BMC Gastroenterology volume 25, Article number: 419 (2025) Cite this article
The Dietary Index of Gut Microbiota (DI-GM) is a newly developed measure for assessing diet quality in relation to the diversity of the gut microbiome. However, whether it is associated with the risk of chronic constipation and chronic diarrhea remains unclear.
We analyzed data from 7,943 U.S. adults aged 20 years and older who participated in the 2007–2010 National Health and Nutrition Examination Survey (NHANES). Weighted logistic regression, subgroup analysis, and restricted cubic spline (RCS) models were used to assess the association between the DI-GM and abnormal bowel symptoms.
A higher DI-GM score was significantly associated with a reduced risk of abnormal bowel symptoms. For each 1-point increase in the DI-GM, the risk of constipation decreased by 12.4% (OR = 0.876, 95% CI = 0.806–0.951, p = 0.002), and the risk of diarrhea decreased by 14.1% (OR = 0.859, 95% CI = 0.789–0.936, p < 0.001). Compared with the lowest DI-GM quartile, the highest quartile showed a markedly lower risk of both constipation (OR = 0.487, 95% CI = 0.340–0.696, p < 0.001) and diarrhea (OR = 0.480, 95% CI = 0.338–0.682, p < 0.001). RCS analysis indicated a significant linear association between the DI-GM and the risks of both constipation (nonlinear p = 0.686) and diarrhea (nonlinear p = 0.136).
The DI-GM was negatively associated with the prevalence of abnormal bowel symptoms. Further longitudinal studies are warranted to confirm these findings and to inform dietary strategies for gut health.
Chronic constipation and chronic diarrhea are common abnormal bowel symptoms, affecting approximately 6.6–11.7% and 1.2–4.7% of the global population, respectively [1]. Disorders of the gastrointestinal tract impose a substantial economic burden, with costs reaching billions of dollars annually in the United States [2]. Chronic constipation can lead to conditions such as anal fissures and hemorrhoids due to straining, and studies have shown that hypertensive patients with constipation have a 34% higher risk of subsequent major adverse cardiac events (MACE) [3, 4]. On the other hand, chronic diarrhea can result in electrolyte imbalances, malnutrition, and intestinal infections [5]. Abnormal bowel symptoms are also commonly linked to mental health issues such as depression and anxiety [6], severely affecting quality of life and placing considerable strain on healthcare systems.
The underlying pathophysiological mechanisms of chronic constipation and diarrhea are complex and not yet fully understood. Contributing factors include abnormal gastrointestinal motility, visceral hypersensitivity, low-grade post-infectious inflammation, alterations in gut microbiota composition, brain–gut axis dysregulation, and genetic predisposition [7]. Among these, the gut microbiota—a dynamic community of microorganisms—has emerged as a key player in gastrointestinal health. Beneficial microbes contribute to intestinal motility, immune modulation, nutrient metabolism, epithelial cell maintenance, and protection against pathogenic invasion [8, 9]. Dysbiosis—characterized by reduced microbial diversity and a decline in beneficial bacteria—has been implicated in the pathogenesis of both constipation and diarrhea, potentially through impaired production of short-chain fatty acids (SCFAs) and altered microbial composition [10,11,12]. These findings highlight the need for tools that can assess the diet–microbiota relationship and its impact on bowel function.
The DI-GM, developed by Kase et al., is an innovative dietary index designed to reflect gut microbiota abundance. Based on a comprehensive evaluation of 106 studies exploring the relationship between diet and the gut microbiome in adults, the index identifies 14 key foods or nutrients associated with microbial diversity [13]. It offers a practical approach for assessing the impact of diet on microbial health and guiding nutritional strategies for microbiota-related diseases. Unlike conventional indices such as the Mediterranean Diet Score (MDS) or the Healthy Eating Index (HEI) [14], which primarily evaluate general dietary quality, the DI-GM is specifically formulated to capture the diversity and richness of the gut microbiota. Its application enables better identification of dietary patterns that may promote gut microbiota abundance. Despite its potential, the association between DI-GM and abnormal bowel symptoms has not yet been fully explored. Therefore, this study aimed to investigate the relationship between DI-GM and the risk of chronic constipation and diarrhea using data from NHANES. The findings may help inform public dietary recommendations to improve gut health and prevent abnormal bowel symptoms.
This study used data from the NHANES, a nationwide program administered by the Centers for Disease Control and Prevention (CDC), and all NHANES data are publicly accessible at https://www.cdc.gov/nchs/nhanes/. Data from two survey cycles (2007–2008 and 2009–2010) were included, totaling 20,686 participants. After applying the following exclusion criteria, 7,943 adults were eligible for analysis. (1) participants aged ≤ 20 years, pregnant, or diagnosed with rectal and/or colon cancer; (2) those lacking bowel health questionnaire data; (3) those missing covariate information. The specific process is shown in Fig. 1. All participants provided written informed consent. The NHANES protocol was approved by the National Center for Health Statistics Institutional Review Board and complied with ethical standards.
The DI-GM was developed based on a systematic review of 106 longitudinal studies published between 2008 and 2021. These studies investigated dietary components linked to gut microbiota diversity, SCFA production, and specific bacterial taxa. Based on literature consensus and NHANES data, 14 components were selected: beneficial components included fermented dairy products, chickpeas, soy, whole grains, dietary fiber, cranberries, avocados, broccoli, coffee, and green tea; adverse components included red meat, processed meat, refined grains, and a high-fat diet (≥ 40% of energy from fat). The index was validated through biomarker associations and correlation with existing dietary indices. In NHANES data, the DI-GM score showed a significant positive correlation with urinary enterodiol and enterolactone, which are indirect markers of gut microbiota diversity. Moderate correlations were observed between the DI-GM and both the HEI-2015 and the MDS, indicating its ability to reflect overall diet quality [13].
The DI-GM score was calculated using dietary recall data from the 2007–2010 NHANES cycles. For gut-friendly foods, participants received 1 point if their intake was equal to or above the sex-specific median; otherwise, they received 0 points. For adverse foods, a score of 0 was assigned if intake exceeded the sex-specific median or, in the case of a high-fat diet, if ≥ 40% of energy was derived from fat; Otherwise, a score of 1 was assigned. The total score of DI-GM ranges from 0 to 13 points. The higher the score, the more conducive the diet is to the diversity and richness of the intestinal microbiota. Among them, the beneficial components range from 0 to 9 points, and the adverse components range from 0 to 4 points [13]. The scores were then categorized into four groups: ≤3, 4, 5, and ≥ 6. In subsequent analyses, when DI-GM was treated as a categorical variable, the group with DI-GM ≤ 3 was used as the reference category [15].
Abnormal bowel symptoms were assessed using the Bowel Health Questionnaire, administered by trained interviewers at the NHANES Mobile Examination Center (MEC), as described in previous studies. The Bristol Stool Form Scale (BSFS) was used for classification. Participants were shown a reference card depicting the seven BSFS types. Those who most frequently reported BSFS Type 1 (separate hard lumps, like nuts) or Type 2 (sausage-shaped but lumpy) were categorized as having chronic constipation. Participants with predominant BSFS Type 6 (mushy stool with ragged edges) or Type 7 (watery, no solid pieces) were classified as having chronic diarrhea. All others were considered to have normal bowel habits [6, 16].
The covariates in this study included demographic characteristics (age, gender, race/ethnicity, education level, marital status, and poverty-to-income ratio) and lifestyle factors (smoking status, alcohol consumption, body mass index (BMI), depressive symptoms, and recreational activity). Age was categorized into 20–39 years, 40–59 years, and 60–80 years. Race/ethnicity was divided into five groups: Mexican American, Non-Hispanic White, Non-Hispanic Black, Other Hispanic, and Other Races. Education level was classified as less than high school, high school or equivalent, and more than high school. Marital status was grouped as married/living with a partner, divorced/widowed/separated, and never married. The poverty-to-income ratio (PIR) was categorized into three groups: <1.3, ≥ 1.3 but < 3.5, and ≥ 3.5. A lower PIR indicates a higher level of poverty. Smoking status was categorized into three groups: never smokers (those who have smoked fewer than 100 cigarettes in their lifetime), former smokers (those who have smoked more than 100 cigarettes in their lifetime but no longer smoke), and current smokers (those who have smoked more than 100 cigarettes in their lifetime and currently smoke). Alcohol consumption was defined as drinking more than 12 alcoholic beverages per year. BMI was grouped into four categories: underweight (BMI < 18.5), normal weight (18.5 ≤ BMI < 24.9), overweight (25 ≤ BMI < 29.9), and obese (BMI ≥ 30). Depressive symptoms were assessed using the Patient Health Questionnaire − 9 (PHQ-9), with a score of ≥ 10 indicating the presence of depressive symptoms. Leisure-time physical activity was categorized into three groups: none, moderate activity (engaging in any moderate-intensity exercise, fitness, or recreational activity that slightly increases breathing or heart rate), and vigorous activity (engaging in any vigorous-intensity exercise, fitness, or recreational activity that significantly increases breathing or heart rate).
All statistical analyses accounted for the complex sampling design of NHANES by applying appropriate sampling weights. Baseline characteristics of participants were described across DI-GM quartile groups. Categorical variables were presented as weighted percentages, and differences among groups were evaluated using the weighted chi-squared (χ²) test.
The association between DI-GM and abnormal bowel symptoms was assessed using weighted logistic regression under three models: Model 1: unadjusted; Model 2: adjusted for age, gender, and race/ethnicity; Model 3: further adjusted for education, marital status, PIR, BMI, alcohol intake, smoking status, depressive symptoms, and physical activity. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Stratified analyses were performed to examine potential effect modification across subgroups defined by key covariates. Interaction terms were tested to evaluate heterogeneity. Finally, RCS regression models were used to assess and visualize the dose-response relationship between DI-GM and the risk of constipation and diarrhea. All statistical analyses were conducted using R software (version 4.4.2), with p-values < 0.05 considered statistically significant.
A total of 7,943 participants were included in this study, comprising 4,047 females (50.9%) and 3,896 males (49.1%). Among them, 628 individuals (7.9%) were classified as having chronic constipation, and 670 individuals (8.4%) as having chronic diarrhea. The racial/ethnic composition included 3,990 Non-Hispanic Whites (50.2%), 1,479 Non-Hispanic Blacks (18.6%), 1,347 Mexican Americans (17.0%), 826 Other Hispanics (10.4%), and 301 individuals (3.8%) of other racial backgrounds, including American Indian/Alaska Native, Pacific Islander, Asian, and multiracial participants. Participants with lower DI-GM scores were more likely to be younger, male, of Non-Hispanic Black ethnicity, have lower education levels, be never married, currently smoke, abstain from alcohol, exhibit depressive symptoms, and engage in vigorous physical activity.
The overall prevalence of chronic constipation and diarrhea was 7.9% and 8.4%, respectively. Stratified by DI-GM quartiles (≤ 3, 4, 5, ≥ 6), the prevalence of chronic constipation was 8.2%, 8.0%, 8.9%, and 6.8%, while the prevalence of diarrhea was 9.4%, 8.8%, 8.6%, and 7.2%, respectively. Participants in the highest DI-GM group (≥ 6) had the lowest prevalence of abnormal bowel symptoms. There were no statistically significant differences across DI-GM quartiles in family income-to-poverty ratio (p = 0.915) or BMI (p = 0.120). A comprehensive summary of baseline characteristics is presented in Table 1.
Multivariable logistic regression results are presented in Table 2. When treated as a continuous variable, the DI-GM was significantly and inversely associated with both chronic constipation (Model 3: OR = 0.876, 95% CI = 0.806–0.951, p = 0.002) and chronic diarrhea (Model 3: OR = 0.859, 95% CI = 0.789–0.936, p < 0.001). When modeled categorically (using DI-GM ≤ 3 as the reference), participants in the highest DI-GM group (≥ 6) had significantly lower odds of constipation (OR = 0.487, 95% CI = 0.340–0.696, p < 0.001) and diarrhea (OR = 0.480, 95% CI = 0.338–0.682, p < 0.001). These associations remained consistent across Models 1 to 3. Notably, the associations were not statistically significant for participants with DI-GM scores equal to 5.
Subgroup analyses were conducted using Model 3 to evaluate the consistency of associations across strata defined by demographic and lifestyle variables. Interaction tests were included to identify effect modifiers.
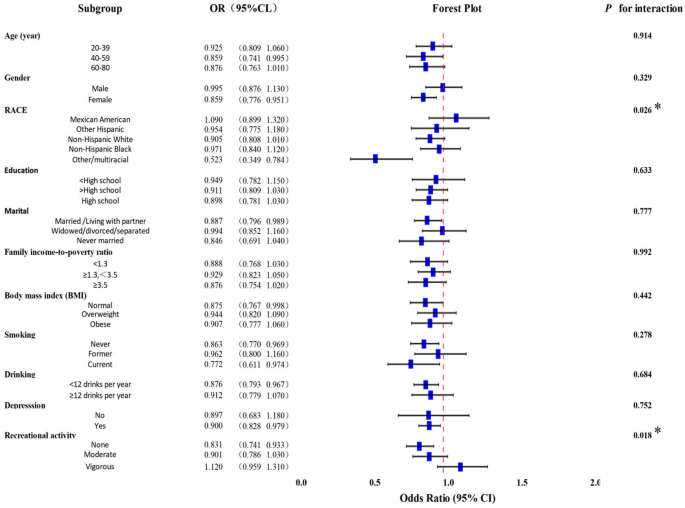
As illustrated in Fig. 2, race (interaction p = 0.026) and recreational activity (interaction p = 0.018) significantly modified the relationship between DI-GM and constipation.In the case of diarrhea (Fig. 3), significant interactions were observed for gender, marital status, education level, smoking status, and recreational activity, suggesting heterogeneity in DI-GM effects across these subgroups.
Forest plot of subgroup analyses of the relationship between DI-GM and constipation. Based on Model IIII: adjusted for age, gender, race, marital, education, Family income-to-poverty ratio, body mass index, smoke, drink, depression, recreational activity. *p < 0.05
Forest plot of subgroup analyses of the relationship between DI-GM and diarrhea. Based on Model III: adjusted for age, gender, race, marital, education, Family income-to-poverty ratio, body mass index, smoke, drink, depression, recreational activity. *p < 0.05
To assess the dose-response relationship between DI-GM and the risks of chronic constipation and diarrhea, RCS models were constructed (Fig. 4). The analysis revealed an approximately linear inverse association for both conditions, as indicated by non-significant nonlinearity p-values (constipation: p = 0.686; diarrhea: p = 0.136). The RCS curves demonstrated a steady downward trend in risk with increasing DI-GM, suggesting a predominantly linear relationship.
Restricted cubic spline of DI-GM vs. constipation and diarrhea probability. The solid line represents the OR, and the shaded area represents the 95% CI. The green line represents the RCS between DI-GM and constipation, while the red line represents the RCS between DI-GM and diarrhea
This study represents the first nationally representative analysis exploring the association between the DI-GM and chronic constipation and diarrhea in U.S. adults. Using weighted logistic regression, we found that higher DI-GM scores were significantly associated with a lower prevalence of abnormal bowel symptoms. This association remained robust after adjusting for potential confounders. Interestingly, compared with the reference group, participants in the third quartile (DI-GM = 5) did not show a statistically significant reduction in risk, while those in the second and fourth quartiles did. This pattern may suggest that only a sufficiently high DI-GM score confers measurable protective effects, or it may reflect random variation or differences in sample distribution across quartiles. Further investigation is warranted to explore this phenomenon.
The DI-GM is based on 14 specific foods or nutrients, each previously shown to influence the abundance of beneficial bacteria (e.g., Bifidobacterium, Lactobacillus) or harmful bacteria (e.g., Clostridium difficile, Escherichia coli) [17, 18]. Higher microbial richness has been consistently linked to better gut health [19]. SCFAs-the key metabolites produced by beneficial gut microbes, including acetate, propionate, and butyrate-enhance gut motility through neuromodulation, maintain mucosal barrier integrity, and suppress inflammatory responses [20,21,22]. These findings support our results that a higher DI-GM is associated with increased gut microbiota abundance, which, in turn, leads to a reduced prevalence of abnormal bowel symptoms.
Over the years, with increasing health awareness, there has been growing attention toward improving quality of life and preventing diseases. In this context, disease prevention through specific foods and dietary patterns has been a key area of public interest. Modern scientific research has continuously unveiled intricate links between nutrition and health. Studies have shown that unhealthy diets, such as those rich in FODMAPs, are considered potential triggers for functional gastrointestinal disorders [23]. A randomized controlled trial demonstrated that a Mediterranean diet helps alleviate constipation symptoms and intestinal inflammation [24]. Another cross-sectional study found that higher HEI-2015 scores were associated with a lower risk of constipation, particularly in individuals with higher levels of physical activity [25]. The results of our study further demonstrate that a higher DI-GM is associated with a lower prevalence of abnormal bowel symptoms. In contrast to conventional dietary pattern development strategies—which primarily rely on epidemiological studies to establish disease-preventive frameworks or on setting thresholds for essential nutrients to define basic dietary standards. The DI-GM was constructed from food components that promote gut microbial diversity and SCFA production, thereby providing a microbiota-targeted example for formulating evidence-based dietary recommendations [13].
The important role of gut microbiota abundance in the disease process has been confirmed by many studies. The gut microbiota plays a critical role in the body’s metabolic, immune, protective, and neurological systems. Its influence is not limited to the gastrointestinal tract but extends to the brain via the enteric nervous system and other pathways, such as the vagus nerve, sympathetic nervous system, and hypothalamic-pituitary-adrenal (HPA) axis [26]. Prior studies have linked higher DI-GM scores to lower depression risk [15]. Beneficial microbes may attenuate stress responses by modulating HPA activity and reducing cortisol release, thereby improving mood and behavior [27]. Furthermore, the diversity of the intestinal microbiota in patients with diabetes and arteriosclerosis was significantly lower than that in the healthy control group [11, 28]. Therefore, considering the role of the gut microbiota in various diseases, it is of great significance to explore dietary intervention strategies that improve the gut microbiota.
In our subgroup analysis, the association between DI-GM and abnormal bowel symptoms varies among different populations or across different lifestyles. For example, a previous Mendelian randomization study demonstrated that smoking alters gut microbiota composition, while gut microbiota, through the gut-brain axis, may in turn influence smoking behavior [29]. Marital status can also affect lifestyle habits, as couples often influence each other’s physical and mental health, dietary habits, and consequently, gut microbiota composition and disease risk. Research has shown similarities in gene expression, immune cell profiles, and gut microbiota among couples [30]. These subtle lifestyle impacts on gut microbiota should also be considered. However, considering the number of interaction tests conducted, the possibility of accidental results resulting from multiple comparisons cannot be ruled out. Therefore, these results should be carefully referred to and regarded as hypothesized.
Nevertheless, there are still several limitations in this study. Firstly, due to the cross-sectional design, it is impossible to clearly infer the causal relationship between DI-GM and intestinal symptoms, and the possibility of reverse causality cannot be ruled out. Secondly, the dietary data of NHANES is based on two non-consecutive 24-hour dietary recalls, which may not accurately reflect the long-term dietary status of the participants. Furthermore, due to the limitations of the database, DI-GM only includes 14 components and fails to fully reflect more components. Secondly, the definition of abnormal intestinal symptoms is based on the single-item self-reported questions of BSFS, failing to assess the frequency and duration of symptoms, and not controlling the impact of drug use on fecal morphology, which may lead to misclassification of outcomes. Although we have adjusted for multiple covariates, there is still the possibility of some unmeasured confounding factors, such as history of gastrointestinal diseases, drug use (such as laxatives, anti-diabetic drugs), and fluid intake, etc. Furthermore, since the population of this study was American adults aged 20 to 80, it is uncertain whether the results are applicable to other dietary cultural backgrounds or the elderly population. DI-GM was also developed based on data from the United States, and its applicability among non-Western populations still needs further verification.
In view of these limitations, the current research results should be interpreted with caution. In conclusion, this study provides a new direction for exploring dietary intervention strategies centered on intestinal microecology and emphasizes the necessity of conducting longer-term longitudinal and clinical trials to verify causal relationships.
This study showed that DI-GM was negatively associated with the incidence of chronic constipation and chronic diarrhea. These findings support the idea that a higher DI-GM score may be associated with a lower risk of abnormal bowel symptoms. Further research, including longitudinal and interventional studies, is warranted to explore whether dietary interventions targeting the gut microbiota can help reduce the risk of bowel disorders and inform future dietary guidelines.
Data for this study were sourced from National Health and Nutrition Examination Survey (NHANES) and available here: https://www.cdc.gov/nchs/nhanes/index.htm.
- NHANES:
-
National health and nutrition examination survey
- DI-GM:
-
Dietary index of gut microbiota
- OR:
-
Odds ratio
- 95% Cl:
-
95% confidence interval
- BMI:
-
Body mass index
- RCS:
-
Restricted cubic spline
- BSFS:
-
Bristol stool form scale
- PHQ-9:
-
Patient health questionnaire - 9
- SCFAs:
-
Short-chain fatty acids
- HPA:
-
Hypothalamic-pituitary-adrenal
- HEI:
-
Healthy eating index
- MDS:
-
Mediterranean diet score
We gratefully acknowledge the contribution of all participants of the present research.
This work was funded by the Jiangsu Province Natural Science Foundation project (No. BK20211085); Suzhou Applied Basic Research (Medical and Health) Science and Technology Innovation Project (NO. SYW2024135); Suzhou People’s Livelihood Science and Technology Project (No. SS2019073). Suzhou Municipal Health Commission (No. LCZX202114); Suzhou Gusu Health Talent Plan (No. GSWS2021046). Suzhou “Science Education and Health” Top-level Project(NO. MSXM2024028).
The study analyzed data downloaded from the National Health and Nutrition Examination Survey public database. The National Center for Health Statistics Ethics Review Committee granted ethics approval. The methods involved in this study were conducted in accordance with relevant guidelines and regulations (Declaration of Helsinki). All individuals provided written informed consent before participating in the study. Details are available at https://www.cdc.gov/nchs/nhanes/irba98.htm. The current study was deemed exempt from further review because the data used are deidentified and publicly accessible.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Shen, S., Feng, H., Wang, C. et al. Association between the dietary index of gut microbiota and abnormal bowel symptoms in U.S. adults: a cross-sectional study based on NHANES 2007–2010. BMC Gastroenterol 25, 419 (2025). https://doi.org/10.1186/s12876-025-04021-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12876-025-04021-8