BMC Nephrology volume 26, Article number: 57 (2025) Cite this article
To promote the application of high-quality frailty risk prediction models in the field of debilitation among Chinese patients undergoing MHD, and to provide a basis for optimisation and improvement of future studies.
A literature search was conducted in Chinese and English databases (PubMed, Web of Science, Cochrane Library, CINAHL, Embase, CNKI, Wanfang, VIP, SinoMed) and the cutoff date for which was April 30, 2024. Literature characteristics, types of studies, predictors, model construction methods and results were analysed and compared.
Ten studies met the inclusion criteria, and seven were focused on model development and validation. A total of 12 predictive models were included across these 10 studies; three of these were solely model development studies, while seven were both model development and validation. The area under the curve (AUC) for the subjects’ operating characteristics was > 0.7 in all ten studies. The most frequently identified predictors in the models included age, nutritional status, the presence of multimorbidity, gender, and depression. While the overall applicability of the ten studies was deemed satisfactory, it is important to note that all studies exhibited a high risk of bias, particularly concerning the data analysis component.
The frailty risk prediction models for patients undergoing maintenance hemodialysis have demonstrated satisfactory applicability; however, they are all associated with a significant risk of bias and lack comprehensive external validation. To develop more accurate and practical prediction models, future studies must rely on large-sample, multicenter prospective cohort studies and adhere to a rigorous study design.
Not applicable.
Chronic kidney disease (CKD) is globally recognized as a significant public health issue. A systematic evaluation conducted in 2022 revealed that the prevalence of CKD in Asia ranges from 7.0 to 34.3%. Notably, China has the highest number of adult CKD patients, with approximately 159.8 million cases [1]. End-Stage Renal Disease (ESRD), a severe progression of Chronic Kidney Disease (CKD), is characterized by irreversible renal failure, which results in disturbances in water, electrolyte, and metabolite balance within the body, severely impacting patients’ quality of life and life expectancy [2, 3]. Maintenance hemodialysis (MHD) is the primary treatment for patients with end-stage renal disease (ESRD) [4]. However, MHD cannot fully replicate the complex metabolic and endocrine functions of healthy kidneys [5]. Patients undergoing long-term hemodialysis often experience a range of complications, with frailty being one of the most common, affecting approximately 46–50% of this population [6, 7]. Frailty is a complex physiological condition characterized by a decline in an organism’s physiological reserves, dysregulation across multiple systems, increased vulnerability, and a diminished capacity to withstand stressors [8]. The presence of frailty significantly increases the risk of adverse clinical outcomes, including falls, disability, cognitive decline, complications related to vascular access, and potentially mortality [9, 10]. Therefore, identifying early risk factors for the development of frailty and implementing targeted interventions are of paramount importance in effectively reducing the incidence of frailty in patients undergoing MHD.
A risk prediction model is a statistical tool that enables individuals to estimate the likelihood of a specific event by combining a variety of predictors that have been assigned appropriate weights [11]. This predictive model, which combines multiple variables to estimate individual risk, can help healthcare professionals screen for people at high risk of frailty MHD patients [12]. In recent years, Chinese scholars have made many efforts in the development of risk prediction models for MHD patient frailty, but most of the existing studies focus on the development or validation of prediction models, and the quality and applicability of the prediction models are still unknown, which makes it difficult for healthcare professionals to choose the appropriate prediction model to accurately identify frailty in MHD patients and to adjust interventions accordingly. In this study, by comprehensively collecting and analysing existing prediction models for the risk of debility in Chinese MHD patients, we systematically summarised, compared and analysed them in terms of their basic features, construction methods, predictive factors, model performance and method quality. It aims to provide reference for clinical healthcare professionals to select appropriate frailty risk prediction models for MHD patients, and to contribute to the optimisation or development of high-quality risk prediction models for subsequent studies.
Following the PRISMA 2020 guidelines [13], we systematically searched five English databases (PubMed, Web of Science, Cochrane Library, CINAHL, and Embase) and four Chinese databases (CNKI, Wanfang, VIP, and CBM). The search period extended from inception to April 30, 2024. We employed English keywords, including: “renal dialysis OR hemodialysis* OR extracorporeal dialyses* OR blood dialysis OR hemodiafiltration OR MHD” AND “frailty OR frail* OR weakness OR debility* OR asthenia OR hyposthenia OR fragile*” AND “prediction model OR prediction tool OR prognostic model OR risk prediction OR risk assessment OR risk score OR partin table* OR Nomograms OR partin nomogram*,” while the Chinese databases were searched using translated versions of the same keywords. Additional relevant studies were identified through the reference lists of included studies.
Inclusion Criteria: (1) Studies published in peer-reviewed journals in English or Chinese; (2) The study population was Chinese maintenance hemodialysis who were 18 years of age or older; (3) The research content is the construction and/or verification of a frailty risk prediction model; (4) The study design was either a case-control study, cross-sectional study, or cohort study.
Exclusion Criteria: (1) < 2 predictors; (2) Studies for which the full text could not be obtained; (3) Inability to obtain complete data from the original article.
The literature screening, data extraction, and cross-checking for this study were conducted independently by two researchers trained in systematic evaluation methodology. In the event of a disagreement, the intervention of a third party was required to make a ruling. The specific steps for literature screening are as follows: First, all literature retrieved using EndNote 20 software was reviewed to eliminate duplicates. Next, the titles and abstracts of the literature were read to exclude any studies not related to the research topic. Finally, a thorough rescreening was conducted through full-text reading. The two researchers utilized a standardized form developed in accordance with the Critical Appraisal and Data Extraction for Systematic Reviews of Prediction Modelling Studies (CHARMS) [14] for data extraction and quality assessment of prediction modeling studies.
The risk of bias and applicability of the included studies were independently evaluated by two investigators using the Prediction Model Risk of Bias Assessment Tool (PROBAST) [15]. The risk of bias evaluation of the included studies was conducted across four aspects: study population, predictors, outcomes, and analyses. Additionally, the suitability evaluation of the included studies was performed in three aspects: study population, predictors, and outcomes.
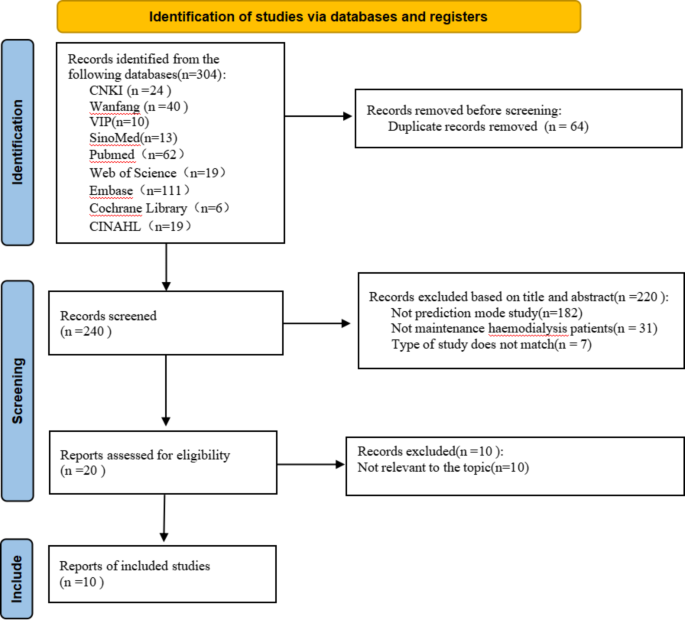
An initial search of 304 documents based on the proposed search strategy resulted in 240 documents remaining after verification using EndNote 20 software. After two rounds of meticulous screening, 10 Chinese-language articles [16,17,18,19,20,21,22,23,24,25] were ultimately selected for research and analysis. The specific steps and processes involved in the literature screening are detailed in Fig. 1.
All ten included studies were published within the last three years, and all were cross-sectional studies. The fundamental characteristics of the included literature are presented in Table 1.
The ten studies included a total of twelve prediction models, of which three [17, 20, 23] were model development studies, and seven [16, 18, 19, 21, 22, 24, 25] were both model development and validation studies. The number of candidate predictors for each model ranged from 16 to 37, while the sample size varied from 145 to 876. Regarding the model-building methods, ten models utilized logistic regression, one [24] model employed a decision tree, and one [24] model applied the random forest algorithm. The details of the model building are presented in Table 2.
The area under the curve (AUC) exceeded 0.7 in ten studies. Four of these studies [16, 18, 22, 23] provided calibration assessments through both calibration plots and goodness-of-fit tests. The models utilized between four and eight independent predictors. The predictor variables that were most commonly identified in these models included age, nutritional status, multimorbidity, gender, and depression.
Among the included studies, four [21, 22, 24, 25] conducted only internal validation, two [16, 19] performed only external validation, and only one [18] utilized a combination of both internal and external validation to assess the model.
In evaluating the risk of bias, all ten included studies were assessed to be at high risk, particularly in the area of analysis. Conversely, the applicability evaluation indicated that all ten documents exhibited a low risk of applicability. The specific evaluation results are presented in Table 3. In the participant domain, two studies [19, 25] were rated as high risk. Both studies collected information using retrospective research methods, which may have introduced a significant risk of bias during the model construction and validation processes. In the prediction domain, two studies [19, 25] could not determine whether the predictors were assessed when the outcome data were unavailable, resulting in an unclear risk of bias in this area. In the outcome domain, all ten studies were rated as having a low risk of bias. The number of events per variable (EPV) was less than 20 in all ten studies, and no further adjustments were made to the parameters. Continuous variables in nine studies [16, 18,19,20,21,22,23,24,25] were converted into categorical variables during the data analysis process. Candidate predictors in nine studies [16, 17, 19,20,21,22,23,24,25] were identified through one-way analyses. Additionally, nine studies [16, 17, 19,20,21,22,23,24,25] did not provide information regarding missing data, while one study [18] opted to remove the missing data entirely. Regarding model performance assessment, one study [24] did not report the model’s calibration, and the evaluation metrics provided were incomplete. In terms of model validation, three studies [17, 20, 23] did not conduct model validation.
Models developed to predict frailty in patients undergoing maintenance hemodialysis demonstrate a substantial overall risk of bias. Despite the increase in the number of studies conducted in China in recent years on predictive modeling of frailty risk in maintenance hemodialysis patients, the overall quality of these studies still requires improvement. In the bias risk assessment, all included studies were assessed as having a high risk for five specific reasons.
Firstly, the number of outcome events is insufficient. According to PROBAST [15], the sample size for model development studies should be determined based on the number of events per variable (EPV). When the EPV is ≥ 20, the likelihood of model overfitting decreases, thereby reducing the associated risk. In the literature included in this study, all studies reported an EPV < 20, resulting in models that are susceptible to overfitting, as well as exhibiting poor stability and reliability. Additionally, two model validation studies [19, 25] included in this research had fewer than 100 outcome events, which may further contribute to bias. Therefore, for future model development, it is recommended to ensure an adequate sample size through multicenter studies.
Secondly, continuous variables were not handled rationally. Specifically, nine studies [16, 18,19,20,21,22,23,24,25] opted to convert continuous variables into categorical variables during their analyses. Transforming continuous variables into ≥ 2 multicategorical variables when constructing a risk prediction model can result in the loss of important information and a reduction in the model’s predictive accuracy [26]. Future researchers are advised to incorporate numerical variables into the model using their original values. If continuous variables need to be categorized, clear grouping criteria should be set first to prevent overfitting caused by arbitrary conversions during the analysis phase. If necessary, adjustments can be made through internal validation and recalibration of regression coefficients [27].
Thirdly, the handling and reporting of missing data are inadequate. A simplistic approach to managing missing data during the research process can result in issues such as an insufficient dataset for modeling and the loss of valuable information. In this study, nine studies [16, 17, 19,20,21,22,23,24,25] failed to report information regarding missing data, while one study [18] opted to remove the missing data entirely. Future researchers should adopt appropriate methods for handling missing data, such as multiple imputation and case-by-case deletion [15], and ensure that these methods are transparently reported in their studies.
Fourthly, predictors were screened using one-way factor analysis. In this study, nine studies [16, 17, 19,20,21,22,23,24,25] initially identified variables significantly associated with frailty through one-way factor analysis and subsequently employed these variables in further regression model analyses. However, this approach does not fully account for the interactions between variables and their intrinsic relationships, which may introduce bias due to the omission of key independent variables. Future researchers should comprehensively consider the existing clinical knowledge, as well as the reliability, consistency, applicability, accessibility, and cost of measuring predictors [28].
Fifthly, there is a lack of assessment regarding model performance. To thoroughly evaluate the predictive performance of a model, researchers must accurately assess both calibration (using the Hosmer-Lemeshow test and calibration plots) and discrimination (measured by the area under the curve, AUC). However, in this study, one study [24] did not report the model’s calibration degree. This omission may hinder a comprehensive evaluation of the accuracy and reliability of the model’s predicted probabilities, which could subsequently impact further optimization efforts for the model.
Sixthly, inappropriate model validation. Three of the included studies [17, 20, 23] did not perform model validation, which may lead to optimism bias in the assessment of model performance. Internal and external validation of models is a crucial step to assess model stability and applicability. However, the current emphasis on model development rather than validation in clinical studies is a prevalent issue, with most studies remaining at the modeling stage. As a result, only a limited number of models are available for clinical practice [29]. Future researchers should consider conducting internal and external validation of existing unvalidated models. Additionally, the Transparency Reporting Interpretation Statement (TRIPOD) [30] should be thoroughly reviewed and strictly adhered to in order to ensure the transparency and reliability of the model development, updating, and validation processes.
Risk factors for frailty in patients with MHD vary and exhibit commonalities across studies due to differences in assessment tools for debility, included predictors, and data sources. The five most common predictors of debility development in MHD patients identified in this study are age, nutritional status, multimorbidity, gender, and depression. Yang [22] found that the occurrence of frailty in MHD patients aged ≥ 60 years was 3.460 times higher than those aged < 60 years, and advanced age was an independent risk factor for the occurrence of debility in MHD patients, which is similar to the findings of Takeuchi [31]. As the duration of dialysis increases, patients with end-stage renal disease undergo a series of complex physiological changes, such as cellular senescence, telomere attrition, mitochondrial dysfunction, and heightened free radical production [32]. These alterations may contribute to sarcopenia, vascular dysfunction, and progressive organ damage, thereby elevating the risk of frailty in these patients [33]. It is suggested that healthcare professionals should pay more attention to the frailty conditions of elderly patients before and after daily dialysis treatment, implement personalised health education to help patients understand the risk factors and preventive measures of frailty, and improve their self-management ability. A study noted that both serum albumin and NRS2002 score were independent risk factors for the development of debility in patients with MHD [19], and both reflected the nutritional status of the patients, in agreement with the findings of Johansen [34]. Inadequate energy intake during dialysis treatment, resulting from decreased appetite and dietary restrictions, along with the loss of essential nutrients such as proteins, water-soluble vitamins, and red blood cells, predisposes patients to malnutrition and significantly heightens the risk of frailty. Healthcare professionals are advised to strengthen nutritional assessment of MHD patients, guide them to rational protein and energy intake, and provide personalised nutritional support through multidisciplinary collaboration in order to improve the nutritional status of the organism and reduce the risk of frailty. The presence of multiple chronic diseases can lead to a reduction in the homeostatic reserves of a patient’s body, diminishing their resistance to external stressors and consequently accelerating the onset of frailty [35]. Therefore, healthcare professionals should strengthen the management of patients’ chronic diseases and rationalise the use of medication, as well as encourage patients to actively participate in self-management in order to enhance the body’s homeostatic reserve and reduce the risk of frailty. Women are more susceptible to frailty, which may be attributed to factors such as generally lower body weight, reduced muscle strength and mass, and increased longevity [36]. It is suggested that healthcare professionals should focus on female MHD patients in hospitals as well as in the community, and that regular frailty screening can be carried out to determine whether frailty is occurring in MHD patients and to intervene as early as possible to avoid aggravation of the degree of frailty. Studies have indicated that the prevalence of frailty among patients with depression can be as high as 40.4% [37]. These individuals often lack adequate psychological and social motivation, which may result in a decreased interest in physical and social activities. Consequently, this increases their risk of physical decline and makes them more susceptible to frailty [38]. Therefore, while treating patients with somatic diseases, healthcare professionals should enhance psychological interventions. This includes providing emotional support and mental health care, as well as teaching patients emotional regulation techniques such as deep breathing, relaxation training, and positive thinking meditation. In summary, these factors are significant predictors of frailty in MHD patients, and clinical staff should improve the assessment and prevention of these key variables.
In addition, we found that while multimorbidity is a common predictor, the Charlson Comorbidity Index (CCI) is typically employed as one of the predictors in existing studies. However, the specific impact of various types of comorbidity on the risk of debilitation has not been thoroughly examined. A study by Takeuchi [31] demonstrated that the risk of frailty in MHD patients with concurrent diabetes was 2.765 times higher than that in patients without diabetes. Additionally, a study by Kutner [39] identified peripheral vascular disease and cardiac disease as independent risk factors for frailty in MHD patients. This indicates that the complexity of comorbidities must be thoroughly considered in risk prediction models to enhance their predictive accuracy. In this context, we recommend that future studies further investigate the specific effects of various types of comorbidities on the risk of frailty and take into account the heterogeneity of these factors during model development.
Models for predicting the risk of frailty in patients with MHD have developed rapidly in recent years, despite their relatively late inception. Overall, existing models demonstrate good differentiation and calibration. However, these models encounter several challenges in clinical application. First, there are methodological limitations in model validation and calibration. Most studies lacked external validation, which restricts the applicability of the model to a broader range of populations. Additionally, some studies evaluated the model’s calibration using calibration plots and Hosmer-Lemeshow (H-L) tests that were not adjusted for specific population characteristics, potentially impacting the model’s predictive accuracy across diverse populations. Therefore, in the future, researchers should enhance the number of external validation studies through multicenter collaborations, gather data from diverse regions and populations, emphasize the implementation of a dynamic calibration strategy tailored to population characteristics, and regularly monitor the model’s performance. Additionally, they should update the model based on new data to ensure its accuracy and relevance. Second, the predictors used in each risk prediction model varied, which may be attributed to the diversity of alternative predictors included in different models and the heterogeneity of the study populations. To optimize the selection of predictors in these models, it is recommended that future researchers identify alternative predictors for inclusion through systematic literature reviews, statistical analyses, and by integrating medical knowledge with expert experience. Additionally, enhancing the representativeness of the models can be achieved by conducting multicenter studies. Third, this study found that the risk of competing events related to death has not been explicitly addressed in the construction of current risk prediction models, which may result in decreased predictive performance. To enhance the accuracy and applicability of these models, future research should explicitly incorporate the risk of competing events by utilizing appropriate statistical methods, such as competing risks modeling. Finally, several frailty assessment tools are currently widely utilized in clinical practice [40, 41]. However, there is no established ‘gold standard’ for these tools due to variations in population heterogeneity and clinical status. It is recommended that a common standard for frailty assessment be established in the future. Such a standard would be practically significant for enhancing the consistency of clinical evaluations and providing a uniform benchmark for the development, validation, application, and interpretation of predictive models. This would ensure that comparisons between different models are both reliable and reproducible.
In this systematic evaluation, we conducted a comprehensive analysis of predictive models for assessing the risk of frailty in patients with MHD. However, it is important to acknowledge several limitations that may have influenced our conclusions: (1) The absence of a search for grey literature may have resulted in the exclusion of certain studies, thereby increasing the risk of publication bias. (2) Variations in predictor selection and classification criteria among the models prevented us from quantitatively analyzing the predictors in the included literature. (3) All risk prediction models included in this study were developed based on limited data from the Chinese population, which may have introduced geographic bias and restricted our understanding of the differences in frailty risk prediction among MHD patients from various countries and regions. Consequently, our conclusions may not fully represent diverse populations worldwide. Despite these limitations, our systematic review offers a valuable reference for understanding the current landscape of frailty risk prediction models for MHD patients and suggests directions for future research.
A total of ten studies on risk prediction models were included in this analysis, which systematically evaluated the characteristics of the models concerning the study population, predictors, outcome indicators, and areas of analysis. The results indicated that while most existing risk prediction models demonstrated good predictive performance, they also exhibited some risk of bias. Future researchers are encouraged to refer to the PROBAST guidelines and adhere to the multivariate prediction model reporting standards to minimize bias and ensure scientific validity and rigor in the model development process. Additionally, prospective cohort studies with multi-center involvement and large sample sizes are recommended to enhance the reliability and generalizability of the models. Future investigations may also consider employing advanced algorithms such as support vector machines, neural networks, and decision trees to optimize model construction and conduct comparative analyses, with the goal of identifying the most suitable prediction models for clinical practice. Furthermore, it is advisable to integrate these predictive models with electronic medical record systems to facilitate their usability by clinical staff.
Data is provided within the manuscript.
Not applicable.
Not applicable.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Zhong, Y., Cao, S., Chen, L. et al. Frailty risk prediction models for patients undergoing maintenance hemodialysis in China: a systematic review. BMC Nephrol 26, 57 (2025). https://doi.org/10.1186/s12882-025-03990-y