BMC Psychiatry volume 25, Article number: 622 (2025) Cite this article
Oxytocin administration is increasingly considered a novel therapeutic support option for alleviating psychological distress in stress-related neuropsychiatric conditions, including anxiety disorders and depression.
However, oxytocin as a stand-alone treatment may fail to consistently target the relevant central autonomic circuitry without a complementary supportive therapeutic context that similarly stimulates stress-regulatory states and behaviors.
Recent findings suggest that the neurophysiological mechanisms underlying stress regulation induced by mindfulness may rely on an activation of the endogenous oxytocinergic system. Accordingly, combining oxytocin with a mindfulness-based training program may enhance efficacy compared to each intervention alone.
A randomized, double-blind, placebo-controlled clinical trial will be conducted in 120 adults with heightened stress complaints, randomly assigned to one of the following four treatment arms (n = 30 per group): (1) oxytocin + mindfulness, (2) mindfulness + placebo, (3) oxytocin, and (4) placebo (control). The oxytocin or placebo nasal spray will be administered four times a week in the morning before each mindfulness session for six weeks, followed by a six-week post-treatment follow-up session.
Primary endpoints include self-reported behavioral measures of stress, depression, and anxiety. Secondary endpoints include self-reported behavioral measures of mood, mindfulness skills, quality of life, sleep quality, and negative thinking. Exploratory measures include (i) electroencephalography (EEG), electrocardiography (ECG), skin conductance, and respiration, measured during rest, meditation, stress induction, and stress recovery; (ii) intervention-induced changes in biological samples, including hormonal levels of oxytocin and cortisol and DNA methylation of the oxytocin receptor gene (OXTR); and (iii) stress reactivity in daily life, assessed through experience sampling and heart rate/sleep monitoring. All outcome measures will be assessed at baseline (T0), immediately post-intervention (T1), and at six-week follow-up (T2).
These findings could provide valuable insights into how combining oxytocin and mindfulness-based interventions might enhance stress regulation, particularly in populations with impaired oxytocinergic function.
This trial was registered in the EU Clinical Trials Register (EU CT 2024–513482-39–00) on 18 March 2025.
In recent years, the increasing prevalence of stress and anxiety among adolescents and adults has driven interest in studying the psychobiological systems underlying stress regulation and homeostasis [1]. Among these, the oxytocinergic system has been identified as playing a crucial role in these regulatory processes [2]. Oxytocin is a neuropeptide and neuromodulator primarily produced in the hypothalamus, acting on receptors widely expressed in both the brain and peripheral tissues, including the heart [2,3,4].
At the neural level, oxytocin is suggested to play a pivotal role in stress buffering and social behaviors [3, 5], mediated through its neuromodulatory effects on the central and autonomic nervous system (ANS). As a neuromodulator, oxytocin is primarily thought to facilitate a neurovisceral state of “immobility without stress or fear”, allowing attunement and social sensitivity as characterized by an increased “rest and digest” response of the parasympathetic branch of the ANS, and a reduced “fight-or-flight” response of the sympathetic branch [6]. Furthermore, higher endogenous oxytocin levels have been related to both reduced psychological distress and anxiety, as well as to attachment-related thoughts, bonding, and social engagement [3, 7,8,9,10,11,12,13]. However, more research is needed to clarify the mechanisms underlying these associations. Furthermore, alterations in the endogenous oxytocinergic system have increasingly been associated with psychological distress complaints and social difficulties associated with neuropsychiatric conditions, including autism [13] and depressive disorders [14], raising questions about whether exogenously administered oxytocin could constitute a novel support option for mediating those complaints.
Indeed, given its stress-regulating and prosocial effects, exogenously administered oxytocin has been proposed as a potential therapeutic approach for alleviating psychological distress in stress-related neuropsychiatric conditions, including anxiety disorders and depression [15]. For example, previous studies from our [16, 17] and other labs [18,19,20,21] have consistently shown that a single dose of oxytocin increases heart rate variability (HRV), an established marker of parasympathetic tone, mediated by the vagus nerve. This marker has also been increasingly linked to both biological and psychological health [22, 23]. While these studies have demonstrated the acute, single dose effects of oxytocin on stress modulation, research into the effects and mechanisms of multiple-dose, chronic oxytocin administration regimens remains limited. These insights are important however, considering that chronic administration regimens more closely reflect how oxytocin administration will be implemented for use in clinical practice. Promising results have been observed with moderate-duration interventions (4–6 weeks) involving intermittent dosing (25–33 international units (IU) every other day) [24,25,26,27,28], although the most effective regimens have yet to be definitively established.
In addition to the duration of oxytocin administration, the efficacy of oxytocin treatment has also been suggested to depend significantly on person-dependent factors, as well as the context in which the treatment is administered [29]. In terms of person-dependent factors, Bartz et al. (2011) [30] put forward the notion that prosocial effects by oxytocin may be more pronounced for individuals with low baseline levels of social proficiency or insecure attachment, whereas for individuals with already high baseline levels of social proficiency, the administration of oxytocin may not stimulate prosocial behavior further [30, 31]. In line with this notion, oxytocin was shown to improve empathic accuracy on an emotion recognition task, but only for less-socially proficient individuals [32, 33]. Also in previous studies from our group, low social responsiveness and low attachment security (predominantly avoidant attachment) were sensitive modulators of oxytocin-induced responses [34,35,36], indicating for example, that oxytocin-induced changes in stress recovery (skin conductance) were most pronounced in individuals with low self-reported social responsiveness [36].
With regard to variations in the context, oxytocin administration as a stand-alone treatment may be suboptimal as opposed to pairing (chronic) oxytocin administration with concomitant standardized psychosocial interventions to amplify its therapeutic effects and clinical efficacy [37]. While the precise bioavailability of exogenously administered oxytocin remains incompletely understood, its short half-life (approximately 3–6 min in plasma and 20 min in cerebrospinal fluid [38]) indicates that some of the effects of exogenously administered oxytocin may be driven by a feed-forward mechanism, stimulating the endogenous release of oxytocin [39]. Therefore, the notion has been put forward that exogenously administered oxytocin’s stress regulatory potential may only be fully capitalized when administered within a therapeutic context that similarly supports and facilitates stress-regulatory processes, i.e., allowing the feed-forward triggering of its own endogenous release. In line with this notion, oxytocin administration in socially supportive settings has been shown to yield improved outcomes [27, 40]. Therefore, the context in which oxytocin is administered is of pivotal importance, with stress-free, supportive contexts being likely most optimal for maximizing its effects [37].
Mindfulness-based intervention (MBI) programs are the most scientifically studied and practiced self-regulatory trainings in medicine [41,42,43]. Prior studies have shown mindfulness training to reduce self-reported stress [44] and boost resilience [45]. Interestingly, given the link between mindfulness training and increased self-regulation, recent studies suggest that the biological mechanism of oxytocin may be involved in mindfulness training [4, 46, 47], with several theoretical accounts suggesting that the neurophysiological-mechanistic underpinnings of stress regulation induced by MBIs may rely on an activation of the endogenous oxytocinergic system. For example, initial lines of evidence demonstrated beneficial behavioral effects paralleled by an induction of higher endogenous oxytocin production post-intervention [48,49,50,51]. Furthermore, albeit evidence is sparse, a handful of initial (single-dose) oxytocin administration studies have shown that intranasal (exogenous) oxytocin can improve components of mindfulness-based self-regulation, including the development of (self-)compassionate behavior and mindfulness-based improvements in negative symptoms in schizophrenia spectrum disorders [52,53,54,55].
Particularly, a recent pilot trial assessed whether administering oxytocin in a positive social setting, namely prior to a mindfulness-based group therapy, could improve empathy and reduce negative symptoms in schizophrenia spectrum disorders compared to mindfulness training administered alone (with a placebo nasal spray) [55]. While no significant improvements in empathy were found, oxytocin showed potential in reducing negative symptoms, alongside reductions in negative affect and stress, thereby providing important proof-of-concept that oxytocin may enhance the beneficial effects of mindfulness-based group trainings.
Here, we introduce a protocol designed to investigate whether the combinatory therapeutic approach of multiple-dose, chronic oxytocin administration (six weeks of intranasal administration of 24 international units (IU), administered 4x/week) paired with a mindfulness-based training program enhances efficacy in regulating stress compared to each intervention as a stand-alone approach.
This study aims to evaluate whether combining oxytocin administration with mindfulness-based training can facilitate stress regulation in adults with heightened stress complaints, compared to each intervention delivered as a stand-alone treatment. A randomized, double-blind, placebo-controlled clinical trial will be conducted, in which 120 adults will be randomly assigned to one of the following four treatment groups (30 participants per group):
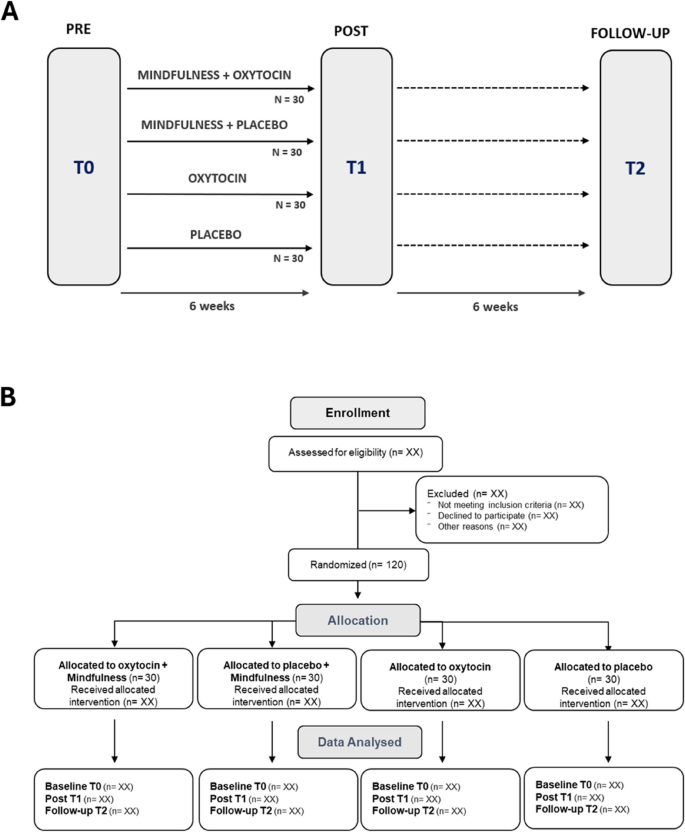
Outcome measures will include both immediate and retention effects on behavioral, neurophysiological, neuroendocrine, epigenetic, and ambulatory stress markers. These will be assessed during three study visits: at baseline (T0), immediately after the six-week nasal spray and/or mindfulness training administration period (T1), and at a follow-up session, six weeks post-intervention (T2) (Fig. 1A).
Trial design. A Outcome measurements will be assessed at baseline (T0), immediately post-intervention (T1), and at a follow-up session six weeks after the intervention period (T2). B CONSORT flow diagram
Figure 1B shows the CONSORT flow diagram as an overview of the planned number of participants enrolled, randomized, and included in the data analysis.
Participants will be primarily recruited by research staff through established contacts with the Flemish Centers for Mental Health Care (Centra voor Geestelijke Gezondheidszorg, CGGs) and adults on waitlists for “Eerstelijnspsychologische zorg” (e.g., ELP Diletti or Vindplaatsen) in Belgium. Additional recruitment will take place at KU Leuven and the university hospital of Leuven through flyers, personal communication, and social media.
Participants meeting the following criteria will be included in the study: 1) sufficient proficiency in Dutch to complete study tasks; 2) age between 18 and 50 years old; and 3) presence of mild to severe stress symptoms, as assessed using the self-report stress subscale of the Depression, Anxiety, and Stress Scale (DASS-21). Exclusion criteria are 1) active use of psychotropic medication within 6 months prior to participation (including antidepressants, anxiolytics, and antipsychotics); 2) active engagement in psychological treatment within 6 months prior to participation (with a psychologist or psychiatrist); 3) substantial experience with meditative practices (including but not limited to mindfulness, yoga, tai chi, or other similar practices) and/or participation in a multi-day meditation retreat or program during the past six months and/or engagement in meditative practices on a weekly basis or more frequently, for at least six consecutive weeks, within six months prior to the study; 4) previous chronic treatment with oxytocin; 5) active use of anti-epileptic medication or has a significant active medical condition including hematological, endocrine, cardiovascular (including any rhythm disorder), respiratory, renal, hepatic, or gastrointestinal disease which influences the metabolism of oxytocin; 6) a history of active epilepsy, defined as individuals experiencing seizures or requiring anticonvulsant therapy within the past 12 months; 7) a known syndrome that interacts with the reproductive hormonal system (e.g. Prader-Willi or Angelman syndrome); 8) for women: pregnancy, breastfeeding, or planning to become pregnant; 9) significant hearing or vision impairments (that cannot be corrected); 10) participation in another clinical trial with an investigational medicinal product; 11) known hypersensitivity to active substance or ingredients of the nasal sprays, including e.g. (history of) latex allergy; and 12) the use of the following medicinal products during the nasal spray administration period: prostaglandins and their analogues, inhalation anesthetics, vasoconstrictors/sympathomimetic drugs, and caudal anesthesia.
Recruitment will begin in September 2025 and is expected to be completed by the end of September 2027. Participants will receive a total compensation of €120 for their participation in the study.
Sample size
In order to examine whether the combinatory treatment yields a superior treatment response compared to the stand-alone treatment arms, which in turn are expected to yield higher treatment responses compared to the placebo treatment arm, this exploratory trial will include a total of 100 + 20 participants (30 individuals/treatment arm), allowing for the detection of a small-to-medium-sized effect (f2 = 0.10; Linear multiple regression: Fixed model, R2 deviation from zero; alpha = 0.05; power = 0.80; number of predictors = 2 estimated using G*power 3.1.9.7), accounting for a potential 15–20% attrition (i.e., n = 100 + 20 attrition). While dropout rates in oxytocin research have generally been low, we adopt a conservative estimate of 15–20% to ensure sufficient statistical power and accommodate potential participant loss throughout the trial. To the best of our knowledge, only one previous study has investigated the combinatory effects of administering oxytocin or placebo, 45 min prior to two sessions of mindfulness training [55]. While in this study, no benefit of oxytocin over placebo was observed for empathy, significant between-group differences favoring oxytocin were found for self-reported negative symptoms, with a reported effect size of f2 = 0.12 (η2ₚ = 0.11). This effect size is within the range that the proposed sample size is expected to detect, as described above. Further, considering that this is an exploratory trial, the planned sample size is anticipated to provide sufficient power to yield proof-of-concept insights that will allow guiding sample size calculations for future combinatory trials.
For the randomization procedure, the pharmacy A15 (The Netherlands), will use Sealed Envelope Ltd. (2022) to implement a permuted-block randomization scheme. Participants will first be randomly assigned in a 1:1 ratio to receive either oxytocin or placebo, using permuted blocks of size 4. Each block will then be randomly assigned to either the mindfulness intervention or no mindfulness using a block-wise randomization procedure, with the constraint that no more than two consecutive blocks are assigned to the same condition.
This randomization procedure will allow obtaining four equal-sized treatment arms (n = 30 each): (1) oxytocin + MBI, (2) oxytocin alone, (3) placebo + MBI, and (4) placebo alone. After all participants have finalized the last follow-up session and the database is locked, the randomization code in the envelope will be opened for analysis of the response data.
All participants will be randomized to receive either oxytocin (Oxytocin CD Pharma®, CD pharmaceuticals AB) or placebo nasal sprays (Physiological water, sodium chloride (NaCl 0.9%) solution, with added preservatives (aqua conservans, methocel)). All experimenters involved in patient contact and data collection, as well as all participants will be blind to the nasal spray assignment. To ensure full blinding, oxytocin and placebo nasal sprays are packaged in identical bottles and labelled with a number. Pharmacy A15 is responsible for preparing the study medication, repackaging it to ensure blinding, and randomization.
Oxytocin/placebo nasal spray administration
The nasal spray will be administered in the morning, prior to the mindfulness training sessions. The training will take place within 2 h after the nasal spray administration to ensure peak oxytocin levels during the session [56, 57]. Participants will be instructed to wait at least 15 min after the nasal spray administration before starting the mindfulness training. Oxytocin will be administered as a single intranasal dose of 24 IU (three puffs in each nostril; 4 IU per puff), 4 times per week for six weeks. The total dose of 24 IU is the standard dose adopted in prior single-dose administration studies in adults and children [58]. The duration and intermittent dosing scheme was chosen to resemble a prior six-week trial with 3–8 year old children [27], in which a similar infrequent dosing regimen was adopted to reduce the possible impact of repeated dosing on receptor desensitization and down-regulation [59, 60]. The schedule could be as follows: administration on Monday, followed by group-based mindfulness training; on Wednesday, Friday, and a weekend day, followed by training using the mobile application.
Mindfulness-based intervention
Half of the participants assigned to oxytocin and half assigned to placebo will receive the nasal spray within the standardized framework of a six-week mindfulness-based intervention. The mindfulness training will take place in groups of 10–15 participants, with a blended approach, such that each week, participants will receive one in-person, group-based mindfulness training (2 h); and three individual, app-based, mindfulness trainings in the participants home-setting (minimum 15 min). This approach is based on a similar blended protocol as developed by Van der Gucht et al. [61].
Sessions will include guided formal meditation exercises (e.g., body scan, mindful movement, sitting meditation, loving kindness/compassion meditation), informal exercises that can be practiced during the day (e.g., mindful eating), experiential exercises, and inquiry. The training will be delivered by a certified trainer with more than 15 years of experience. The training is supported by the use of homework exercises and audio material available on their smartphone via a mobile application developed at the Leuven Mindfulness Consortium (LMC), Leuven, Belgium [61,62,63]. Attendance to the live sessions and compliance with the app-based trainings will be monitored via the app. In case participants are not able to attend one of the live sessions (and are not able to join another group in the same week), they will be provided with recorded audio material of the session to complete the session (and concomitant administration of nasal spray) at a later time. Prior to the six-week training, a meet-and-greet session will be organized (without nasal spray administration) to create a safe and familiar environment among the group participants. To assess the therapeutic relationship toward the whole group, other individual participants, and the mindfulness trainer, the Group Questionnaire GQ (30 items) [64] will be assessed at the third and sixth weeks of the training.
All outcome assessments will be performed at the baseline session before randomization (T0), immediately after the six-week intervention period (T1), and at a six-week follow-up session (T2). Prior to the start of any trial assessments, participants will sign the informed consent form during an intake session. The session will also include eligibility screening and the collection of participant demographics and medical history, including details of any background treatments or psychoactive medication.
The primary endpoints are assessments of self-perceived emotional stress, measured using the Dutch versions of the Perceived Stress Scale (PSS) [65] and the Depression Anxiety Stress Scale (DASS-21) [66]. The PSS is a self-report questionnaire designed to measure the perception of stress in individuals. The questionnaire was originally developed by Cohen et al. in 1983 and asks participants to rate how often they find their lives to be unpredictable, uncontrollable, and overloaded within the past month [65]. It consists of 10 items that are rated on a 5-point Likert scale. Example items include: “In the last month, how often have you felt that you were unable to control the important things in your life?” and “In the last month, how often have you felt nervous and ‘stressed’?” Higher scores indicate greater perceived stress. The presence and severity of symptoms of emotional distress are measured using the DASS-21, developed by Lovibond and Lovibond in 1995 [66]. The DASS-21 is a measure of distress that distinguishes between symptoms of anxiety, stress, and depression. The three subscales have demonstrated good convergent and discriminant validity and high internal consistency both in clinical and nonclinical samples [66]. Example items include: “I found it hard to wind down” (stress subscale), “I felt scared without any good reason” (anxiety subscale), and “I felt that life was meaningless” (depression subscale). Items are scored on a 4-point Likert scale, where high scores indicate higher levels of symptoms of stress, anxiety, and depression. In this study, we will use the total score as a measure of emotional distress.
The secondary endpoints include assessments of the following self-report questionnaires: State Adult Attachment Measure (SAAM) [67] to assess feelings of (secure) attachment/bonding towards others, Self-Compassion Scale-Short Form (SCS-SF) [68] to assess self-compassion, Pittsburg Sleep Quality Index (PSQI) [69] for sleep quality, Quality of life World Health Organization Five (WHO-5) Well-Being Index for quality of life [70], Perseverative Thinking Questionnaire (PTQ) [71] for repetitive negative thinking, and the Three-Facet Mindfulness Questionnaire-Short Form (TFMQ-SF) [72] for trait mindfulness. During the six-week intervention period, participants will additionally be asked to complete the Profile of Mood State (POMS) [73] at the end of each weekly group training session.
Aside from standardized behavioral (self-report) assessments of stress, the study will also include exploratory assessments of stress neurophysiology and biological samplings. Since most prior studies predominantly focus on assessing oxytocin administration effects on clinical scales and questionnaires, the current inclusion of stress neurophysiology assessments and biological samplings will allow to gain important mechanistic insights into the bio-physiological aspects that are anticipated to underlie or precede the clinical improvements.
Stress neurophysiology assessments
As an exploratory outcome, intervention-induced changes in stress neurophysiological recordings will be acquired at each assessment session (T0, T1, T2) during rest, meditation, stress induction, and stress recovery.
Resting-state recording: During the resting-state recording, participants will be instructed to sit still, keep their eyes closed and patiently wait for a duration of 5 min while neurophysiological measures are taken. Auditory cues will signal the start and end of the recording.
Meditation: During the meditation recording, participants will be instructed to engage in 10 min of focused-attention meditation. Specifically, they will be instructed to sit still with closed eyes and to focus their attention on an anchor point (i.e., the contact point between their bottom and the chair [74]). Participants will be encouraged to notice whenever distractions arise and gently redirect their attention back to the anchor point. Auditory cues will signal the start and ending of this recording.
Stress induction: The socially evaluated cold-pressor test (SECPT [75]) will be used to trigger a physiological stress response. This test involves a physiological stressor (immersing one’s hand in cold water) combined with socially-evaluative elements (observation by the experimenter and facing a camera). Specifically, participants will be instructed to immerse their hand and wrist into near-freezing (2°C) water without moving or making a fist, while facing a camera recorder and being observed by the experimenter. After a duration of 3 min, participants will be instructed to remove their hand from the water, although this duration will not be disclosed beforehand. If the participant cannot tolerate the temperature of the water any longer and takes their hand out of the water, the camera and the evaluation by the experimenter will still continue until the three minutes are over. Research shows that the SECPT leads to reliable increases in subjective stress levels, autonomic arousal, and cortisol [76].
Stress recovery: Following the stress induction procedure, participants will be left alone in the room for an initial eyes-closed recovery phase of 17 min during which participants are instructed to wait patiently and let their thoughts wander freely, similar to the resting-state recording phase. This initial recovery phase will be followed by a subsequent recovery phase of 40 min during which participants are asked to open their eyes. During this remaining waiting time, participants will be watching a neutral and muted video (i.e. BBC documentary Spy in the wild; as used in the study by De Calheiros Velozo et al., 2021 [77]).
The following neurophysiological recordings will be conducted during the specified experimental conditions using the Nexus-32 device with BioTrace software (V2018A1) (Mind Media, The Netherlands).
Electroencephalography. EEG recordings will be conducted using a 19-electrode EEG cap (plus two reference electrodes and one ground electrode) positioned according to the 10–20 system. Vertical [vertical electro-oculogram (VEOG)] and horizontal [horizontal electro-oculogram, (HEOG)] eye movements will be recorded using pre-gelled foam electrodes (Kendall, Germany) placed above and below the left eye, as well as next to the left and right eye (sampling rate of 1024Hz). Skin abrasion and electrode paste (Nuprep) will be applied to reduce the electrode impedances during the recordings. The EEG signal will be amplified using a unipolar amplifier with a sampling rate of 512 Hz.
Electrocardiography. ECG will be measured by placing one ECG electrode below the left rib cage and another electrode just below the right collarbone.
Electrodermal recordings. Electrodermal recordings will be performed using two silver chloride (Ag–AgCl) electrodes attached to the middle and ring fingers of the left hand. A low current will be applied to the electrodes to measure skin conductance.
Respiration. Respiration will be measured using a belt with a respiration sensor on the chest, measuring the relative expansion and contraction of the chest. The belt can be applied on top of the clothing of the participant.
Intervention-induced changes in biological samples (oxytocin and cortisol hormonal levels; epigenetics)
Oxytocin and cortisol hormonal levels. Salivary samples for hormonal assessments will be collected using Salivette cotton swaps (Sarstedt AG & Co., Germany) at each assessment session (T0, T1, T2) to explore levels of peripheral (endogenous) oxytocin and cortisol as indicative of variations of arousal/ (social) stress. Analyses of the oxytocin levels will be performed by using Oxytocin Enzyme-Linked Immunosorbent Assay (ELISA) kits from Enzo Life Sciences, Inc., USA. The analyses of the cortisol levels will be performed by applying the Salivary Cortisol ELISA kits by Salimetrics, USA. For each participant, salivary samples will be acquired at four time points during the same day: a sample, acquired at home, in the morning, within 30 min after awakening and before breakfast, and three additional samples, acquired right before the start of the stress induction task, and respectively, 20 and 60 min later. Sample concentrations (100 µl/well) will be calculated according to plate-specific standard curves.
Epigenetic variation. In the healthy population, imaging genetic studies assessing OXTR (Online Mendelian Inheritance in Man entry 167,055) methylation provided consistent evidence of a relation between OXTR methylation and individual attachment-related behaviors [78, 79]. We aim to explore these topics further by characterizing OXTR methylation in the current population and to explore whether variations in OXTR methylation relate to possible variations in oxytocin treatment responses. At the baseline assessment (T0) and at every post-administration assessment (T1, T2), an additional saliva sample will be collected right before the start of the stress induction task, using the Oragene DNA (OG-500) kit, to specifically explore the level of DNA methylation of the oxytocin receptor gene (OXTR) (epigenetic variations).
All outcome measures will be assessed by the study researchers, including PhD students and research staff, who are trained in the relevant data collection procedures. The assessments will be conducted at the Brainshub facility at KU Leuven, Belgium. Since the acquisition of some of these assessments may be challenging, they are considered exploratory. Each testing session will last no longer than 3 h, with adequate breaks provided to avoid fatigue.
Stress reactivity in daily life (experience sampling and ambulant physiology recording).
Experience sampling method (ESM). The experience sampling method will be used to assess self-reported stress reactivity in daily life. This is a momentary assessment method that allows for repeated and ecologically valid measurements of participants’ mood state by collecting in-the-moment data [80]. Here, participants will be prompted on their smartphone at semi-random moments during the daytime to indicate how they are feeling in daily life for 4 consecutive days and will receive 10 prompts a day. The experience sampling will be administered using the m-path app [81]. At each beep, participants will be asked to indicate their current experience of emotional distress, attachment, self-compassion, mindfulness skills, and negative feelings. Before answering the ESM questions, they will be prompted to indicate whether they are at home, at work, or elsewhere. This 4-day experience sampling protocol will be administered at T0, before the first nasal spray administration; at T1, starting two days prior to the last nasal spray administration; and at T2, six weeks after the intervention, allowing to calculate changes in psychological resilience following the nasal spray administration.
Whoop wristband. A subset of participants will be asked to wear a WHOOP wristband (WHOOP, Inc., Boston, MA, USA), mobile sensor for ambulatory recordings of heart rate and sleep architecture, including measures of deep sleep stages, total sleep duration, sleep onset latency, sleep efficiency, and sleep fragmentation. These recordings will be collected three times over 4 consecutive days, following the same schedule as the ESM, allowing for the assessment of changes in ambulatory stress physiology.
It is hypothesized that the combined treatment of pairing mindfulness and oxytocin will result in better stress regulation than either intervention alone or placebo, particularly at each timepoint (baseline, post-intervention, and 6-week follow-up). Specifically, it is expected that participants receiving both interventions will show the greatest improvement in stress regulation, followed by those receiving only one of the interventions (mindfulness + placebo or oxytocin alone), with the placebo group showing the least improvement. The expected order of effectiveness is as follows: [mindfulness + oxytocin] > [mindfulness + placebo] = [oxytocin] > [placebo].
While changes in stress regulation will also be explored over time (baseline, post-intervention, and 6-week follow-up), the primary interest lies in comparing the effects of the combined treatment to each stand-alone treatment and placebo at each timepoint.
Intervention effect
To assess whether the combinatory treatment (mindfulness + oxytocin) leads to greater improvements in stress regulation than either intervention alone or placebo, a linear mixed-effects model (LMM) will be used.
Primary analyses will be conducted separately at post-intervention (T1) and 6-week follow-up (T2), including fixed effects for mindfulness (yes/no), oxytocin (yes/no), and their interaction (mindfulness × oxytocin). Baseline (T0) scores will be included as a covariate to account for pre-treatment individual differences. This approach will also allow examining whether the oxytocin or mindfulness stand-alone treatments are superior to the other for particular outcomes.
As an exploratory analysis, a LMM including all three time points (baseline, post-intervention, and 6-week follow-up) will be performed to examine how treatment effects evolve over time. This model will include fixed effects for mindfulness, oxytocin, time, and their two- and three-way interactions. Baseline (T0) scores will be included as a covariate to account for pre-treatment differences.
Cohen's d or f2 effect sizes will be reported for the treatment variable.
The primary analysis will be conducted according to a modified Intention-to-Treat (mITT) principle, based on the Full Analysis Set (FAS). The FAS will include all randomized participants, except participants who received no treatment (i.e., less than one nasal spray administration) and provided no post-baseline data.
Per protocol set is planned, which will be fully defined after completion of all data entries, but before database lock and unblinding. Protocol violations under consideration will include, but are not necessarily limited to, the following: insufficient compliance regarding study medication (defined as use of less than two-thirds of the prescribed dose); and post-randomization violations or the inclusion or exclusion criteria.
All efficacy analyses will be performed for both the FAS and per protocol set, whereby the analysis of the FAS will be considered of primary importance and per protocol set will be considered a sensitivity analysis.
The planned LMM is sufficiently robust to accommodate moderate amounts of missing data, provided they are missing at random. To examine whether data were missing at random, patterns of missing data will be explored descriptively, and baseline characteristics of participants with and without missing follow-up data will be compared. If substantial differences are observed, sensitivity analyses using multiple imputation or pattern-mixture models may be performed to evaluate the impact of missing data on the results.
All statistical analyses will use a two-sided significance level of α = 0.05. Primary outcomes will be assessed at this threshold without correction for multiple comparisons. Secondary outcomes will be reported both with and without correction for multiple testing, using the false discovery rate (FDR) approach where applicable.
Exploratory analysis. Variation in person-dependent characteristics is highlighted to be an important factor for explaining heterogeneity in treatment responses [26]. For further translation, it is important to delineate possible subpopulations of participants/patients that may benefit the most from receiving allocated treatments. To examine the potential impact of distinct person-dependent variables, exploratory analyses will examine whether inter-individual variations in symptom load (e.g., higher stress/anxiety), higher baseline stress neurophysiology, and/or lower endogenous oxytocin levels (at baseline) impact treatment outcome. Also, the effects of age or biological sex will be examined. To do so, general linear model analysis, including these variables as covariates in the model, will be performed.
During the course of the intervention, the participant will be asked to keep a trial diary for logging the time point of the day of the nasal spray administration. Participants will be supported by research staff for adhering to and logging of the timings.
Upon receiving the supply of nasal sprays, participants will receive a side-effect report form on which they can record any possible adverse events associated with the administration of the nasal sprays during the nasal spray administration period.
On completion of the treatment period, participants will be asked to return the used nasal sprays to measure the amount of dispensed fluid for monitoring of compliance. While adherence will be monitored tightly and is expected to be high, skipped nasal spray administrations will be monitored tightly and will be included as dimensional covariates in data analyses. A protocol deviation will be recorded if less than two-thirds of the expected volume of oxytocin nasal spray fluid is used by the participant over the entire administration period. Here, secondary analyses are envisaged, conducting analyses with and without participants with less than two-thirds of the administered oxytocin nasal spray volume.
Next to monitoring nasal spray compliance, adherence to mindfulness training will also be tracked. In addition to logging the timings of their mindfulness sessions in a trial diary, the m-path app will track whether the home-based individual trainings have been completed.
Finally, at each post-treatment assessment session (T1, T2), participants will report whether they believe they received the verum oxytocin or placebo nasal spray.
While the use of psychoactive medications constitutes an exclusion criterion for participation, participants will not be asked to halt their participation or be excluded from the trial if their medication use changes during the course of the study. However, participants will be asked not to change their psychoactive medication or psychosocial treatment during the six weeks of nasal spray administration. If a change in psychoactive medication or psychosocial treatments is necessary during this period, participants must contact the study doctor to monitor the change. No list of prohibited or non-banned medications will be provided, as there are no known contraindications or interactions between other medications and the oxytocin nasal spray. Antiepileptic medication forms an exception, as the use of antiepileptic medication within the last 12 months is considered an indicator of active epilepsy (i.e., an exclusion criterion). Simultaneous participation in another clinical trial involving an approved or non-approved investigational medicinal product is prohibited.
The current protocol version is 1.0 (18 March 2025). Recruitment has not yet started but is expected to begin in September 2025 and conclude in September 2027.
No datasets were generated or analysed during the current study.
- ANS:
-
Autonomic Nervous System
- DASS-21:
-
Depression Anxiety Stress Scale
- ECG:
-
Electrocardiography
- EEG :
-
Electroencephalography
- GQ:
-
Group Questionnaire
- HPA axis :
-
Hypothalamic–Pituitary–Adrenal axis
- HRV:
-
Heart Rate Variability
- IU :
-
International Units
- LMC:
-
Leuven Mindfulness Consortium
- MBI:
-
Mindfulness-Based Intervention
- OXTR:
-
Oxytocin Receptor Gene
- POMS:
-
Profile of Mood States
- PSQI:
-
Pittsburg Sleep Quality Index
- PSS:
-
Perceived Stress Scale
- PTQ:
-
Perseverative Thinking Questionnaire
- SAAM:
-
State Adult Attachment Measure
- SCS-SF:
-
Self-Compassion Scale-Short Form
- SECPT:
-
Socially Evaluated Cold-Pressor Test
- TFMQ-SF:
-
Three-Facet Mindfulness Questionnaire-Short Form
- WHO-5:
-
World Health Organization Five Well-Being Index
Not applicable.
The work is funded by the Research Council KU Leuven, with the C2-project Grant C2M/24/074 and the Flanders Fund for Scientific Research (FWO), G018525N. As part of the funding approval process, the study outline was subject to independent peer review by external reviewers during the evaluation procedures of both grant applications. The funders had no role in the design of the study, collection, analysis, or interpretation of the data, and in writing the manuscript.
This study was approved by Commissie Medische Ethiek AZ Delta of Belgium through the Clinical Trials Information System (CTIS) (EU CT number: 2024–513482-39–00). The protocol and informed consent forms adhere to the principles outlined in the latest version of the Declaration of Helsinki and the ICH-GCP E6(R2) guidelines. Any subsequent protocol amendments will be submitted for approval to the Competent Authority and Ethical Committee of Belgium.
Written informed consent will be obtained from all participants by trained research staff before any study-related procedures. The consent process will include a verbal and written explanation of the study’s purpose, procedures, potential risks and benefits, and participants' rights, ensuring adequate time for questions. All trial staff involved in obtaining consent will be trained in Good Clinical Practice (GCP) and receive study-specific training before interacting with participants.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
van Weert, E., De Vuyst, H., Van der Gucht, K. et al. Exploring the mechanistic link between the oxytocinergic system and mindfulness training in adults with heightened stress: study protocol for a double-blind, randomized, placebo-controlled trial (MOX-MIND). BMC Psychiatry 25, 622 (2025). https://doi.org/10.1186/s12888-025-07077-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12888-025-07077-8