BMC Medicine volume 23, Article number: 306 (2025) Cite this article
Early menarche is associated with lifelong health implications, including heightened risks for obesity, type 2 diabetes (T2D), cardiovascular disease, and overall mortality. This study explored the associations that link early menarche, major adiposity indices, and T2D in a group of multi-ethnic Asian women.
A prospective, hospital-based study was conducted in Singapore. Two thousand seven hundred fifteen women were recruited from 2014 to 2016 (45–69 years old) and 1201 women were followed up from 2021 to 2023. At baseline, age at menarche (AAM) was divided into sub-categories: < 12 (early AAM), 12–13, 14–15 (reference), > 15 years. Major adiposity indices and glycemic profiles were assessed, including fat mass index (FMI), visceral adipose tissue (VAT), and HOMA-IR (homeostatic model assessment for insulin resistance). At the 6.6-year follow-up, T2D was assessed. One-way ANOVA and chi-square were performed for continuous and categorical variables, respectively. Multivariable regression analysis was performed to determine the association between AAM and primary outcomes, including adiposity measurements (FMI, VAT) and metabolic assessments (HOMA-IR) at baseline. Modified Poisson regression was performed to assess relative risk (RR) between AAM and T2D at follow-up. Serial mediation analysis was performed to determine potential mediators underlying the link between AAM and T2D. All analyses accounted for major confounders including age, ethnicity, and education.
Women with early AAM had significantly greater values in adiposity assessments, including increments in FMI (10.9 vs 10.3, p < 0.001), VAT (134 vs 113 cm2, p = 0.05) and HOMA-IR (1.20 vs 1.03, p = 0.08) at baseline. Early AAM (< 12 vs. 14–15 years) was associated with a 60% increased risk of developing T2D (RR 1.60 (95%CI: 1.04, 2.45)). Serial mediation analysis suggested a significant pathway underlying early AAM and T2D, which was firstly mediated by FMI, followed by VAT and lastly by HOMA-IR (p < 0.05).
Our study provided valuable insight into the pathophysiology of T2D development amongst mid-life women with early AAM. The findings could potentially indicate strategies to target FMI and VAT among Asian women in the menopausal phase with early AAM, to prevent the development of T2D.
Diabetes prevalence has risen to epidemic proportions on a global scale. The World Health Organization (WHO) reported that 246 million individuals had diabetes in 2007, with this statistic expected to increase to 380 million by 2025 [1]; 95% of individuals with diabetes have type 2 diabetes mellitus (T2D) which is caused by chronic hyperglycemia and insulin resistance, resulting in increased risks for cardiovascular disease, renal failure, blindness, lower limb amputations, and premature mortality [2]. It is estimated that 286 billion dollars are spent on annual healthcare costs for diabetes alone, which places an additional burden on the world’s economy. Traditional risk factors for developing T2D include lifestyle, e.g., obesity, and genetics, such as a family history of having diabetes. Certain ethnicities, notably Asians, exhibit a higher diabetes prevalence of T2D due to increased deposition of visceral fat compared to Caucasians [3]. Moreover, emerging evidence underscores the role of early age at menarche (AAM) as an additional risk factor for the development of T2D, shedding light on how hormonal and reproductive influences play a role on T2D risk [4].
Menarche refers to a woman’s first menstruation at adolescence, occurring between 10 to 16 years of age, with an average age of onset at approximately 13 years old [5]. Early AAM has been reported to be associated with health implications later on in a woman’s life, including an increased risk for diabetes [6], metabolic syndrome [7], hypertension [8], cardiovascular diseases [9], and all-cause mortality [10]. A large meta-analysis of 28 studies demonstrated a negative linear relationship between early AAM and T2D, with a 9% decrease (RR = 0.91, 95% CI 0.83–0.93) in T2D risk for every subsequent delay in AAM year [4]. The majority of the cohort studies present in the literature are predominantly from Caucasian participants, such as from North American and European populations. Asian ethnicities are under-studied and have been under-represented in large cohort studies. In addition, the underlying pathophysiology of early AAM and T2D remains largely understudied.
Specific biological mechanisms and potential mediators remain elusive. Traditionally, body mass index (BMI) was considered to be the underlying mechanism governing T2D development. However, emerging evidence has shown that visceral fat or visceral adipose tissue (VAT), instead of BMI, could possibly be a more plausible cause for T2D. Several recent studies have shown that FMI (fat mass index), VAT, and HOMA-IR (homeostatic model assessment for insulin resistance) are individually associated with T2D; however, the serial mediation between these factors and T2D development has not been clearly established. Furthermore, in middle-aged women, fat distribution and muscle strength are affected by gradual estrogen withdrawal due to the impending onset of menopause [11]. It is also noteworthy that BMI, while a convenient metric used to report health risks, is unable to differentiate between bone mass, muscle mass, and fat distribution in the body. Therefore, we aim to increase the resolution and understand the interplay of features within the generalized BMI metric, namely FMI, VAT, ALM (appendicular lean mass), and HOMA-IR, underlying the known link between AAM and T2D.
Prior to menopause, women typically have a lower risk of cardiometabolic diseases, such as heart disease and T2D, as compared to men [12]. This difference is largely attributed to the protective effects of estrogen, a hormone that plays a significant role in maintaining cardio-metabolic health in women. Despite the fact that postmenopausal women lose the advantages conferred by estrogen protection, resulting in increased risks of cardiometabolic diseases, there remains a paucity in research focusing on mitigating the cardiometabolic risk factors in mid-life women undergoing menopause. Thus, elucidating such associations to determine potential remediable causes of cardiometabolic diseases could potentially mitigate the adverse health consequences driven by menopause.
In order to investigate the association between early AAM and the development of T2D in more depth, with plausible pathophysiological mechanisms, and also to close the gap on the lack of evidence in Asian populations, we utilized a multi-ethnic Singaporean population and studied the association between early AAM and 6.6-year T2D development among mid-to-late aged women, via a mediation analysis factoring in all potential anthropometric biometrics. We hypothesize that AAM and 6.6-year T2D development could potentially be mediated by a set of adiposity-related parameters which affect metabolic regulation.
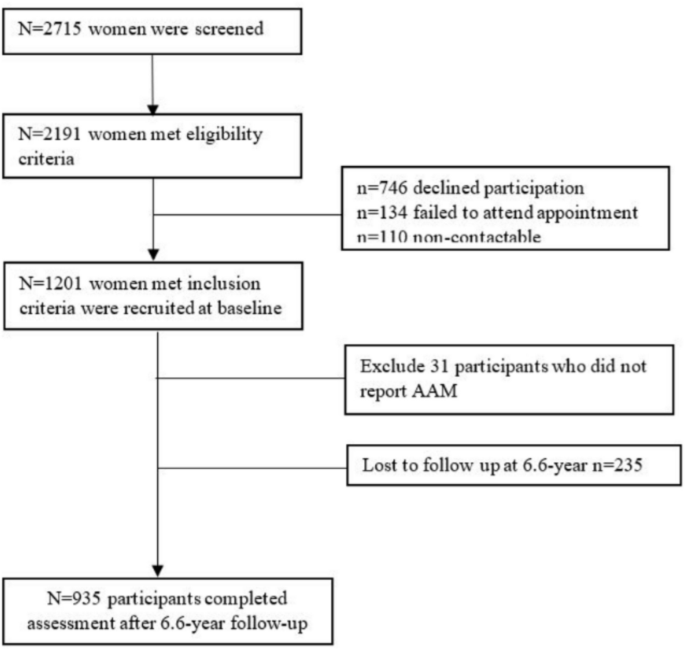
The Integrated Women’s Health Program (IWHP) is a longitudinal study examining key health issues among mid-life Singaporean women [13]. Healthy women aged 45–69 years old receiving routine gynecological follow-up at women’s health clinics at the National University Hospital were recruited from September 2014 to October 2016, and again followed up from February 2021 to July 2023. The protocol was approved by the Domain Specific Review Board of the National Healthcare Group, Singapore (reference number: 2014/00356 and 2020/00201) and all participants provided written informed consent. Two thousand seven hundred fifteen women were screened and 2191 met the eligibility criteria. Exclusion criteria included pregnancy or being severely ill. Women with type 1 diabetes were also excluded from the study. Subsequently, 746 participants, 134 participants, and 110 participants declined participation, failed to attend the appointment, and were non-contactable, respectively, leaving a final sample of 1201 women. Among these 1201 women, 1170 reported their AAM. The same group of 1170 women were contacted again after 6.6 years. Two hundred thirty-five women were lost to follow-up, leaving 935 women with follow-up information. One hundred ninety-nine women were recorded to have T2D at the 6.6-year follow-up (6.6 year follow-up refers to the 6 years and 7.2 months in between the baseline and follow-up visit), as shown in Fig. 1.
AAM was collected within the clinical demographics questionnaire [13] using the specific question “About how old were you when you had your first menses?” and women were subsequently divided into 4 categories: < 12 years, 12–13 years, 14–15 years (reference), and > 15 years old. The reference group of AAM was 14–15 years, in tandem with previous studies of menarche from China [14], Japan [15], and the USA [16]. Following other landmark studies, early AAM was defined as < 12 years old [17, 18].
Participants were classified as having diabetes if they had fasting blood glucose levels (overnight fast of 8–10 h) ≥ 7.00 mmol/L, were on anti-diabetic medications, or self-reported physician-diagnosed diabetes via validated questionnaires [13]. Participants were classified as prediabetic if fasting glucose levels were between 5.60 and 6.90 mmol/L. The process of determining if a participant had diabetes at baseline or at 6.6-year follow-up was the same.
Height and body weight were recorded using SECA 769 Electronic Measuring Station [13]. Waist and hip measurements at both the baseline and 6.6-year follow-up were recorded. They were measured twice using a standardized measuring tape, and the average was taken. If the difference between the first and second waist/hip measurement > 1 cm, third and fourth measurements were taken. Waist circumference was measured at the end of expiration assuring the tape was level. Weighing was conducted without shoes and participants were not explicitly requested to empty their bladders. Average values were calculated. BMI was computed as the body weight divided by height squared (kg/m2). Whole-body composition scans were performed and a fasting blood sample was collected for participants on arrival. Appendicular lean mass (ALM; kg/m2) was measured using DXA (Hologic Discovery Wi, Apex software 4.5) [13]. VAT (cm2) and fat mass index (FMI; kg/m2) were identified by automated software algorithms from DXA [13].
After the fasting blood draw, a series of questionnaires, biophysical measurements, and physical function tests were performed with the full visit lasting approximately 90 min. Blood pressure was measured thrice while in a seated position using the OMRON Intellisense device (HEM7211), with the average values calculated. Hypertension was defined as systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg or use of anti-hypertensive drugs or self-reported physician-diagnosed hypertension via questionnaire. Clinical demographics including age, ethnicity, education level, age of menarche, menopausal status, smoking status, and alcohol consumption were self-reported using a validated questionnaire. Serum biomarkers (glucose and insulin) were obtained from fasting blood samples and measured using ELISA assay kits (DRG International, Inc. USA), chemiluminescence immunoassays (ADVIA Centaur Analyzer, Siemens Healthcare Diagnostics), and colorimetrics (Beckman Coulter, Inc., USA). Homeostatic model assessment for insulin resistance (HOMA-IR) at baseline was obtained through the combination of fasting insulin and glycemic profile following the following formula: fasting insulin (microU/L) × fasting glucose (nmol/L)/22.50 [19].
One-way ANOVA and Kruskal–Wallis test were used to examine the relationship between AAM subgroups and continuous variables. Chi-square and Fisher’s exact test were used to test the significant differences between groups in terms of categorical variables, depending on the numbers of cases in subgroups. Partial Spearman Rank test was used for assessing the association between AAM and inflammatory, hormonal biomarkers, and anthropometric indices after adjusting for age at study entry, ethnicity, and highest education level in our participants. Skewed variables were subjected to a logarithmic transformation.
Multivariable regression analyses were used for assessing the association between AAM and the primary outcome T2D development at 6.6 years, after adjusting for age at study entry, ethnicity, highest education level, ALM, and HOMA-IR at baseline. We also identified potential mediators between AAM and T2D using multiple regression analyses.
Mediation analysis with multi-categorical independent variable which was proposed by Hayes and Preacher [20] was used to test the significance of the mediation effect. This multi-categorical mediating model was established using ordinal least squares regression to estimate both the direct and indirect effects of AAM on T2D. Subsequently, we conducted serial mediation analysis to investigate the relationships between FMI, VAT and HOMA-IR, based on evidence a priori, aiming to establish connections between AAM, FMI, VAT, HOMA-IR, and T2D. While prior groups have shown direct associations between FMI and T2D, VAT and T2D, and HOMA-IR and T2D [21,22,23], the mediating effects between these variables remain unclear. Non-parametric bootstrapping procedures were used to test the statistical significance of indirect effects of the proposed mediating variables. The significance of each relative indirect path was tested by using the bootstrapping method (5000 samples). Point estimates and confidence intervals for direct and indirect effects were derived from the bootstrapped samples. Following the approach outlined by Hayes and Preacher, we considered mediation to be supported if the 95% bias-corrected confidence interval did not include zero. Ratios of relative direct or indirect effect corresponding to relative total effect will not be reported as suggested by the methods’ developer [20].
As the independent variable AAM was a categorical variable with four levels, three dummy-coded variables (D1, D2 and D3) were created for the purpose of multiple mediation analysis [20]. In this study, the group of AAM = 14–15 years was the reference category. D1 represents comparisons between AAM < 12 years with the reference group; D2 represents comparisons between AAM = 12–13 years with the reference group; D3 represents comparisons between AAM > 15 years with the reference group. Relative direct and relative indirect effects were reported. Adjustments were applied, including ethnicity, education, study entry age, systolic blood pressure, and ALM when conducting the mediation analyses.
Additionally, we performed sensitivity analyses to investigate whether factors such as menopausal status, parity, breastfeeding history, history of hypertension, and physical performance might influence the magnitude of the mediating effects. Effect modification was also examined to identify potential modifiers in all observations investigated mentioned above. All statistical analyses were performed using R 4.2.2 version. PROCESS for R Version 4.3 was used for mediation analyses and bootstrapping procedures [24].
We applied a modified Poisson regression model—an approach which is better than Poisson regression with no difficulty with converging, yet which provides similar estimates in prospective data [25]—to examine the temporal relationship between age at menarche, adiposity, and metabolic features and T2D that occurred at the 6.6-year follow-up. To test the robustness of the findings, we performed sensitivity analyses by additionally adjusting for the following variables, including ALM, sarcopenia, hypertension, and heart conditions at baseline, as AAM was previously reported to be associated with heart conditions, hypertension, and sarcopenia in terms of comorbidity with T2D [26, 27].
The final cohort included 935 women with complete variables required for our analyses. The mean AAM was 13.10 years old, categorized into four AAM groups: < 12 years (n = 96), 12–13 years (n = 525), 14–15 years (n = 254), and > 15 years (n = 60). The crude associations between ethnicity, sociodemographic variables, reproductive variables, anthropometric variables, and serum biomarkers are shown in Table 1. No significant differences were observed among these subgroups in terms of demographics, such as ethnicity, household income, smoking status, alcohol use, or reproductive history. However, there were significant variations between AAM groups in mean age at entry (55.90 vs. 56.10 vs. 56.40 vs. 58.30; p < 0.01) and highest education level (p < 0.001). Specifically, 26.00% of women in the AAM < 12 years group attained a university degree or higher, compared to 22.90% in the AAM 12–13 years group, 19.30% in the AAM 14–15 years group, and 15.00% in the AAM > 15 years group.
Blood pressure measurements (systolic or diastolic) and reproductive history when compared across the four AAM categories did not show any significant difference. Of interest, body composition measurements were significantly different among AAM subgroups, such as ALM, FMI, BMI, and VAT. Women with early AAM had the highest BMI, VAT, FMI, and ALM, compared with other groups.
In terms of serum biomarkers, fasting insulin and HOMA-IR were statistically significant when comparing across the four AAM categories at the 6.6-year follow-up. At 6.6-year follow-up, women with AAM < 12 years had a mean insulin level of 7.20 mU/L and HOMA-IR 1.76, which were both higher compared to the reference group, which had a mean insulin level of 6.60 mU/L and HOMA-IR of 1.53 (p < 0.05). In the analysis of comorbidities, early AAM was not associated with hypertension, sarcopenia, or heart conditions at baseline in our cohort (p = 0.63, 0.89, and 0.22, respectively).
We conducted multiple regression models to investigate the associations between AAM and our primary outcome, T2D. The results revealed that early AAM significantly increased the risk of developing T2D (RR: 1.60, 95% CI: 1.04–2.45). These associations remained significant after adjusting for factors such as ethnicity, education, study entry age, and household income, as shown in Table 2 (aRR: 1.61, 95% CI: 1.02–2.54). Furthermore, individual mediation analysis showed that early AAM was also significantly linked to higher VAT (β: 0.36, 95% CI: 0.14–0.64), higher FMI (β: 0.05, 95% CI: 0.004–0.24) and higher HOMA–IR (β: 0.57, 95% CI: 0.22–1.01) (Supplementary Table 1 and Supplementary Fig. 1). Consistent with findings from previous studies, both VAT and FMI emerged as important predictors for T2D and higher HOMA-IR levels. Consequently, we proceeded to conduct a serial mediation analysis with VAT, FMI, and HOMA-IR as potential mediators in the relationship between AAM and T2D.
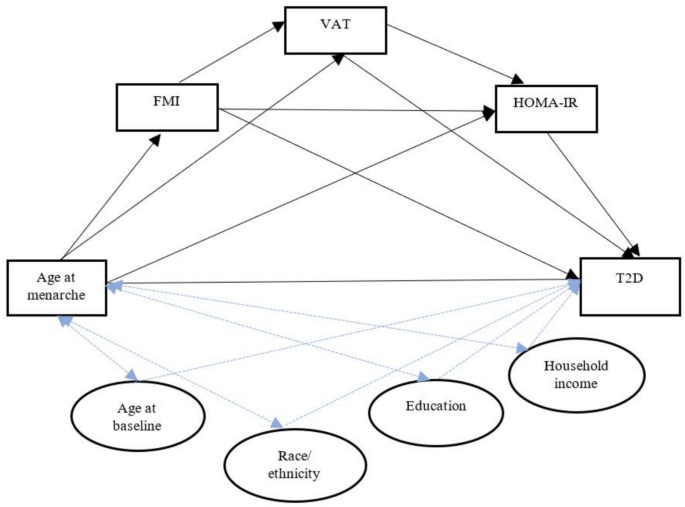
Serial mediation analysis observed that a statistically significant path coefficient existed between early AAM (< 12 years vs. 14–15 years) and T2D through an indirect effect from FMI to VAT to HOMA-IR (β = 0.089 (0.01, 0.34)) as shown in Table 3, after a full adjustment of ethnicity, education, and study entry age. Figure 2 represents the directed acyclic graph capturing the study exposure (early menarche), outcome (T2D), mediators (FMI, VAT, HOMA-IR), and confounders (age, ethnicity, education, household income).
For the sensitivity analysis, we further adjusted for ALM, sarcopenia, hypertension and heart conditions at baseline, which did not significantly change the effect size of observed associations, as shown in Supplementary Table 2. We also examined the interaction between AAM and menopausal state in relation to T2D, and no significant interaction was found.
In this study, we show for the first time that early AAM (< 12 years old) is associated with a 60% increased risk of development of T2D during the menopausal phase, compared with women with AAM at 14–15 years. This temporal relationship was significantly mediated firstly by the increment of overall fat mass, followed by increased visceral fat volume and lastly by insulin resistance.
Early menarche has been associated with higher BMI through the consequences of greater leptin production [28]. Leptin secreted by adipose tissue has been shown to act directly on the hypothalamus and stimulate gonadotrophin-releasing hormone (GnRH), which subsequently causes an increase in the secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary gland as well as a surge in sex hormone production by the ovaries (estrogen, progesterone) [29]. These concerted changes within the hypothalamus–pituitary–ovary axis eventually result in a woman’s first menstrual cycle, also termed menarche. Of interest, early AAM has been reported to cause premature closure of the epiphyseal plates, resulting in these women being of shorter stature, which could account for higher BMI tendencies [30].
BMI, a composite index of total body weight accounting for height, is a widely used surrogate for body adiposity in public health practice [31, 32]. Obesity has typically been diagnosed using BMI ≥ 25 kg/m2 for overweight individuals and BMI ≥ 30 kg/m2 for obese individuals. For Asian ethnicities, a modified BMI of > 23 would categorize individuals as overweight and BMI > 27.50 as obese, as this took into consideration the differences in body build and muscularity between Caucasians and Asians [33]. However, BMI alone has been subsequently noted to be an unspecific metric which is unable to discern between fat, muscle, or bone mass [34]. Additional indices such as waist circumference (WC), waist–hip ratio (WHR), lipid accumulation product index (LAP) and relative fat mass (RFM) were subsequently used to more accurately report the fat distribution in the body [35]. A global review of T2D risk showed that in almost all regions and ethnicities, a larger WC was strongly and linearly associated with T2D risk [36]. However, the exact biological mechanisms linking anthropometric measures to T2D development remained elusive.
We show for the first time that early AAM predisposes women to higher insulin resistance, a quantitative marker for T2D, via higher FMI, and then higher VAT accumulation. While other groups have shown individual links between fat mass and T2D, HOMA-IR and T2D as well as BMI and T2D [37, 38], this study aims to unravel the potential sequential events of T2D development, through a serial mediation analysis. For example, analysis from the BioCycle Study reported that early AAM was associated with unfavorable metabolic phenotypes as compared with the average age of onset of menarche, with the concurrent observation of reduced insulin sensitivity and β-cell function [37]. In addition, it was shown that body composition and BMI were complex metrics, containing several variables, which could not specifically account for fat composition in certain individuals, for example, those with normal BMI [38]. In addition, insulin resistance, measured through HOMA-IR, has been reported to be associated with increasing BMI [39]. However, normal-weight individuals have been known to be insulin resistant, suggesting that BMI is not the sole determinant of insulin resistance. Therefore, it is increasingly more likely that there exists another intermediary factor linking BMI to insulin resistance. Recently, insulin resistance has been associated with ectopic fat deposition, especially in the liver and muscle, with a tendency toward the development of a vicious cycle driven by overnutrition and improper dietary choices [40]. In metabolic syndromes, greater associations between insulin resistance and central adiposity were conferred [41]. Therefore, our model tracing the path connecting early AAM to FMI, VAT and HOMA-IR provides new knowledge in the collective understanding of the biological mechanisms governing metabolic disorders, such as T2D. In addition, the majority of the studies linking AAM to T2D have been performed in Caucasian populations. In Asian populations, where VAT and T2D incidence are high, the findings of our study are especially relevant. Furthermore, this study provides new insight into a woman’s unique mid-life stage, coinciding with the menopausal transition which involves a cascade of hormonal and physiological changes, marking a shift from the reproductive years to menopause (post-reproductive years). Extreme hormonal shifts at mid-life have been associated with an increase in fat mass, an increase in waist circumference and a decrease in skeletal mass [42]. The menopausal transition has also been associated with increased cardiovascular risk due to hormonal changes and fat redistribution, independent of aging [11]. While midlife has been reported as a vulnerable timeframe, recent studies have shown that it is also highly dynamic and amenable to meaningful change [43]. Therefore, midlife is a critical window as a target of interventions, as several limitations commence at midlife, such as reduced estrogen and behavioral changes (changes in diet, exercise and sleep patterns due to age), with visible health trajectories which persist into late adulthood [44].
As compared with blood-based biomarkers which tend to fluctuate and change frequently, VAT is relatively stable and has the potential to be a robust biomarker for metabolic aberrations, including insulin resistance [45]. Health screening during mid-life, including FMI, VAT, and HOMA-IR assessment could provide early indicators and a potential window phase for intervention to mitigate T2D development. Though one cannot control the age at menarche, the results of this study suggest that women with early menarche could benefit from maintaining a healthy lifestyle through balanced nutrition and moderate exercise to reduce fat mass. As fat tissues build up over time, early interventions to reduce VAT could mitigate the predisposition to insulin resistance and potentially diabetes development later in life. This intervention and emphasis on healthy nutrition and lifestyle before a girl reaches menarche, with continuous control against the accumulation of visceral fat into midlife, would ensure optimal cardiometabolic health during her reproductive, midlife, and possibly even into her postmenopausal years.
A major strength of this study is that it is the first to provide direct evidence of the interactions between age at menarche, adiposity features, insulin resistance, and diabetes development within a multi-ethnic Asian cohort. Selection bias was minimal in this study, as the IWHP recruited all mid-life consenting multi-ethnic Asian female participants from Singapore who presented to the National University Hospital for routine gynecological care, thus maximizing the generalizability of our findings, taking into account the sample size, sampling procedures, and participation rate.
This is a prospective hospital-based study. As with all human participant research studies, there is a limitation of patient recall, although it has been reported that women will tend to remember their age at menarche as it marks a significant life event [46,47,48]. Future longitudinal validation studies recruiting patients at their age at menarche and continuing into adulthood are clearly warranted [49]. As our cohort focused on a multi-ethnic Asian population, future studies should also include other ethnicities and populations to be able to extrapolate these findings on a global scale. Residual confounding due to unmeasured variables such as diet during pregnancy, intrauterine life and genetic factors represent other limitations. However, despite certain limitations, this is the first longitudinal study that provides compelling evidence that early menarche is a marker of excess body fat accumulation, which can cause metabolic complications later in a woman’s life.
This study offers a distinct contribution to the field, as it is one of the rare works in a multi-ethnic Asian population that has revealed a link between early menarche and developing diabetes later in a woman’s life, through increased fat mass, visceral adipose tissue deposition, and insulin resistance. Studies such as these could equip healthcare providers on the long-term effects of early menarche as well as modifiable risk factors which could potentially improve a woman’s health trajectory, with the emphasis to begin healthy nutrition from childhood as well as optimal control of visceral fat accumulation during midlife.
No datasets were generated or analysed during the current study.
- AAM:
-
Age at menarche
- AGM:
-
Abnormal glucose metabolism
- ALM:
-
Appendicular lean mass
- BMI:
-
Body mass index
- DXA:
-
Dual energy x-ray absorptiometry
- ELISA:
-
Enzyme linked immunosorbent assay
- FMI:
-
Fat mass index
- FSH:
-
Follicle stimulating hormone
- GnRH:
-
Gonadotropin releasing hormone
- HOMA-IR:
-
Homeostatic model assessment for insulin resistance
- IWHP:
-
Integrated Women’s Health Programme
- LAP:
-
Lipid accumulation product index
- LH:
-
Luteinizing hormone
- T2D:
-
Type 2 diabetes mellitus
- VAT:
-
Visceral adipose tissue
- WC:
-
Waist circumference
- WHR:
-
Waist–hip ratio
This project was funded in part by NUS Bia-Echo Asia Center for Reproductive Longevity and Equality (ACRLE). The IWHP was supported by the Singapore National Medical Research Council (NMRC/CSA-SI/0010/2017 and NMRC/CSA-SI/MOH-000670-01 were awarded to Prof Yong Eu-Leong).
Ethics approval and participant consent was obtained for this study (Domain Specific Review Board of the National Healthcare Group, Singapore, #2014/00356 and #2020/00201).
NA.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material 2: Supplementary Table 1. Individual mediation analysis of VAT, FMI and HOMA-IR on association between age at menarche and T2D, after adjusting for maternal age at study entry, race/ethnicity, maternal education at study entry and house income at study entry.
Supplementary Material 3: Supplementary Figure 1. Comparison of relative indirect effects of VAT, FMI and HOMA-IR individually on the association between age at menarche and T2D, after adjusting for maternal age at study entry, race/ethnicity, maternal education at study entry and house income at study entry.
Supplementary Material 4: Supplementary Table 2. Serial mediation analysis of FMI, VAT, HOMA-IR on association between age at menarche and T2D.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Benny, P., Yang, Q., Wong, B.WX. et al. Exploring the link between age at menarche, anthropometry and body fat composition with type II diabetes in a Singapore multi-ethnic cohort. BMC Med 23, 306 (2025). https://doi.org/10.1186/s12916-025-04145-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-025-04145-4