meta-analysis offers valuable insights into the impact of surgical techniques on postoperative wound infections, it is not without limitations. One notable constraint is the potential for inherent selection bias within the included studies, as patients undergoing VATS procedures may have been pre-selected based on lower operative risks or smaller tumor size. Additionally, the variability in surgical expertise and postoperative care protocols across different institutions may have influenced the outcomes, but these factors were not consistently reported. Furthermore, the number of studies analyzed was relatively small, which could limit the generalizability of our findings. Lastly, our analysis did not account for all possible confounders, such as patient comorbidities or the use of adjuvant therapies, which could affect the risk of postoperative infections. Future research should explore the timing and causative agents of postoperative infections for a deeper understanding. Investigating the criteria and risk factors influencing the decision to choose thoracoscopic surgery would provide valuable context for these findings. The role of antibiotic prophylaxis in reducing infection rates across different surgical techniques warrants further examination. Finally, distinguishing between uniportal and multiportal VATS in future studies could provide further insights into their impact on postoperative complications.
Our meta-analysis concludes that in lung cancer patients, open thoracotomy procedures significantly increase the risk of postoperative wound infections compared to VATS procedures. This evidence highlights the importance of considering surgical approach in clinical decision-making to enhance patient outcomes and minimize postoperative complications.
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
We appreciate the technical support provided by our laboratory’s entire research personnel and students.
Not applicable.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Wang, JQ., Ma, ZJ. Impact of video-assisted thoracic surgery versus open thoracotomy on postoperative wound infections in lung cancer patients: a systematic review and meta-analysis. BMC Pulm Med 25, 159 (2025). https://doi.org/10.1186/s12890-025-03589-x