BMC Surgery volume 25, Article number: 288 (2025) Cite this article
To evaluate the effectiveness of preoperative 3D reconstruction combined with intraoperative augmented reality fluorescence guidance system in laparoscopic liver surgery by establishing a retrospective cohort study.
A retrospective cohort study was conducted from March 2023 to December 2024, with patients' data from the medical record system. Patients were divided into two groups according to their surgical protocols: 46 cases in the control group (conventional laparoscopic liver surgery) and 50 cases in the observation group (preoperative 3D reconstruction combined with intraoperative augmented-reality fluorescence guiding system in laparoscopic liver surgery). We compared perioperative indexes (operation time, intraoperative bleeding, time to first flatus, drainage tube removal time, hospitalization time), preoperative and postoperative liver function indexes [alanine aminotransferase (ALT), albumin (ALB), total bilirubin (TBIL)], stress indexes [angiotensin II (AT II), norepinephrine (NE), epinephrine (AD)], and complication rates between the two groups.
The operation time of patients in the observation group was shorter than that of patients in the control group (110.75 ± 20.56 vs. 122.35 ± 20.48 min, 95% CI of difference: 2.52–20.68 min, p = 0.013), and the amount of intraoperative bleeding was less (300.80 ± 32.70 vs. 320.76 ± 35.84 mL, 95% CI of difference: 7.62–32.30 mL, p = 0.002). There was no statistically significant difference in the comparison of time to first flatus, drain removal time, hospitalization time and complication rate between the two groups (p > 0.05). Preoperatively, the comparison of ALT, ALB, TBIL, AT II, NE, AD levels of patients in the two groups were not statistically significant (p > 0.05); postoperatively, the AT II, NE, AD levels of patients in the observation group were lower than those of patients in the control group, with statistically significant differences (p < 0.001), while the differences in ALT, ALB, TBIL levels were not statistically significant (p > 0.05). For malignant cases, the R0 resection rate was similar between groups (92.3% vs. 89.5%, p = 0.724).
Preoperative 3D reconstruction combined with intraoperative augmented reality fluorescence guidance system is potentially beneficial for laparoscopic liver surgery, which can modestly shorten the operation time, reduce intraoperative bleeding, and alleviate postoperative stress reactions.
Numerous tumors occur in the liver, including benign tumors such as cavernous hemangioma and focal nodular hyperplasia; primary malignant tumors such as hepatocellular carcinoma and intrahepatic cholangiocarcinoma; and secondary malignant tumors that metastasize from other organs, such as colorectal cancer, breast cancer, and pancreatic cancer [1]. For these liver tumors, surgical resection is usually the treatment of choice. With the development of minimally invasive techniques, laparoscopic hepatectomy has gradually become the recommended procedure for treating liver tumors due to its reduced trauma, faster recovery, and tumor eradication effect comparable to open surgery [2]. However, as the largest solid organ in the abdominal cavity, the liver possesses complex vascular and biliary systems, which complicate laparoscopic surgical approaches. Additionally, some hepatic tumors are deeply located and cannot be accurately localized laparoscopically, further increasing the surgical difficulty [3]. Currently, there is a lack of noninvasive real-time navigation systems for laparoscopic liver surgery to help surgeons accurately locate tumors and perform precise resections, which would improve tumor resection rates and reduce postoperative complications.
Most previous hepatic resection strategies were formulated based on CT or MRI two-dimensional imaging, which have several limitations: difficulty in accurately determining intrahepatic vascular structural variations, inability to clarify the relationship between tumors and blood vessels from multiple perspectives, and challenges in accurately calculating residual liver volume [4, 5]. In recent years, with the development of three-dimensional visualization technology, surgeons can use 3D reconstruction to observe liver vascular anatomy and variations more clearly before surgery, understand the relationship between tumors and blood vessels, accurately calculate residual liver volume, and simulate hepatic resection plans on computers, thereby making resections more accurate and personalized [6, 7]. Luo X et al. [8] found that compared with traditional CT images, 3D stereoscopic images provide surgeons with more straightforward and clearer visualization, facilitating the development of appropriate surgical plans. Matsumoto K et al. [9] found that in some patients with hepatocellular carcinoma, segment 8 of the liver is close to the diaphragm, which limits space for laparoscopic instrument manipulation. Additionally, the lack of clear surface demarcation on segment 8 and the complexity of portal vein branches within it make precise localization and dissection difficult, resulting in challenges for accurate laparoscopic anatomical resection of segment 8 and increased risk of intraoperative hemorrhage. Laparoscopic augmented reality fluorescence guidance systems can fuse 3D liver models with the real surgical field and, when combined with indocyanine green (ICG) fluorescence, enable effective surgical navigation in various complex laparoscopic hepatectomies, an approach that has been well-received in clinical practice [10, 11].
However, current research on the application of preoperative 3D reconstruction combined with intraoperative augmented reality fluorescence guidance in laparoscopic liver surgery is relatively limited. While several studies have demonstrated the potential benefits of either 3D reconstruction or AR guidance separately, fewer have examined their combined effect across a range of liver pathologies. Building upon previous work in this field, this study aims to evaluate the application of preoperative 3D reconstruction combined with intraoperative augmented reality fluorescence guidance system in laparoscopic liver surgery by establishing a retrospective cohort study. We hypothesize that this approach can improve surgical planning and intraoperative navigation, thereby reducing unnecessary tissue resection, operative trauma, and operative time, speeding postoperative recovery, and ultimately improving surgical success and patient outcomes. The findings could provide data to inform further technological development in liver surgery.
This study was approved by our Institutional Review Board and Ethics Committee. Given that this study was retrospective and only de-identified patient data were used, informed consent was not required as there was no risk or adverse effect on patient care. This waiver is in line with regulatory and ethical guidelines related to retrospective studies.
This retrospective cohort study included 96 patients who underwent laparoscopic liver surgery between March 2023 and December 2024 at our institution. Patient data were obtained from the medical record system. The study utilized a consecutive time period allocation design. From March 2023 to June 2024, patients underwent conventional laparoscopic liver surgery (control group, n = 46), while from July 2024 to December 2024, following the institution's acquisition of the AR fluorescence system, patients underwent surgery with preoperative 3D reconstruction and intraoperative AR guidance (observation group, n = 50). This temporal allocation helped minimize selection bias, as patients were not specifically selected for either technique based on their case complexity or surgeon preference. Importantly, the same team of surgeons performed all operations in both groups. The surgeons had completed a standardized training program on the AR guidance system before its clinical implementation, including at least 10 supervised case simulations.
A power analysis was performed prior to data collection. Assuming a moderate effect size (Cohen's d = 0.6) for the primary outcomes of operative time and blood loss, with α = 0.05 and power = 0.8, a minimum sample size of 45 patients per group was required. Our final sample exceeded this minimum requirement.
Inclusion criteria: (1) all benign and malignant liver lesions such as hepatocellular carcinoma, liver abscess, intrahepatic bile duct stones or hepatic hemangiomas that met the indications for laparoscopic liver surgery; (2) if malignant tumors, no distant metastasis was found in preoperative examination and intraoperative exploration; (3) first-time hepatectomy; (4) age ≥ 18 years; (5) preoperative liver function Child-Pugh grading [12] of grade A; (6) complete clinical data.
Exclusion criteria: (1) conversion to open in laparoscopic surgery; (2) in case of malignant tumors, not primary tumors of the liver; (3) those who could not tolerate surgery or did not need to undergo surgery; (4) those who were allergic to ICG or iodine; and (5) the presence of uncontrollable underlying diseases.
Laparoscopic liver surgery was routinely carried out in the control group as follows: the surgery was carried out in strict accordance with the standard procedure, and the specific details were based on the patient's lesion location, obesity level, abdominal width and other factors. Tracheal intubation general anesthesia was routinely used, head-high-feet-low supine split-leg position was routinely used, CO2 pneumoperitoneum pressure was maintained at 12–14 mmHg, and the five-hole method was routinely used, with the operating holes distributed in a fan-shaped manner around the diseased liver lobes (segments), and the umbilical hole was used as a 3D laparoscopic observation hole. Hepatoportal blocking band was routinely pre-positioned during the operation, and hepatic portal blocking was performed intermittently during liver parenchyma dissection. If the lesion location is deep and the localization is not clear, the localization can be assisted by intraoperative laparoscopic ultrasound system. Different surgical methods were selected according to the actual situation of each patient, including anatomical hepatectomy and local resection, and the specimens were routinely sent for pathological examination after surgery.
The observation group applied preoperative 3D reconstruction combined with intraoperative augmented reality fluorescence guidance system to carry out laparoscopic liver surgery:
For preoperative 3D reconstruction, we used 64-row helical CT (Siemens SOMATOM Force, Siemens Healthineers, Erlangen, Germany) to perform epigastric scanning and three-phase augmentation scanning of the liver to obtain two-dimensional data. Three-dimensional reconstruction was then carried out using a specialized three-dimensional visualization software system for abdominal medical images (IQQA-Liver, EDDA Technology, Princeton, NJ, USA). The 3D reconstruction model was carefully observed from multiple angles to clarify the anatomical relationship of the intrahepatic vascular system and whether there were any variations. Simulated surgery was performed using the 3D visualization system, and the whole liver volume, standard liver volume, tumor volume, pre-resection liver volume, and residual liver volume were calculated. The percentage of residual liver volume (percentage of residual liver volume = residual liver volume/standard liver volume × 100%) was also calculated.
Preoperatively, two liver surgeons with senior titles (each with >15 years of experience in hepatobiliary surgery and >100 laparoscopic liver resections) independently evaluated the case and planned the surgical approach, then collaboratively developed the optimal surgical plan. The planning process typically required 60–90 min per case.
For the ICG fluorescence procedure, patients received ICG (Diagnogreen; Daiichi-Sankyo, Tokyo, Japan) at a dose of 0.5 mg/kg body weight. For tumor identification, ICG was administered intravenously 24 h before surgery to achieve positive staining of tumors through the enhanced permeability and retention effect. For vascular delineation, ICG (0.25 mg/mL) was injected intraoperatively after clamping the relevant portal vein branch to identify hepatic segment boundaries (negative staining). The overall success rate of tumor staining was 92% (46/50 cases), while the success rate for vascular delineation was 97% (48/50 cases). For the six patients in whom ICG staining failed, we immediately resorted to intra–operative ultrasound localisation; this approach was successful and did not lead to conversion or additional morbidity.
The laparoscopic augmented reality surgical navigation system (DaVinci Surgical Navigation System; Software Copyright No.: 2018SR840555; Hunan DiKe Intelligent Technology Co., Ltd., Changsha, China) and ICG fluorescence imaging system (IRIS-L; Zhuhai DiPu Medical Technology Co., Ltd., Zhuhai, China) were used for real-time surgical navigation. Intraoperatively, the three-dimensional model of the liver, the intraoperative laparoscopic scene, and ICG fluorescence images were fused and aligned to achieve an augmented reality display. The surface contour of the liver, gallbladder, and inferior vena cava fossa were used as marker points for the alignment, combined with intraoperative ultrasound guidance.
Calibration of the AR system was performed at the beginning of each case and rechecked every 30 min or if any discrepancy was noted. Tracking errors occurred in 8% (4/50) of cases, primarily due to liver deformation during manipulation. These were corrected through recalibration using anatomical landmarks. Under AR guidance, the surface boundaries of the liver and the projected positions of important veins were labeled, and the operation paths for anatomical hepatic resection were determined. The surgery was then carried out in strict accordance with these planned paths. No case exceeded a 3 mm deviation after the second calibration, demonstrating adequacy of error mitigation.
The same anesthesia and postoperative analgesic protocols were used for both groups to minimize confounding factors in the assessment of stress responses. All patients received a standardized enhanced recovery after surgery (ERAS) protocol.
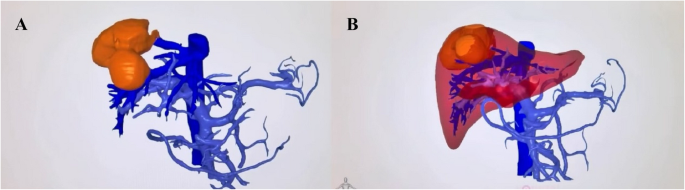
Figure 1 demonstrates the preoperative 3D reconstruction and virtual surgical planning used in this study. This visualization technology enables precise understanding of the tumor-vessel relationships and facilitates planning of optimal resection approaches before surgery.
Preoperative 3D reconstruction and surgical planning. This figure demonstrates the key aspects of preoperative 3D planning: A 3D liver reconstruction model showing liver parenchyma (semi-transparent), vascular structures (color-coded), and tumor location (yellow) (B) Virtual surgical resection planning showing projected resection plane and calculated remnant liver volume
General demographic data of the patients were collected through the medical record system, including age, gender, Body Mass Index (BMI), American Society of Anesthesiologists Physical Status Classification (ASA) [13], hypertension (defined according to the Japanese Society of Hypertension guidelines [14]), diabetes (defined according to the Chinese Expert Consensus on Diabetes Classification [15]), hyperlipidemia (defined according to the Japan Atherosclerosis Society guidelines [16]), and the location of the surgery.
The perioperative indicators of the patients were collected through the medical record system, including the operation time, intraoperative bleeding, time to first flatus (indicating return of bowel function), drainage tube removal time and hospitalization time. Time to first flatus was operationally defined as the interval from tracheal extubation to the first documented passage of gas (flatus) or stool, whichever occurred first.
Before and 1 day after the operation, 3 mL of venous blood was extracted from the two groups in fasting state in the early morning, and the serum was separated by centrifugation at the speed of 3000r/min for 10 min. The level of Alanine Aminotransferase (ALT) was detected by the method of enzyme activity, Albumin (ALB) was measured by immunoturbidimetric method, and Total Bilirubin (TBIL) was measured by spectrophotometric method.
Before and 1 day after the operation, 3 mL of venous blood was extracted from the two groups in the fasting state in the early morning, and the serum was separated by centrifugation at a speed of 3000r/min for 10 min. The levels of angiotensin II (AT II), norepinephrine (NE) and adrenaline (AD) were measured by enzyme-linked immunosorbent assay.
These specific stress biomarkers were selected based on their validated role as indicators of surgical stress response in hepatobiliary surgery [17,18,19]. These catecholamines and AT II are well-established markers of the sympathetic-adrenal-medullary axis activation during surgical stress and have been shown to correlate with the extent of surgical trauma in liver surgery. Blood samples were collected at a standardized time (24 h post-operation) to minimize diurnal variation in these hormones. All patients received standardized anesthesia and post-operative pain management to reduce confounding effects on these measurements.
The complication rate of patients was collected through the medical record system, which mainly contained bile leakage, pleural effusion, fever, and ascites. Complication rate = number of cases with complications/total number of cases × 100%.
Statistical analysis
SPSS25.0 statistical software was used to analyze the data. The count data were expressed as [n(%)], and the χ2 test or Fisher's exact test (for low event counts) was adopted for comparison between groups. The measurement data conforming to normal distribution were expressed as (x̄ ± s), with 95% confidence intervals (CI) reported for key outcomes. The independent sample t-test was adopted for comparison between groups. Due to the multiple comparisons performed, we acknowledge the increased risk of type I error, though no formal correction was applied as this was primarily an exploratory study. Differences were considered statistically significant at p < 0.05.
To control for potential confounding factors, we performed stratified analyses by tumor type, size, and location, though the sample size limited the power of these subgroup analyses. We also conducted adjusted analyses controlling for key baseline variables using multivariable linear regression for continuous outcomes.
Comparison of the two groups in terms of age, gender, BMI, ASA classification, hypertension, diabetes mellitus, hyperlipidemia, and surgical location showed no statistically significant differences (p > 0.05), see Table 1.Tumor‑related baseline characteristics of the two groups are summarized in Table 2.
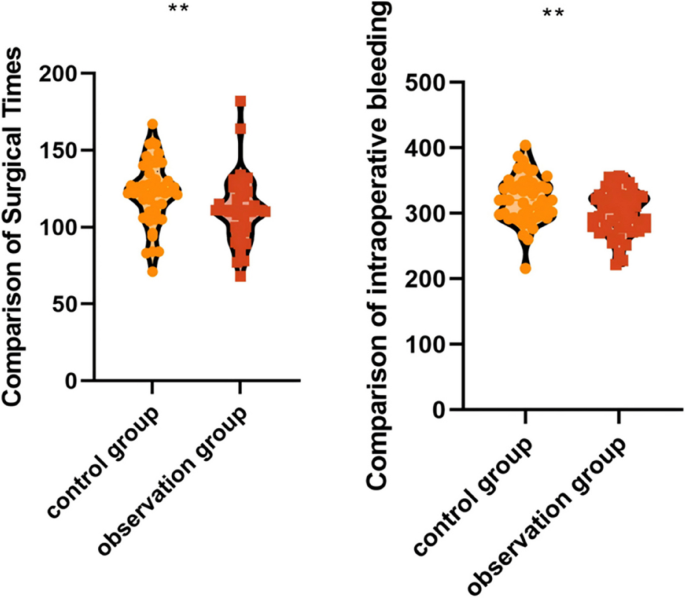
Surgical time is used to assess surgical efficiency, intraoperative bleeding is used to assess surgical trauma, time to first flatus, drainage tube removal time and hospitalization time are used to assess postoperative recovery; short surgical time represents high surgical efficiency; low intraoperative bleeding represents low surgical trauma; short time to first flatus, drainage tube removal time and hospitalization time represent quicker postoperative recovery.
Comparison of time to first flatus (2.10 ± 0.45 vs. 2.02 ± 0.56 days), drain removal time (7.32 ± 1.52 vs. 7.18 ± 1.64 days), and hospitalization time (9.40 ± 1.85 vs. 9.24 ± 1.96 days) between the two groups showed no statistically significant differences (p > 0.05), see Table 3.
The operation time of patients in the observation group (110.75 ± 20.56 vs. 122.35 ± 20.48 min, 95% CI of difference: 2.52–20.68 min, p = 0.013) was shorter than that of patients in the control group, and intraoperative bleeding (300.80 ± 32.70 vs. 320.76 ± 35.84 mL, 95% CI of difference: 7.62–32.30 mL, p = 0.002) was less than that of patients in the control group. While statistically significant, the absolute differences represent a relatively modest reduction of approximately 9.5% in operative time and 6.2% in blood loss. This indicates that the application of preoperative 3D reconstruction combined with intraoperative augmented reality guidance system to carry out laparoscopic liver surgery can modestly shorten the operating time of patients and reduce intraoperative bleeding (Fig. 2).
ALT, ALB, TBIL are used to assess the state of liver function, and the elevation of ALT and TBIL and the decrease of ALB suggest that there may be hepatic function injury. Comparison of preoperative ALT (245.96 ± 28.74 vs. 246.30 ± 30.62 U/L), ALB (42.84 ± 5.96 vs. 42.28 ± 5.82 g/L), and TBIL (13.29 ± 2.74 vs. 13.45 ± 2.80 μmol/L) levels between the two groups of patients showed no statistically significant differences (p > 0.05).
ALT (278.66 ± 32.95 vs. 280.75 ± 33.14 U/L), ALB (38.21 ± 3.54 vs. 37.66 ± 3.75 g/L), and TBIL (18.44 ± 3.30 vs. 18.60 ± 3.42 μmol/L) levels were elevated in the postoperative period of patients in the two groups, but the differences between groups were not statistically significant (p > 0.05), suggesting that the application of preoperative 3D reconstruction combined with intraoperative augmented reality fluorescence guidance system to carry out laparoscopic liver surgery did not affect the liver function of the patients differently than conventional surgery (Table 4).
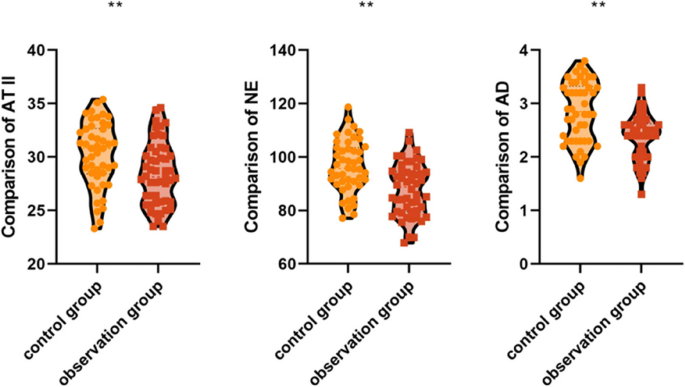
AT II, NE, and AD levels reflect the physiological stress response; higher levels indicate a more severe stress reaction. Comparison of preoperative AT II (25.36 ± 3.44 vs. 25.57 ± 3.56 pg/mL), NE (61.74 ± 8.22 vs. 62.34 ± 8.98 ng/mL), and AD (1.98 ± 0.45 vs. 1.95 ± 0.40 pg/mL) in the patients of the two groups showed no statistically significant differences (p > 0.05) (Table 5).
The postoperative AT II (28.46 ± 2.95 vs. 30.24 ± 3.10 pg/mL, p < 0.001), NE (87.90 ± 9.80 vs. 96.55 ± 9.72 ng/mL, p < 0.001), and AD (2.40 ± 0.42 vs. 2.85 ± 0.56 pg/mL, p < 0.001) levels of the patients in the observation group were lower than those of the patients in the control group, and the differences were statistically significant. This indicates that the application of the preoperative 3D reconstruction combined with the intraoperative augmented reality fluorescence guidance system for laparoscopic liver surgery may reduce the patients'postoperative stress response (Fig. 3).
Complications are used to assess the surgical outcome, and the lower the rate of postoperative complications, the more favorable the surgical outcome. Comparison of the complication rates of bile leakage, pleural effusion, fever and ascites between the two groups (8.70% vs. 4.00%) showed no statistically significant difference (p > 0.05), which suggests that the application of preoperative 3D reconstruction combined with intraoperative augmented reality fluorescence guiding system to perform laparoscopic liver surgery did not increase the risk of complications (Table 6).
For patients with malignant tumors (hepatocellular carcinoma and intrahepatic cholangiocarcinoma), we evaluated the resection margin status. In the control group, 17 of 19 malignant cases (89.5%) achieved R0 resection (microscopically negative margins), while in the observation group, 24 of 26 malignant cases (92.3%) achieved R0 resection. This difference was not statistically significant (p = 0.724). The remaining cases in both groups had R1 resections (microscopically positive margins), but none had R2 resections (macroscopically positive margins).
Laparoscopic liver surgery offers advantages like reduced trauma and faster recovery; however, it has limitations, including the lack of tactile feedback, a restricted visual field, and challenges in formulating an optimal surgical plan preoperatively [20,21,22]. In recent years, with the wide application of three-dimensional reconstruction, image navigation, indocyanine green fluorescence guidance, virtual reality, and augmented reality technologies, surgeons can better visualize the complex liver anatomy, precisely locate tumors intraoperatively, design surgical plans, monitor and timely correct hepatic parenchyma dissection planes, and carry out precise hepatic resections. These technological advances enhance surgical safety and compensate for the limitations in tactile sensation and visual field, effectively extending the capabilities of human vision and tactile perception [23,24,25]. In the present study, we found that the application of preoperative 3D reconstruction combined with intraoperative augmented reality fluorescence guidance system in patients undergoing laparoscopic liver surgery not only modestly reduces operative time and intraoperative bleeding but also reduces postoperative stress reactions, suggesting the potential benefit of this approach for laparoscopic liver surgery.
Our study found that the operation time in the observation group was shorter than in the control group, and intraoperative bleeding was less, suggesting that preoperative 3D reconstruction combined with intraoperative augmented reality fluorescence guidance system can modestly improve surgical efficiency and reduce blood loss. The study by Xu C et al. [26] found that preoperative evaluation of the liver typically uses two-dimensional imaging methods such as CT and MRI, which cannot accurately delineate complex three-dimensional anatomical relationships. Preoperative 3D reconstruction can address these limitations and effectively reduce surgical bleeding and operation time, which is consistent with the findings of the present study. Huettl F et al. [27] found that fluorescence imaging technology can clearly demonstrate the relationship between hepatocellular carcinoma and the hepatic vascular structure and accurately predict the extent of hepatic resection for hepatocellular carcinoma, which improves the safety of laparoscopic hepatectomy, further supporting our findings.
The three-dimensional liver model obtained through preoperative 3D reconstruction can be rotated in multiple directions, and the transparency of corresponding organs can be adjusted to observe from any angle, providing surgeons with a more intuitive understanding of the relationships between tumors and intrahepatic vascular structures. This improves the assessment of tumor resectability and resection range and facilitates management of key surgical steps. Additionally, simulated surgery can be performed using the 3D visualization system, increasing the surgeon's preparedness, familiarizing them with the surgical process, shortening the surgical time, and avoiding damage to important blood vessels, thereby reducing intraoperative bleeding [28, 29]. The intraoperative augmented reality fluorescence guidance system further enhances the accuracy of hepatic resection, particularly during deep liver parenchyma dissection, by improving tumor localization and determining the optimal dissection plane, which may reduce unnecessary surgical bleeding and improve surgical efficiency [30, 31]. Therefore, preoperative 3D reconstruction combined with intraoperative augmented reality fluorescence guidance system may shorten operating time and reduce intraoperative bleeding by enabling surgeons to familiarize themselves with the surgical procedure in advance and accurately localize and resect liver tissue.
It should be noted, however, that while the differences in operative time and blood loss were statistically significant, the absolute reductions were modest (approximately 12 min and 20 mL, respectively). These differences, representing a 9.5% reduction in operative time and a 6.2% reduction in blood loss, may have limited clinical significance in routine cases but could potentially be more meaningful in complex resections or high-risk patients. The clinical relevance of these findings should be interpreted cautiously, especially considering the resource investment required for implementing this technology.
Our current study showed that the postoperative levels of AT II, NE, and AD in the observation group were lower than those in the control group, suggesting that preoperative 3D reconstruction combined with intraoperative augmented reality fluorescence guidance system for laparoscopic liver surgery may reduce postoperative stress reactions. The study by Nozaki A et al. [17] found that the application of 3D reconstruction technology and augmented reality navigation systems can clearly demonstrate important anatomical structures such as blood vessels, allowing surgeons to perform operations more accurately and reduce the stress response caused by intraoperative manipulations, which is consistent with our findings.
By combining preoperative 3D reconstruction with intraoperative augmented reality fluorescence guidance, the preoperative simulation of the surgical path reduces tissue manipulation and mechanical damage caused by repeated intraoperative exploration, thus reducing the secretion of stress markers. Precise vascular localization ensures accurate liver resection and reduces persistent activation of stress reactions triggered by significant blood loss. Additionally, precise resection minimizes ischemic injury to healthy liver tissues, maintains mitochondrial functional integrity, reduces the generation of free radicals (e.g., superoxide anion, lipid peroxides), and attenuates oxidative stress [18, 19]. Therefore, combining these technologies may inhibit the systemic stress response triggered by surgical trauma through precise anatomical localization, reduction of tissue damage, and protection of liver function.
However, there was no statistically significant difference between the two groups in terms of time to first flatus, drain removal time, hospitalization time, postoperative liver function, or complication rate. The lack of significant differences in these outcomes may be related to the small sample size, single-center design, or the fact that both techniques were performed by experienced surgeons with standardized perioperative care protocols. These findings suggest that while the combined AR and 3D reconstruction approach offers some advantages in terms of operative parameters and stress response, it may not substantially alter the overall recovery trajectory or complication profile in the immediate postoperative period.
Our study has several limitations that should be acknowledged:
First, the retrospective, non-randomized design introduces potential selection bias despite our consecutive time period allocation approach. Although baseline characteristics appeared similar between groups, unmeasured confounders may still have influenced the results. The temporal allocation of patients to either conventional or AR-guided surgery means that improvements might be partially attributable to increasing surgical team experience over time rather than solely to the technology itself. Additionally, the learning curve associated with adopting the AR system may have influenced the outcomes; the benefits observed might underestimate the potential advantages once surgeons become more proficient with the technology.
Second, our study was conducted at a high-volume center with specialized equipment and experienced hepatobiliary surgeons. The outcomes might differ in less specialized settings with limited resources or technical expertise. The additional resources, time, and expertise required for 3D reconstruction and AR setup (approximately 60–90 min of preoperative planning per case) were not factored into our analysis. This investment in preoperative planning time may offset some of the intraoperative time savings, and the cost-effectiveness of this approach remains to be evaluated. In lower‑volume hospitals, adoption will require remote 3‑D reconstruction support and an estimated learning curve of approximately 15–20 cases.
Third, our patient cohort included a mix of different types of liver resections and pathologies. The benefits of AR + 3D technology likely vary based on the complexity and type of procedure, with potentially greater advantages in complex anatomical locations like segment 8 or near major vascular structures. Our sample size did not allow for meaningful subgroup analyses to determine which specific surgical scenarios would benefit most from this technology.
Fourth, our study focused only on immediate perioperative outcomes with limited follow-up. For malignant cases, while we reported R0 resection rates, important long-term oncological outcomes such as disease-free survival, overall survival, and recurrence patterns were not assessed. Similarly, functional liver outcomes beyond the immediate postoperative period, quality of life measures, and 30-day readmission rates were not evaluated. These longer-term outcomes would provide a more comprehensive understanding of the technology's impact.
Finally, it is important to note that the field of AR in surgery is rapidly evolving, and there is a possibility of publication bias, with positive results more likely to be published than negative or equivocal findings. Our modest improvements align with the generally positive literature, but confirmatory studies, particularly randomized controlled trials in diverse settings, are needed before widespread adoption can be recommended. Because no multiplicity adjustment was performed, these findings should be regarded as exploratory; future confirmation with FDR or Bonferroni correction is warranted.
Therefore, prospective, large-sample, multicenter, randomized controlled trials should be conducted in the future to increase follow-up time for evaluating long-term prognosis, collect more comprehensive data on specific patient subgroups, and assess cost-effectiveness. Such studies would provide more objective, comprehensive, and accurate results to better guide clinical practice. P-values should be interpreted in conjunction with effect sizes and cost-benefit considerations.
In summary, our study demonstrates for the a real-time, in-vivo augmented-reality workflow that dynamically couples pre‑operative 3‑D models with intra-operative anatomical updates. This dynamic guidance extends beyond previously reported static navigation systems by providing continuous visual support during parenchymal transection.
In this retrospective cohort study, preoperative 3D reconstruction combined with intraoperative augmented reality fluorescence guidance system demonstrated the potential to modestly shorten operative time, reduce intraoperative bleeding, and alleviate postoperative stress in patients undergoing laparoscopic liver surgery. While the absolute improvements in surgical parameters were statistically significant, their clinical significance requires further evaluation, particularly in relation to the additional resources required for implementation. Future research should focus on identifying the specific surgical scenarios that would benefit most from this technology and evaluating long-term outcomes and cost-effectiveness.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
PWW, SFW, PL declare that they have no conflict of interest.
No funding was received.
Given that this study was retrospective and only de-identified patient data were used, informed consent was not required as there was no risk or adverse effect on patient care. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University.
Not Applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Wang, P., Wang, S. & Luo, P. Evaluation of the effectiveness of preoperative 3D reconstruction combined with intraoperative augmented reality fluorescence guidance system in laparoscopic liver surgery: a retrospective cohort study. BMC Surg 25, 288 (2025). https://doi.org/10.1186/s12893-025-02989-4