Journal of Translational Medicine volume 23, Article number: 394 (2025) Cite this article
Clinical implications of metagenomics next-generation sequencing (mNGS) in sepsis have not been fully evaluated. This study aimed to determine the diagnostic, therapeutic, and prognostic impacts of mNGS in sepsis.
This multicenter prospective study was conducted at 19 sites in China from 2020 to 2021, and 859 adult patients hospitalized with sepsis were enrolled. The advantages, challenges, knowledge gaps and privacy risks of mNGS were carefully introduced to all participants, and participants chose on their own to either receive conventional microbiological test (CMT) alone (conventional-test-only group, n = 394) or receive mNGS test along with CMT (combined test group, n = 465). For prognostic analysis, the primary endpoint was 28-day mortality. Secondary endpoints included 7-day mortality and average per-day hospital cost. Inverse probability of treatment weighting was used to balance covariates between groups. Concurrent CMT and mNGS results from patients in the combined test group were used for diagnostic analyses. Therapeutic impact of mNGS was evaluated based on subsequent antibiotic adjustment.
Compared with composite reference standard, the positive percent agreement of mNGS among infected site samples was significantly higher than that of CMT (92.0% [95% CI, 88.7 to 94.5] vs. 51.1% [95% CI, 45.9 to 56.2], p < 0.001), while the negative percent agreement of mNGS was inferior to that of CMT (39.6% [95% CI, 29.5 to 50.4] vs. 69.2% [95% CI, 58.7 to 78.5], p < 0.001). The mNGS test identified causal microbes in 344 (74.0%) patients, and concomitant antibiotic changes occurred in 136 patients (29.2%). Death by day 7 occurred in 24 of 465 (5.2%) patients in the combined test group and in 34 of 394 (8.6%) patients in the conventional-test-only group (hazard ratio, 0.44 [95% CI, 0.26 to 0.77], p = 0.004). However, no significant difference in 28-day mortality was observed between two study groups (hazard ratio, 0.82 [0.56 to 1.20], p = 0.300).
The mNGS test of infected site samples exhibited 40% higher pathogen detection rate than CMT in patients with sepsis, which led to improved etiological diagnosis and tailored antibiotic therapy. Additional use of mNGS halved the risk of early death in 7 days, but did not improve 28-day survival in patients with sepsis.
chictr.org.cn Identifier: ChiCTR2000031113. Registered 22 March 2020.
Sepsis is a life-threatening organ dysfunction triggered by a dysregulated host response to an underlying infection [1]. Sepsis remains a leading cause of morbidity and mortality worldwide, with an annual estimate of 49 million incident cases and 11 million related deaths [2]. Timely and appropriate antibiotics are crucial for improved outcomes [3], but pathogen identification can be challenging under traditional modality due to low diagnostic yields and long turnaround times. Previous reports demonstrated increased proportion of culture-negative sepsis, which is associated with greater acute organ failure and mortality [4, 5]. Notably, atypical pathogens, fastidious bacteria, and opportunistic organisms are difficult to culture [6]. Antibiotic exposure can further reduce culture-based pathogen detection rate [7], while the inability to detect mixed infections is another thorny problem [8]. Moreover, conventional microbiological tests (CMT) usually take 2 to 5 days to identify causative pathogens and measure antibiotic susceptibility, which may contributes to preventable deaths [9]. As for serological or polymerase chain reaction (PCR) tests, a presumptive microbiological diagnosis has to be made by the physician first before a specific test can be ordered.
Recent years, new technological advancements have revolutionized the modalities of disease diagnosis and treatment [10, 11]. Metagenomic next-generation sequencing (mNGS) is a molecular-based process in which all nucleic acids in a sample are amplified and sequenced in an unbiased manner, enabling the identification of microbes without prior knowledge of potential pathogens [12, 13]. The clear advantages in speed and sensitivity makes mNGS a potent diagnostic tool for infectious diseases. Previous literature revealed that mNGS yielded a higher pathogen detection rate in suspected sepsis [14, 15], pneumonia [16, 17], encephalitis and/or meningitis [18, 19], infections in immunocompromised hosts [20, 21], and was less affected by antibiotic exposure [22]. However, there is still a large gap between research evidence and daily clinical practice. Comprehensive evaluation of the diagnostic and therapeutic impacts, as well as the cost-effectiveness of mNGS among large sepsis cohorts are limited. Besides, it remains unknown whether the turnaround time of mNGS would be sufficiently short to make such results actionable, and whether earlier and more precise etiological diagnosis would lead to improved patient outcomes. Hence, in this study, we aimed to explore the role and cost of mNGS in detecting causative pathogens, guiding antibiotic therapies, and improving prognosis in a large multicenter sepsis cohort.
This study was conducted at 19 hospitals in China between March 2020 and October 2021. Adult patients were eligible for the study if they were hospitalized for sepsis and fulfilled the Sepsis-3 criteria [1]. Patients were excluded if they chose do-not-resuscitate, participated in other interventional trials, refused to give informed consent, or failed to have infected site samples readily available for microbiological tests within 24 hours of sepsis diagnosis. The study was composed of a non-randomized controlled trial and a diagnostic test study, and the study flowchart can be found in Fig. 1.
The potential benefits and risks of the use of additional mNGS test were carefully explained to the participants or their authorized trustee (Fig. 2). Participants then chose the study group to which they would like to be allocated. For patients in the conventional-test-only group, only CMT would be performed on infected site samples as in routine clinical care. For patients in the combined test group, both CMT and mNGS would be conducted on infected site samples. The investigator paid for all mNGS tests in the study, and the cost of mNGS was also included in the calculation of hospitalization expenses during data analysis. Selection of the appropriate conventional microbiological testing methods, and interpretation of the conventional and mNGS test results were only at the discretion of the treating clinicians. No test results were disclosed outside the treating team to prevent interference. Given the study design, practitioners were necessarily unblinded. The rationale for having a non-randomized design for this study is that at the moment, mNGS is still an investigational technique under evaluation and the use of mNGS can raise complicated privacy and ethical challenges [23]. This study complied with the Declaration of Helsinki and was approved by the institutional review board at each study site. All participants provided written informed consent. All study-related information including sequencing data was stored securely at the study site with access restrictions to protect confidentiality.
Conventional microbiological testing methods used in this study include culture, smear microscopy, acid-fast staining, Grocott’s methenamine silver staining, serological tests for cytomegalovirus (CMV), Epstein-Barr virus (EBV), mycoplasma pneumoniae and cryptococcus, serum (1,3)-β-D-glucan test (G test), serum galactomannan test (GM test), bronchoalveolar lavage fluid (BALF) GM test, PCR test for CMV and EBV, nucleic acid test for influenza A and B, respiratory syncytial virus, adenovirus, rhinovirus, and mycoplasma pneumoniae, T-SPOT.TB and Xpert MTB/RIF. Notably, due to local ‘Dynamic COVID-zero’ strategy during the study period, patients who test positive for COVID-19 were quarantined in designated hospitals and were not able to get admitted to any study center.
The mNGS platform used in the study was Illumina NextSeq 550. Detailed descriptions of the laboratory workflow are provided in the supplementary materials. Briefly, after sample preparation and nucleic acid extraction, 30 µL DNA was used to generate libraries with the Nextera DNA Flex kit (Illumina, San Diego, USA), and 10 µL purified RNA was used for complementary DNA (cDNA) generation and library preparation with the Ovation Trio RNA-Seq Library Preparation Kit (NuGEN, CA, USA). Purification and size selection were carried out following the double-sided bead purification procedure. The library was prepared by pooling a 1.5 pM concentration of each purified sample equally for sequencing on an Illumina NextSeq 550 sequencer using a 75-cycle single-end sequencing strategy. Library quality was assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, USA).
Due to lack of gold standard of causative microbes, in the diagnostic accuracy study, microbiological test results were compared to a clinically-adjudicated composite reference standard. When the participant was discharged from hospital or deceased, a committee composed of a critical care medicine specialist, an infectious disease physician and a clinical microbiologist performed the clinical adjudication following a standardized algorithm [14]. Complete medical records, including patient history, physical examination, laboratory investigations, all microbiological test results, imaging data, medical treatment, response assessment, and clinical outcomes were reviewed. Another senior physician would be involved in the adjudication in case of disagreement.
The therapeutic impact of mNGS tests was evaluated based on the following criteria. Escalation of treatment was defined as initiation or escalation of appropriate therapy (antibiotics, antivirals, or antifungal therapy). De-escalation of treatment was defined as discontinuation or de-escalation of unnecessary therapy. Medication unchanged applied to situations where treating physicians perceived no need to change the therapy.
In the prognostic study, the primary outcome was death from any cause by day 28. Secondary outcomes included death from any cause by day 7, changes in Sequential Organ Failure Assessment (SOFA) and Acute Physiology And Chronic Health Evaluation II (APACHE II) scores by day 7, 28-day hospital-free days, and average per-day hospital cost. Average per-day hospital cost of the combined test group were calculated as the sum of total hospitalization expenses and the cost of mNGS, divided by the hospital length of stay.
Based on the previous Chinese Epidemiological Study of Sepsis (CHESS) study conducted in China, a 28-day all-cause mortality rate of 24.3% in the conventional-test-only group was assumed in this trial [24]. According to the results of a multicenter retrospective cohort study, 16.4% sepsis-associated death were judged as definitely or moderately-likely preventable [25]. Hence, we estimated that a sample size of 858 patients would provide a power of 80% for a 2-sided 0.05 significance level on an assumption that the 28-day mortality would be 20.3% in the combined test group.
Categorical variables were summarized as numbers and percentages, and were analyzed using the chi-square test. Continuous variables were summarized as medians and interquartile ranges (IQR), with Mann-Whitney U test used to assess the difference between groups when the assumptions of normality of t-test were not met. Confidence intervals (CI) were calculated according to the percentile method. McNemar’s test was used to examine paired dichotomous data. The primary analysis used inverse probability of treatment weighting (IPTW). To calculate the inverse probability weights, we estimated each patient’s propensity to receive combined microbiological tests using a logistic regression model including twenty-four confounding variables (see Table 1 for those variables). Stabilized weights were used to reduce the variability of estimates. Kaplan-Meier curves were plotted and log-rank test results were reported based on the inverse-probability-weighting weights. Hazard ratios were estimated using weighted Cox proportional-hazards models, stratified by study center and adjusted for clinical covariates. Several sensitivity analyses were performed to assess the robustness of results, including multivariable adjustment and propensity score matching (PSM). To conduct PSM, 1:1 nearest neighbour method with a caliper of 0.2 was applied to create a matched control sample. Balance among covariates were assessed by standardized mean difference and a difference of 10% or less was considered well-balanced. We analyzed changes in SOFA and APACHE II scores, as well as hospital free days and hospitalization costs in the framework of a linear model. Statistical analyses for secondary end points were not adjusted for multiplicity, so the findings should be interpreted as exploratory. Similarly, the widths of CI have not been adjusted for multiplicity and should not be used in place of hypothesis testing. The statistical analyses were performed using R, version 4.3.0 (R Foundation), with a two-sided P value of less than 0.05 considered as significant.
A total of 1368 patients were screened and 859 patients were eventually enrolled, of whom 394 chose to enter the conventional-test-only group and 465 chose to enter the combined test group (Fig. 1). Baseline characteristics of the two groups before and after IPTW and PSM are shown in Table 1 and supplementary table S1, respectively. Other important laboratory features were summarized in supplementary table S2. Patients who chose to receive additional mNGS test were older, had higher Charlson comorbidity index, SOFA and APACHE II scores, and were more likely to suffer from shock or receive invasive mechanical ventilation. The covariates were well balanced after weighting or matching with all standardized differences less than 10%.
Of the 465 patients in the combined test group, the majority were diagnosed with pulmonary infections (310 [66.7%]), followed by urinary tract infections (66 [14.2%]) and intra-abdominal infections (53 [11.4%]). Accordingly, most of the infected site samples belonged to the respiratory system, with 293 (63.0%) from BALF or sputum, followed by urine (66 [14.2%]), pus from deep abscess (30 [6.5%]), peritoneal effusion (22 [4.7%]), pleural effusion (17 [3.7%]), and blood (14 [3.0%]) (Fig. 1). The median turn-around time were 24.5 (IQR, 24.0–25.0) hours for mNGS and 48.0 (IQR, 27.0–72.0) hours for culture (supplementary figure S1).
For the 465 patients in the combined test group, causative pathogens were identified in 374 (80.4%) patients when composite reference standard was used as the criteria. At per-patient level, the conventional and mNGS test of infected site samples successfully detected causative pathogen(s) in 191(41.1%) and 344 (74.0%) patients, respectively. The overall positive percent agreement of mNGS was significantly higher than that of CMT (92.0% [95% CI, 88.7 to 94.5] vs. 51.1% [95% CI, 45.9 to 56.2], p < 0.001) (Table 2) and the benefit was maintained across all sample types and clinical subgroups, yet an even larger difference favoring mNGS could be observed in immunocompromised hosts, in patients with lower SOFA scores and without invasive mechanical ventilation (supplementary figure S2). The overall negative percent agreement of mNGS was inferior to that of CMT (39.6% [95% CI, 29.5 to 50.4] vs. 69.2% [95% CI, 58.7 to 78.5], p < 0.001), mainly due to the unsatisfactory performance of mNGS among respiratory samples (27.8% [95% CI, 17.9 to 39.6] vs. 62.5% [95% CI, 50.3 to 73.6], p < 0.001) (Table 2). The negative predictive value of mNGS was higher than that of CMT (54.5% [95% CI, 41.8 to 66.9] vs. 25.6% [20.3 to 31.5], p < 0.001), while the positive predictive values of the two tests were comparative (86.2% [95% CI, 82.4 to 89.4] vs. 87.2% [95% CI, 82.1 to 91.3], p = 0.588) (Table 2).
Per-pathogen level analysis yielded an overall positive percent agreement of 89.3% (95% CI, 86.2 to 91.9) for mNGS and 45.1% (40.7 to 49.6) for CMT (p < 0.001). The positivity rate of mNGS outperformed that of CMT in pulmonary infections of P. aeruginosa (26/29 vs. 15/29, p = 0.013), S. aureus (17/18 vs. 7/18, p = 0.013), E. faecium (13/14 vs. 0/14, p < 0.001), H. parainfluenzae (11/11 vs. 1/11, p = 0.002), E. faecalis (8/8 vs. 1/8, p = 0.016), anaerobes (7/7 vs. 1/7, p = 0.031), C. psittaci (6/7 vs. 0/7, p = 0.031), L. pneumophila (6/6 vs. 0/6, p = 0.031), H. influenzae (6/6 vs. 0/6, p = 0.031), P. jiroveci (20/20 vs. 2/20, p < 0.001), and Aspergillus spp. (14/15 vs. 4/15, p = 0.006). Details of the pathogens and the concordance between tests are shown in supplementary table S3–6.
Among the 465 patients in the combined test group, mNGS results led to medication changes in 136 (29.2%), including escalation of antimicrobials in 40 (8.6%), de-escalation in 58 (12.5%), and concurrent escalation and de-escalation in 38 (8.2%). (supplementary figure S3). Commonly added antimicrobials based on mNGS results included glycopeptides (17.9%) for treatment of gram-positive cocci, quinolones (5.1%) for treatment of atypical bacteria, sulfamethoxazole/trimethoprim (SMZ/TMP) (14.1%) and antifungals (28.2%). Meanwhile, the most commonly de-escalated antibiotics were carbapenems (22.9%), followed by glycopeptides (21.4%) and quinolones (16.8%) (supplementary table S7-8).
In subgroup analysis, more medication changes happened in immunocompromised patients than in immunocompetent ones (escalate: 23.3% vs. 15.8%, de-escalate: 28.3% vs. 19.5%), and in patients presented without shock than those with shock (escalate: 18.2% vs. 14.2%, de-escalate: 22.3% vs. 17.8%). As for infection sites, mNGS showed the least clinical impact in patients with intra-abdominal infections, with escalation of therapy in 9.4% and de-escalation in 15.1% patients (supplementary figure S4).
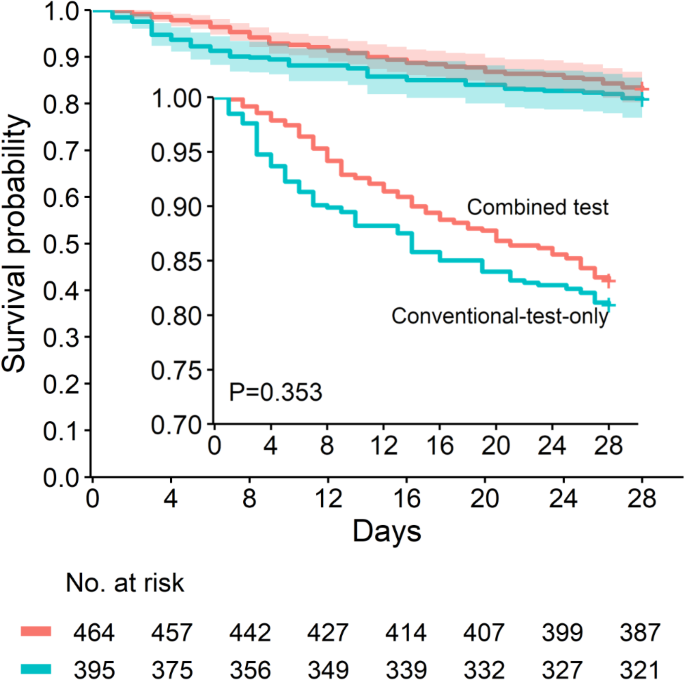
By day 28, death had occurred in 66 of 394 patients (16.8% [95% CI, 13.4 to 20.8]) in the conventional-test-only group and in 89 of 465 patients (19.1% [95% CI, 15.8 to 23.0]) in the combined test group. In the primary multivariable analysis with IPTW, there was no significant difference in 28-day mortality between groups (hazard ratio, 0.82 [0.56 to 1.20], p = 0.300) (Table 3). Weighted 28-day survival curves are plotted in Fig. 3. In sensitivity analyses, alternative analytic strategies yielded consistent results on 28-day mortality, including multivariable Cox regression in crude data (hazard ratio, 0.83 [95% CI, 0.57 to 1.19], p = 0.303) and PSM analysis (hazard ratio, 0.77 [95% CI, 0.51 to 1.17], p = 0.228) (Table 3). In the predefined subgroup analyses, younger participants aged 55 years or below showed a greater benefit from additional mNGS test when compared to older participants (supplementary figure S5).
Inverse probability weighted Kaplan-Meier survival curves censored at 28 days for 859 patients with sepsis. Shaded bands represent the 95% confidence interval. P value was calculated using log-rank test. The at-risk table shows the number of patients at risk after inverse probability treatment weighting. The inset shows the same data on an enlarged y axis
As for secondary outcomes, death from any cause by day 7 occurred in 34 of 394 of patients (8.6% [95% CI, 6.2 to 11.8]) in the conventional-test-only group and 24 of 465 patients (5.2% [95% CI, 3.5 to 7.6]) in the combined test group (hazard ratio, 0.44 [95% CI, 0.26 to 0.77], p = 0.004). Patients in the combined test group showed a more significant decrease in SOFA scores at day 7 (adjusted difference: -0.50 [95% CI, -0.86 to -0.14], p = 0.007) and tended to have a more obvious decrease in APACHE II scores at day 7 (adjusted difference: -0.58 [95% CI, -1.27 to 0.11], p = 0.097), though significance was not reached. No substantial difference was found between groups in 28-day hospital-free days or average per-day hospital cost (Table 3).
In this study involving a wide range of samples from patients with sepsis, mNGS was proved to be a rapid and sensitive method for identifying causative pathogens, thus serving as an important reference for tailored antimicrobial treatment. However, the additional use of mNGS test did not improve 28-day survival in patients with sepsis.
Few large, multicenter studies have comprehensively evaluated the clinical impact of mNGS in sepsis. In a cohort of 350 patients with a sepsis alert, the identification of sepsis etiology was significantly higher for mNGS than for all CMT methods combined [14]. Another multicenter study, involving 277 patients with suspected sepsis, reported that blood mNGS showed better diagnostic performance than blood culture, especially in those at earlier stage of infection [15]. However, previous studies had relatively small sample size and focused mainly on blood mNGS. Our study further demonstrated that mNGS was more sensitive than CMT across various sample types. Subgroup analysis showed that the causative pathogen detection rate of mNGS exceeded that of CMT by an absolute difference of nearly 50% points in those with lower SOFA scores, and in those without septic shock or mechanical ventilation. Given the unsatisfactory diagnostic performance of CMT in patients with early stage of the disease, mNGS may be considered as an upfront diagnostic test in those patients, rather than as a last resort.
The effects of a diagnostic modality on patient outcomes are generally mediated by subsequent therapeutic interventions. In the current study, antimicrobial therapy was optimized for roughly 30% of patients based on mNGS results, which is similar to the findings of Liang et al. on respiratory samples in patients with pulmonary infections [26], yet higher than those of Hogan et al. on plasma samples in patients with suspected infections [27]. A probable reason is that pathogen detection rate is much higher for infected site samples than for blood samples, suggesting that it may be better to use the former for mNGS testing. Notably, the early initiation of broad-spectrum antibiotics in sepsis may provide no reason for antibiotic escalation even if specific pathogens were later determined by mNGS. This is especially the case in patients with abdominal infections, the majority of whom were infected by gram-negative bacilli and treated with appropriate empirical antibiotics in our study, suggesting that there may be bare necessity to use mNGS in abdominal sepsis. Besides, de-escalation is no easy job for physicians in the real world due to fear of false negative results [28]. It is also worth noting that the use of mNGS could be even more helpful for immunocompromised hosts in identifying pathogens and tailoring treatment, which is consistent with prior reports [29,30,31,32]. This was probably due to the advantage of mNGS in detecting opportunistic microbes [33, 34], indicating potential application of mNGS in well-selected patients.
It remains debated to which extent earlier etiological diagnosis could have mitigated poor outcomes in sepsis [35]. It has been long believed that early identification of causative pathogens is crucial for favorable outcomes, but direct evidence was lacking. Recent studies demonstrated that appropriateness of initial empirical antibiotics was not associated with better prognosis, probably due to higher compliance with the surviving sepsis campaign bundle [36, 37]. However, methodologic limitations existed in evaluating the appropriateness of antibiotics. The current study found that additional mNGS test resulted in clinically-important modifications to empirical antimicrobial therapy in approximately 30% of patients, particularly impactful in those infected with gram-positive cocci, atypical bacteria, or opportunistic pathogens. However, mNGS-guided treatment measures only held prognostic value within 7 days, while no significant survival benefit was observed at day 28. Several reasons may explain the discrepancy. First, other factors can impact in-hospital outcomes, including delay in source control, hospital-acquired infections, medication-related adverse events, and procedural complications. Second, only a small proportion of sepsis-associated deaths were regarded as potentially preventable under optimal clinical care, while most underlying causes of death were related to severe chronic comorbidities [25]. In our study, patients who chose to enter the combined test group had more comorbidities and worse disease status at baseline, which could result in increased mortality. Noteworthy, our data was also in line with the findings of prior meta-analysis results, which implied the pooled odds ratio of inappropriate administration of empirical antibiotics were higher for 7-day mortality than for 28-day mortality [38]. Additionally, current guidelines suggest using shorter (5–8 days) over longer (10–14 days) duration of antimicrobial therapy in adult sepsis with adequate source control, which underscored the pivotal role of appropriate antibiotics in early phase of the disease [39]. Hence, it is reasonable that additional mNGS and mNGS-guided treatment measures can only impact on early patient outcomes. Given the non-randomized design, however, the current study should not be taken to rule out the long-term benefit of mNGS in sepsis. Our findings illustrated that mNGS can provide a valuable treatment window for sepsis, so long as the cost and availability of the test do not hamper its application. Further studies in larger populations are warranted.
Another interesting finding was that additional mNGS test provided a 28-day mortality benefit over CMT in patients aged 55 years or below. Further analysis showed that younger patients had lower Charlson Comorbidity Index, while no significant difference in baseline SOFA score or antibiotic adjustment rate was observed between two age groups (supplementary table S9 and figure S4A). Hence, it can be assumed that age-related comorbidities and chronic disease may greatly impaired the prognostic value of mNGS. Of note, our results is also in line with the previous study that reported the interaction between age and benefits of antibiotics, which was associated with significant improvements in 28-day mortality in younger sepsis patients and lessened as age increased [40]. Further study is warranted to investigate the survival benefit of mNGS in different age groups.
To our knowledge, this is the first multicenter prospective study on the prognostic impact of mNGS in sepsis. Undoubtedly, a randomized trial, which minimizes the unmeasured confounding and bias, is the best approach to determine whether benefit can be ascribed to the diagnostic method. However, the rationale for having a non-randomized design is that at the moment mNGS is still an investigational technique under evaluation and use of mNGS can raise complicated privacy and ethical challenges. In real world clinical practice, physicians also explain potential benefits and risks of additional mNGS test to patients and their families, and patients or their authorized trustee chose whether to receive additional mNGS test or not after careful consideration. Hence, the results of the study are pragmatic and can be generalized to the real world in that they may reflect the decisions that are made in the real world [41]. With the analytic approaches used in the examination of our cohort, we have tried to minimize possible confounding in a variety of ways. In the main analysis, a multivariable regression model with IPTW was used. The application of propensity-scored weights to the study population creates a pseudo-population in which measured confounders are equally distributed across groups. Compared with PSM, in which unmatched individuals are discarded from the analysis, IPTW is able to retain most individuals in the analysis, increasing the effective sample size [42], and also allows for the estimation of marginal hazard ratios with minimal bias [43]. Notably, we also performed a series of analysis using multivariable regression and PSM. The consistency of the results across multiple sensitivity analyses made our findings reassuring.
An important nontechnical issue regarding mNGS is the cost of sequencing, which was one of the main reasons that limit the wide application of mNGS in clinical routine. Although the cost of sequencing has dropped sharply in the past decade, it is still much higher than that of any single conventional test [21]. Besides, the inability to distinguish colonization, which may lead to overuse of antibiotics and increased costs were another concern. In our study, no significant difference in average per-day hospital cost could be observed between the two intervention arms, suggesting that additional mNGS test improved short-term survival outcomes not at the expense of increased healthcare costs.
Our study had some limitations. First, non-randomized nature of the study and patient self-assignment to intervention arm can result in selection bias and decreased internal validity, as well as unequal groups and loss of blinding. Second, despite the extensive adjustment for covariates, there is still a chance of unmeasured confounding as with all non-randomized trials. Likewise, the treatment regimens in response to pathogen detection from multi-centers may be an important factor affecting the estimates of outcomes. Third, interpretation of mNGS results may be subjective and all studies on mNGS have the same bias of arbitrarily assigning a causative pathogen. In the case of respiratory diseases, entanglement between dysbiosis and infection is even harder to illustrate. Future research is needed in the following fields: (1) randomized controlled trial evaluating the outcomes of additional mNGS test in sepsis; (2) predictive enrichment selecting patients who are more likely to benefit from additional mNGS test; (3) pragmatic study exploring whether to routinely perform mNGS with conventional tests or to consider mNGS as a salvage method.
In conclusion, the mNGS test of infected site samples exhibited higher pathogen detection rate than CMT in patients with sepsis, which led to improved etiological diagnosis, tailored antibiotic therapy, and better early prognosis. With technology improvements, reduction of sequencing costs and careful handling of data protection issues, mNGS can be used as a valuable supplement to conventional tests in sepsis.
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
- mNGS:
-
Metagenomics next-generation sequencing
- CMT:
-
Conventional microbiological test
- PCR:
-
Polymerase chain reaction
- BALF:
-
Bronchoalveolar lavage fluid
- SOFA:
-
Sequential Organ Failure Assessment
- APACHE II:
-
Acute Physiology And Chronic Health Evaluation II
- IQR:
-
Interquartile ranges
- CI:
-
Confidence interval
- IPTW:
-
Inverse probability of treatment weighting
- PSM:
-
Propensity score matching
The authors would like to thank all the patients and the frontline staff who participated in the study. The authors deeply appreciate the support from Prof. Li Chen (Fudan University, Shanghai, China) on methodology and Dr. Zhenzhen Lu (Department of Biostatistics, Zhongshan Hospital, Fudan University, Shanghai, China) on data analysis. The authors also acknowledge the contributions and cooperation of Vision Medicals Center for Infectious Diseases, Guangzhou, China.
This research was funded by National Key Research and Development Program of China (NKPs) (2021YFC2501800 to Zhenju Song; 2023YFC0872500 to Zhenju Song, 2023YFC3043507 to Zhenju Song), National Natural Science Foundation of China (NSFC) (82072214 to Zhenju Song); Science and Technology Commission of Shanghai Municipality (STCSM) (23Y31900100 to Zhenju Song and 21MC1930400 to Zhenju Song), and Clinical Characteristic Discipline Construction Project of Shanghai Pudong New Area Health Commission (PWYts2021-17 to Dongfeng Guo).
The research related to human use has complied with all the relevant national regulations, institutional policies. The study was conducted in compliance with the Declaration of Helsinki and its later amendments, and was approved by the institutional review board at each site. All participants provided written informed consent.
Not applicable.
The authors declare that they have no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Xu, F., Chen, C., Lu, S. et al. Impact of metagenomics next-generation sequencing on etiological diagnosis and early outcomes in sepsis. J Transl Med 23, 394 (2025). https://doi.org/10.1186/s12967-025-06332-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12967-025-06332-6