BMC Pediatrics volume 25, Article number: 370 (2025) Cite this article
Lower urinary tract symptoms (LUTS) are common in pediatric patients and significantly affect children’s well-being and family-related quality of life. However, no validated Chinese assessment tool for LUTS exists. This study aimed to develop and validate a Chinese version of the International Consultation on Incontinence Questionnaire-Pediatric Lower Urinary Tract Symptoms (ICIQ-CLUTS).
The ICIQ-CLUTS was translated following the Beaton cross-cultural adaptation guidelines. A total of 192 children with and without LUTS and their parents were enrolled between June and October 2024. Psychometric evaluation was performed using multiple approaches. Reliability was assessed using Cronbach’s alpha coefficient for internal consistency. Validity was assessed by expert content review and exploratory factor analysis. Clinical diagnoses were made using standardized assessment protocols including medical history, physical examination, and laboratory tests (urinalysis and uroflowmetry) based on the International Children’s Continence Society (ICCS) criteria. Using these clinical diagnoses as the reference standard, the diagnostic accuracy was evaluated using receiver operating characteristic (ROC) curve analysis.
The study included 192 participants (70.8% male) divided into two age groups: 5–9 years (n = 139) and 10–14 years (n = 53). The Chinese ICIQ-CLUTS showed acceptable internal consistency (Cronbach’s alpha = 0.718 for children’s version; 0.706 for parents’ version) and distinct factor structure. The diagnoses encompassed various LUTS manifestations including monosymptomatic nocturnal enuresis (MNE), non-monosymptomatic nocturnal enuresis (NMNE), and overactive bladder (OAB). Both versions exhibited high diagnostic accuracy (AUC = 0.948 for children’s version; 0.933 for parents’ version), with superior performance observed in the 10-14-year age group (AUC = 0.963 and 0.960, respectively). The optimal cut-off points were 13.5 for both versions, with sensitivity of 0.90 and specificity of 0.86 for the children’s version, and sensitivity of 0.89 and specificity of 0.85 for the parents’ version.
The Chinese version of ICIQ-CLUTS shows good psychometric properties and diagnostic accuracy, making it a useful screening tool for pediatric LUTS in Chinese-speaking populations.
LUTS encompass a spectrum of disorders that manifest at any stage of urination due to anatomical and/or functional changes in the urinary system [1]. It represents a common reason for pediatric urology consultations, accounting for up to 40% of outpatient consultations [2]. The clinical manifestations of LUTS include increased voiding frequency, urgency, daytime urinary incontinence, and nocturnal enuresis. Epidemiological studies have reported varying prevalence rates for individual LUTS manifestations, ranging from 3.1 to 75% depending on the specific symptom and study population [3,4,5]. LUTS significantly impact both physical and psychological well-being of children and adolescents [6, 7].
The International Children’s Continence Society (ICCS) standardized the terminology for pediatric lower urinary tract dysfunction in 2016, advocating for multimodal assessment approaches using minimally invasive diagnostic procedures [8, 9]. The European Association of Urology-European Society for Pediatric Urology (EAU-ESPU) also recommends the use of standardized, validated questionnaires for symptom assessment [10, 11]. These standardized assessment tools have become increasingly important for initial diagnosis, symptom quantification, and treatment monitoring [12].
Several validated assessment tools have been developed and widely used in clinical practice, including the Dysfunctional Voiding Symptom Survey (DVSS), the Dysfunctional Voiding and Incontinence Scoring System (DVISS), and the Incontinence Symptom Index-Pediatric (ISI-P) [13,14,15]. While these instruments have contributed significantly to LUTS evaluation, certain limitations regarding age-specific applicability and population-specific adaptations have been identified [16].
The International Consultation on Incontinence Questionnaire for Children with Lower Urinary Tract Symptoms (ICIQ-CLUTS), developed by ICCS in 2010 [17], has been widely recognized as a comprehensive assessment tool for children aged 5–18 years. This non-invasive self-reporting tool allows both parent and child participation, and stands out for its alignment with ICCS guidelines, broad coverage of LUTS symptoms, and its design to minimize literacy and judgment reliability issues [2]. The ICIQ-CLUTS is currently available in English, Italian, and German [8], clinical studies have demonstrated its good correlation with clinical impressions and robust reliability in pediatric LUTS assessment [11]. However, despite China’s large pediatric population and the increasing recognition of LUTS in clinical practice, a validated Chinese version of ICIQ-CLUTS is not yet available, limiting its application in Chinese-speaking regions and hindering standardized assessment in both clinical practice and research settings.
This study aims to develop and validate a Chinese version of the ICIQ-CLUTS through translation, cross-cultural adaptation, and comprehensive psychometric evaluation.
This cross-cultural adaptation and validation study was conducted at Shenzhen Children’s Hospital, a tertiary pediatric center in Southern China. The study followed both the COSMIN (Consensus-based Standards for the selection of health Measurement Instruments) [18] and the principles of the Declaration of Helsinki. The protocol was approved by the Ethics Committee of Shenzhen Children’s Hospital, Guangdong, China (approval number: 202317002, date: January 5, 2024). Written permission for cross-cultural adaptation was obtained from the original authors prior to the study.
Using convenience sampling, children aged between 5 and 18 years and their parents were recruited from the Pediatric Urology Outpatient Clinic and Pediatric Nephrology Specialty Outpatient Clinic of Shenzhen Children’s Hospital (SCH), a tertiary pediatric hospital in Guangdong Province, China. These Outpatient clinics specialize in the diagnosis and management of pediatric LUTS. Sample size estimation, based on at least 10 respondents per item for factor analysis [19], required a minimum of 150 child-parent pairs for the 12-item ICIQ-CLUTS.
The inclusion criteria for children were: (a) being aged between 5 and 18 years, (b) having or not having LUTS. Children were excluded if they were: (a) postoperative urological patients, (b) patients with uncontrolled diabetes, or (c) patients with anatomical or neurological disorders. Asymptomatic controls (children without LUTS) were recruited from the same specialty Outpatient clinics for conditions unrelated to voiding dysfunction. Eligibility required: (1) physician-confirmed absence of LUTS through clinical assessment, (2) parental report of no urinary symptoms in the past six months, and (3) no documented voiding disorders in electronic health records.
As the original ICIQ-CLUTS consists of both child and parent versions, participation of parents was essential for questionnaire validation. For parents, the inclusion criteria were: (a) being the legal guardian living with the child, (b) being able to understand and complete the questionnaire independently, and (c) being willing to participate in the study. Parents who met the inclusion criteria were excluded if they could not provide accurate information about their child’s daily symptoms.
Original questionnaire: The ICIQ-CLUTS consists of 12 questions: items 1 and 2 (age and gender), item 3 (urinary tract infection), and items 4 to 12, each scored from 0 to 4. The total score ranges from 9 to 36, with answers based on experiences over the past four weeks. Higher scores indicate more severe LUTS in children. The ICIQ-CLUTS prioritizes detection of any clinically significant LUTS (symptomatic vs. asymptomatic distinction) rather than subtype differentiation, aligning with its primary care screening purpose.
Medical diagnosis: All children underwent standardized clinical assessment by pediatricians, including medical history, physical examination, and relevant laboratory tests (urinalysis, uroflowmetry, etc.). The diagnoses were made based on the standardization document of the International Children’s Continence Society (ICCS) [8]. Common diagnoses included monosymptomatic nocturnal enuresis (MNE), non-monosymptomatic nocturnal enuresis (NMNE), and overactive bladder (OAB).
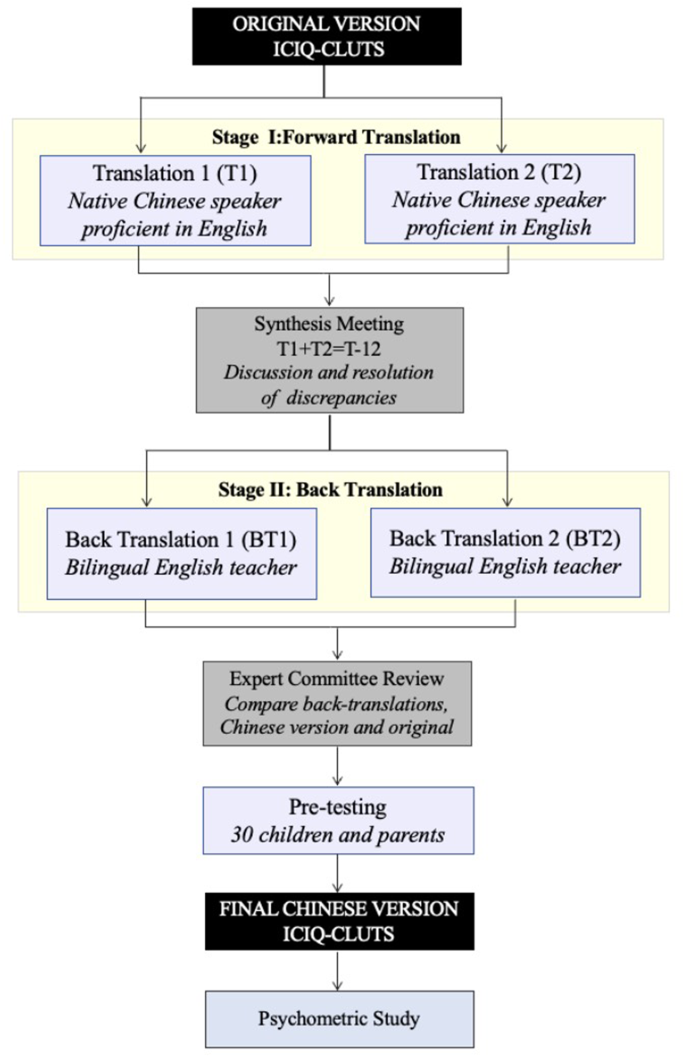
The translation and cultural adaptation process followed Beaton et al.‘s [20] standardized guidelines (Fig. 1). The process consisted of five Phases:
Phase I (Forward Translation): Two independent forward translations (T1 and T2) were performed by native Chinese speakers with professional English proficiency.
Phase II (Synthesis): The two translations (T1 and T2) were compared and synthesized through discussion and consensus between the translators, producing a unified Chinese version (T-12).
Phase III (Back Translation): Two native English speakers with advanced Chinese proficiency, blinded to the original questionnaire, independently translated T-12 back into English (BT1 and BT2).
Phase IV (Expert Committee Review): A multidisciplinary committee was established, consisting of pediatric urology nurse specialists, all translators involved in the previous stages, methodologists, and linguistic experts. The committee performed cross-cultural adaptation by evaluating semantic, idiomatic, experiential, and conceptual equivalence between the original, Chinese, and back-translated versions. All discrepancies were documented and resolved through structured consensus meetings. The original authors reviewed both the synthesized Chinese version (T-12) and its back-translations (BT1 and BT2).
Phase V (Pre-testing): Pre-testing was conducted with 30 children and their parents, who were invited to complete the questionnaire and provide feedback to identify potential ambiguities. Following the feedback analysis, the final simplified Chinese version was established for psychometric evaluation.
Data collection was conducted using paper-based questionnaires from June to October 2024, with participant eligibility confirmed through direct interaction and medical record review. Prior to data collection, written informed consent was obtained from all parents. For children, written informed consent was obtained from children older than 8 years, while for children aged 8 years or younger, verbal assent was obtained from the children themselves with written informed consent provided by their parents. During data collection, children and parents completed the questionnaires independently. A standardized protocol was implemented for young participants to ensure the quality of responses. The support framework encompassed providing sufficient time for recall, with appropriate breaks offered when children exhibited decreased attention. Moreover, when children encountered difficulties understanding specific items, age-appropriate examples, illustrations, and analogies were provided without suggesting answers. Trained research assistants were present throughout the process to ensure that both children and parents could participate simultaneously and complete the questionnaires independently. This approach was implemented to overcome potential literacy barriers while maintaining the standardization of the assessment process. Completed questionnaires were reviewed for completeness, typically taking about 5 to 10 min per participant.
Statistical analyses were performed using SPSS® version 27.0 and Microsoft® Excel®. Statistical significance was defined at p < 0.05. Descriptive statistics were used to summarize participant characteristics, with means and standard deviations for continuous variables and frequencies and percentages for categorical variables. The Kolmogorov-Smirnov test was used to assess the normality of data distribution.
Reliability analysis included internal consistency assessed by Cronbach’s alpha (α > 0.70) and parent-child agreement evaluated using Spearman’s rank correlation coefficient (SRCC) for both total scores and individual items [21,22,23]. Content validity was evaluated by an expert panel using a 5-point Likert scale, with item-level content validity index I-CVI ≥ 0.78 and scale-level content validity index S-CVI ≥ 0.90 indicating good content validity [24]. Structural validity was examined through exploratory factor analysis (EFA) using principal axis factoring with direct oblimin rotation. Factors were retained based on eigenvalues > 1.0 and explained variance > 10%. The Kaiser-Meyer-Olkin (KMO) measure (acceptable range: 0.7-1.0) and Bartlett’s test of sphericity (p<0.001) were used to verify sampling adequacy [25]. Using the medical diagnosis of LUTS as a reference for positive cases, through receiver operating characteristic (ROC) curves and area under the curve (AUC) values to evaluate the questionnaire’s ability to distinguish symptomatic from asymptomatic children. Additionally, sensitivity, specificity, and likelihood ratios were calculated from the ROC analysis [26].
A total of 192 participants were enrolled, divided into two age groups: 139 children aged 5–9 years, and 53 children aged 10–14 years. The median age was 7 years in the younger group and 10 years in the older group. The overall sample included 70.8% males and 29.2% females. All participants completed the questionnaires without missing data. Clinical assessments identified 93 children with LUTS, with NMNE and OAB being the most common diagnosis. Questionnaire scores varied slightly between age groups and respondent types, with detailed statistics provided in Table 1.
The ICIQ-CLUTS showed acceptable internal consistency for both child (α = 0.718) and parent versions (α = 0.706). The reliability remained stable across age groups, with slightly higher coefficients in the older group (10–14 years) (Table 2).
SRCC analysis revealed significant associations between children’s and parents’ responses to questionnaire items. Table 3 presents the correlation coefficients across different age groups, ranging from 0.661 to 0.954, with Item 4 demonstrating the strongest parent-child agreement (ρ = 0.954).
Content validity
Content validity indices for the Chinese version of the ICIQ-CLUTS demonstrated high values, with I-CVI ranging from 0.85 to 1.00 for children and S-CVI at 0.99, and for parents, both I-CVI and S-CVI achieved a perfect score of 1.00.
Factor analysis
Exploratory factor analysis (EFA) was conducted using principal axis factoring with direct oblimin rotation. Sampling adequacy was confirmed by KMO values of 0.750 for the child version and 0.731 for the parent version, with Bartlett’s tests (p < 0.001) supporting the appropriateness of factor analysis.
The child version revealed a three-factor structure, explaining 63.05% of the total variance, with items clustering into distinct factors. In contrast, the parent version exhibited a four-factor structure, accounting for 74.34% of the variance, with some items loading independently. Detailed factor loadings and variance explained are presented in Table 4.
ROC curve analysis demonstrated high diagnostic accuracy for both versions of the Chinese ICIQ-CLUTS. The child version achieved an AUC of 0.948 (95% CI: 0.917–0.978) and the parent version an AUC of 0.933 (95% CI: 0.899–0.966), with an identical optimal cut-off point of 13.5 for both versions. Sensitivities and specificities were 0.90 and 0.86 for the child version, and 0.89 and 0.85 for the parent version, respectively. Subgroup analysis showed slightly higher AUC values in the 10-14-year age group compared with the 5-9-year group for both versions. Detailed diagnostic performance metrics, including likelihood ratios and Youden index, are provided in Table 5.
The LUTS group demonstrated significantly elevated scores compared to non-LUTS controls in both child (r = 0.39) and parent reports (r = 0.36), with detailed discrimination parameters in Supplementary Table S2.
Diagnostic subgroup analysis revealed the MNE group scored markedly lower than NMNE/OAB subgroups (p < 0.001), while NMNE and OAB showed comparable severity levels (p > 0.78). Parent-child concordance remained strong across all diagnostic categories (ICC = 0.85–0.92), consistent with our primary reliability findings (Supplementary Table S1).
This study completed the cross-cultural adaptation and validation of the ICIQ-CLUTS in China. The results demonstrate that the Chinese version of ICIQ-CLUTS is a valid and reliable instrument for assessing lower urinary tract symptoms in children aged 5–14, maintaining excellent psychometric properties comparable to the original version while exhibiting unique cultural characteristics.
Regarding reliability, both versions showed acceptable internal consistency. Both the children’s version (Cronbach’s α = 0.718) and parent’s version (α = 0.706) exceeded the acceptable threshold of 0.7, comparable to the original validation study [17] (children’s α = 0.719, parent’s α = 0.690) and Turkish version [27] (children’s α = 0.709, parent’s α = 0.710). This cross-cultural consistency confirms the robustness of ICIQ-CLUTS as an assessment tool.
Factor analysis revealed different structural patterns between versions. The child version showed a three-factor structure aligned with the original version, suggesting cross-cultural consistency. The parent version yielded a four-factor structure, explaining 74.34% of the total variance. The additional factor (bowel-related) in the parent version may reflect different assessment perspectives: children typically report immediate physical sensations (urgency and frequency), while parents observe both urination and defecation patterns over time. These complementary perspectives could enhance the comprehensive assessment of lower urinary tract symptoms.
Diagnostic accuracy analysis revealed high AUC values (children’s version = 0.948, parent’s version = 0.933), comparable to the Spanish version [28] (AUC > 0.95) and higher than the original study [17] (children’s AUC = 0.890, parent’s AUC = 0.900). The optimal cut-off values in our study were lower than those reported in the Spanish [28] and Turkish versions [27], but closer to the original version, potentially reflecting cultural differences in symptom reporting. The 10–14 age group showed higher diagnostic accuracy (AUC > 0.96) and better child-parent agreement, consistent with the Turkish study findings. Additionally, younger children demonstrated satisfactory questionnaire completion when provided with structured guidance, extending the tool’s applicability beyond previous studies.
The questionnaire demonstrated moderate effect sizes (r = 0.36–0.39) in distinguishing LUTS/non-LUTS groups, fulfilling its core function as a sensitive screening tool for initial clinical triage. This stepped design prioritizes detecting significant symptoms over subtype classification, reserving precise differentiation for subsequent specialist assessment.
Exploratory subgroup analysis revealed two contextual findings: (1) MNE cases showed attenuated symptom profiles consistent with their clinical definition, though this observation requires confirmation in larger samples; (2) The NMNE-OAB symptom overlap mirrors current continuum models of bladder dysfunction [29], with 63% of our cohort exhibiting combined day-night symptoms - a pattern aligning with European epidemiological data [30]. These secondary results should be interpreted as hypothesis-generating given the study’s validation focus.
Analysis of child-parent consistency showed correlation coefficients ranging from 0.661 to 0.954, compared to the original study’s ICC of 0.848. This consistency might reflect both the standardized testing environment and the close family interactions common in Chinese households. The findings suggest two practical applications: the reliability of both child and parent reports provides flexibility in symptom assessment, while the effectiveness of structured support during questionnaire completion offers a framework for clinical implementation.
During the questionnaire administration to 192 participants, we observed that while children could accurately state their current age, many had difficulty providing their exact date of birth. Although this limitation did not compromise the questionnaire’s validity and reliability due to research assistants’ timely data verification, this observation suggests that future pediatric assessment tools should consider simplifying demographic information collection, particularly for chronological data that children may find challenging to recall.
Several limitations warrant attention in this study. Resource and time constraints prevented the completion of test-retest reliability assessment. While structured support enhanced data collection, these optimized testing conditions may not represent routine clinical practice. The study’s age range (5–14 years), restricted by Chinese pediatric department policies, fell short of the intended 5–18 years target population, limiting result generalizability to adolescents. Furthermore, the significantly lower scores in the MNE subgroup (n = 4) should be interpreted with caution due to insufficient statistical power, necessitating validation in larger cohorts. The sample’s uneven distribution in gender and age groups also affects result representativeness. Future research should focus on validating the questionnaire’s test-retest reliability, expanding validation to diverse clinical settings with larger and demographically balanced samples, and conducting comparative studies to better understand the influence of cultural factors on questionnaire performance.
In conclusion, following standardized translation procedures and comprehensive validation, the Chinese version of the ICIQ-CLUTS has demonstrated acceptable structural validity, as well as satisfactory internal consistency in Chinese children aged 5–14 years, supporting both clinical screening and research applications in the Chinese healthcare context.
The ICIQ-CLUTS questionnaire (Supplementary File 1) and aggregated dataset (Supplementary File 2) are publicly available. However, data containing sensitive variables are not publicly available due to legal/ethical reasons. Researchers with valid ethical approval may request access to these data by contacting the corresponding author at [email protected].
- ICIQ-CLUTS:
-
International Consultation on Incontinence Questionnaire-Children’s Lower Urinary Tract Symptoms
- LUTS:
-
Lower Urinary Tract Symptoms
- ICCS:
-
International Children’s Continence Society
- DVSS:
-
Dysfunctional Voiding Scoring System
- DIVSS:
-
Dysfunctional Voiding and Incontinence Scoring System
- ISI-P:
-
Incontinence Symptom Index-Pediatric
- ROC/AUC:
-
Receiver Operating Characteristic/Area Under the Curve
- OAB:
-
Overactive Bladder
- MNE:
-
Monosymptomatic Nocturnal Enuresis
- NMNE:
-
Non-Monosymptomatic Nocturnal Enuresis
The authors thank Shenzhen Children’s Hospital for their institutional support. We are grateful to all team members and nursing staff for their valuable contributions to data collection and clinical coordination. In preparing this manuscript, we utilized Claude 3.5 Sonnet to enhance language clarity and readability. All content has been thoroughly reviewed and edited by the authors, who assume full responsibility for the final manuscript.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Shenzhen Children’s Hospital, Guangdong, China (Approval No. 202317002; Date: January 5, 2024). Written informed consent was obtained from all participating children’s parents/legal guardians prior to their inclusion in the study.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Tan, Y., Xu, Y., Liang, D. et al. Cross-cultural adaptation and psychometric properties of the International Consultation on Incontinence Questionnaire Pediatric Lower Urinary Tract Symptoms (ICIQ-CLUTS): a Chinese validation study. BMC Pediatr 25, 370 (2025). https://doi.org/10.1186/s12887-025-05706-5