BMC Pulmonary Medicine volume 25, Article number: 201 (2025) Cite this article
Early in the pandemic, corticosteroids became standard treatment for patients with critical COVID-19 infections. This study aimed to investigate the possible long-term pulmonary consequences after corticosteroid treatment in patients with critical COVID-19 requiring ventilatory support.
This observational single-center cohort study included patients treated for critical COVID-19 requiring ventilatory support between March 1, 2020, and August 1, 2021, with a 6-month follow-up after discharge from the intensive care unit. Corticosteroid treatment was defined according to the RECOVERY trial (6 mg dexamethasone daily or equivalent dose of another corticosteroid, initiated within eight days of hospital admittance and continued for at least one day) Pulmonary function was assessed by diffusion capacity for carbon monoxide. Health-related quality of life was measured with the questionnaire RAND-36. General linear regression was used to present mean score differences with 95% confidence intervals.
Among the 456 (69%) critically ill COVID-19 patients who survived at least 90 days after ICU discharge, 286 (63%) attended the follow-up six months later. The groups were balanced regarding invasive ventilation; 47% received invasive ventilation in both groups. Corticosteroid treatment was associated with a lower diffusion capacity for carbon monoxide (MSD − 8.3, 95% CI: -14.2 to -2.4) 6 months after ICU discharge (change > 10% were regarded as clinically significant). There were no differences in health-related quality of life between the groups.
Corticosteroids might negatively impact pulmonary function after critical COVID-19. The decrease did not seem to influence health-related quality of life. Future studies are needed to confirm the results.
The disease spectrum of SARS-CoV-2 infection ranges from asymptomatic disease to critical illness with acute respiratory failure requiring invasive respiratory support [1]. Critical COVID-19 is associated with a high degree of inflammation and immunomodulators have been added to the regimen. Following the press release of the RECOVERY trial in June 2020, which demonstrated reduced mortality with corticosteroid treatment, corticosteroids became the cornerstone of the anti-inflammatory regimen for critically ill patients requiring respiratory support [2,3,4].
Long-lasting functional impairment has been reported among intensive care unit (ICU) survivors in the first year following hospital discharge [5,6,7,8]. Survivors of severe COVID-19 show a higher proportion of impairment of diffusion capacity for carbon monoxide (DLCO) compared to predicted values and chest CT visualizes remaining abnormalities at 3-month follow-up [9]. Except for the variation in the severity of disease due to viral strains [10], there may be different causes of pulmonary sequelae between patients during different periods as treatment recommendations evolved. For example, during the latter part of the pandemic, the diagnosis of secondary bacterial pneumonia increased coinciding with the introduction of immunomodulation [11, 12].
Corticosteroid treatment in COVID-19 is given in a phase where inflammation is believed to dominate and viral replication declines [13]. The effect of corticosteroid treatment in acute respiratory distress syndrome (ARDS) is likely explained by its anti-inflammatory effect [14, 15]. Little is known about serious adverse events of corticosteroid treatment, where findings are inconsistent and difficult to interpret because of potential confounders. Therefore, this study aimed to investigate the possible long-term pulmonary consequences after corticosteroid treatment in patients with critical COVID-19 requiring ventilatory support.
This study is a prospective, single-centre cohort study including surviving patients with critical COVID-19, defined as those requiring intensive care due to respiratory failure, at a large emergency hospital in Sweden between March 1, 2020, and August 1, 2021. Critically ill adult (≥ 18 years) patients with a positive polymerase chain reaction test for COVID-19 who were treated for respiratory failure with high-flow treatment nasal oxygen (HFNO), non-invasive ventilation (NIV), and/or invasive ventilation in the ICU were eligible for inclusion in the study. Exclusion criteria were either already on corticosteroid treatment for other reasons or corticosteroid treatment not fulfilling the definition of the RECOVERY trial [13] defined as six mg dexamethasone daily or an equivalent dose of another corticosteroid, initiated within eight days of hospital admittance and continued for at least one day. Reporting of the study followed the STROBE checklist [16].
The total number of ICU beds was 16 before the pandemic, expanding to a maximum of 60 beds during the first wave in Sweden (March-August 2020) and 33 later in the pandemic [6]. Staffing during the study period was a patient-to-nurse ratio of 1:1–2 and a patient-to-physician ratio of 1:4–6, but the proportions decreased during the peaks of the pandemic. At the turn of May/June, after the peak of the first wave, corticosteroids were introduced and had previously only been given in isolated cases. Later in the pandemic treatment with remdesivir and tocilizumab were introduced, see Supplementary Table 1.
All ICU survivors treated with ventilatory support due to COVID-19 were invited to attend a hospital follow-up visit and complete a questionnaire assessing their health-related quality of life (HRQL), six months after ICU discharge. Non-responders received two reminders about the follow-up.
Data were recorded from electronic medical records regarding age, sex, body mass index (BMI), smoking habits, and comorbidities i.e. diabetes mellitus, hypertension/cardiovascular disease and chronic lung disease [17]. Further data collected from medical journals were the severity of illness according to Simplified Acute Physiological Score III (SAPS 3) [18], length of ICU stay, and corticosteroid treatment.
The primary outcome was pulmonary function, measured as the capacity for gas exchange, and the secondary outcome was HRQL approximately six months after ICU discharge.
To evaluate pulmonary impairment, the capacity for gas exchange, i.e. DLCO (uncorrected value, i.e. not corrected for lung volume or hemoglobin value) was measured with standard methods [19] and in accordance with reference values (Hedenström) [20, 21].
HRQL was assessed with the 36-item questionnaire RAND-36 Item Health Survey (RAND Corp). The instrument has 36 items and measures eight domains of HRQL. The different domains together with the number of items of each domain are: Physical Functioning (limitations in physical activities because of health problems), Role physical (limitations in work or daily activities), Bodily pain (limitations due to pain), General health (subjective assessment of overall health), Vitality (evaluates energy levels and fatigue), Social functioning (limitations in social activities due to physical and emotional health problems), Role emotional (limitations on work and daily activities due to emotional problems) and Mental health (assesses general mental health status) [22]. There is also one item about reported health transition and one general item about perceived health. The items are answered using Likert scales and summarized using the Likert method of summated ratings. Questionnaire responses were linearly transformed into scores between 0 and 100, where a higher score represented a higher HRQL [22, 23]. The Swedish version of RAND-36 has been validated as a reliable instrument for HRQL in the general and ICU population [24,25,26].
Descriptive statistics were presented as counts (n), proportions (%), and medians with interquartile range (IQR) according to the type and distribution of data. Potential differences between groups were analyzed by Fisher´s Exact Test for categorical data and Mann-Whitney U-test for continuous variables. Multivariable linear regression models were used to estimate mean score differences (MSD) with 95% confidence intervals (CI) to assess lung function changes (DLCO) and health quality of life (RAND-36 scores) between those treated with corticosteroids and those without. The models were adjusted for presumed clinically relevant variables, age > 65 years (yes/no), sex (male/female), chronic lung disease (yes/no), tobacco smoking habits (ever/never), and length of ICU stay (continuous variable). In an additional model, SAPS 3 (as a continuous variable) and invasive ventilation (yes/no) were adjusted for. According to American Thoracic Society guidelines, clinically important differences in DLCO were set to 10% between groups [27]. For RAND-36 scores, MSDs of 5 points between groups were considered as a clinically relevant difference [23, 28]. Statistical significance was only tested for if the MSDs were clinically relevant. Missing items were accounted for with automatic calculation of the scores, according to the RAND-36 scoring protocol [22]. If more than half of the scores in one domain were missing, no result was presented. Statistical analyses were performed with Jamovi version 2.3.19 and an experienced biostatistician validated all analyses.
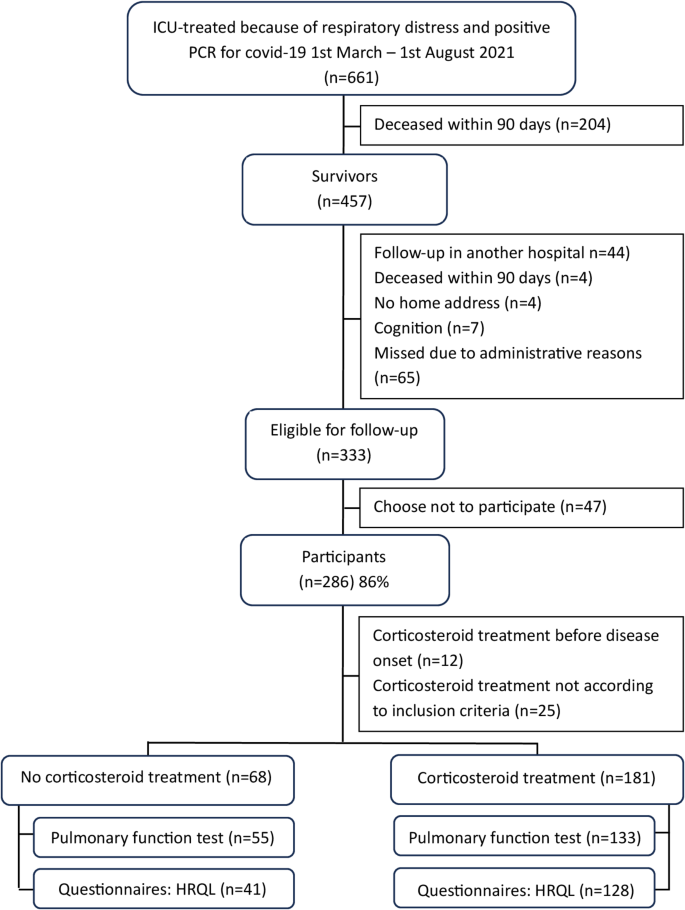
During the study period, 456 (69%) critically ill COVID-19 patients survived for at least 90 days after ICU discharge, and of those 286 (63%) attended the follow-up approximately six months after ICU discharge (Fig. 1). Among these, 68/286 (24%) had not received treatment with corticosteroids and they were all survivors of the first wave. There were 181/286 (63%) patients who received treatment with corticosteroids according to inclusion criteria. The group of patients who had not received corticosteroid treatment had lower calculated mortality risk in hospital and shorter ICU stay in comparison to those treated with at least 6 mg dexamethasone or an equivalent dose daily (Table 1).
Flowchart of study inclusion, left shows patients without corticosteroid treatment and right corticosteroid treatment
When comparing the group without corticosteroid treatment with those who received treatment within the first wave, there was no difference in the frequency or length of invasive ventilation between the groups, the median for the whole group was 12 days with invasive ventilation.
The patients with corticosteroid treatment performed spirometry at a median time of six months and those without at five months (p < 0.05).
Those who received corticosteroid treatment had a statistically significant worse pulmonary outcome at 6 months post-ICU measured as diffusion capacity DLCO (MSD − 8.3, 95% CI: -14.2 to -2.4) compared to those who did not receive corticosteroids (Table 2). Similar results were seen for those with complete data regarding DLCO and SAPS 3, n = 162, with adjustment also for SAPS 3 and invasive ventilation (MSD − 8.0, 95% CI: -14.2 to -1.9).
Among participants who reported HRQL data, there were no statistically significant differences between the groups (Table 3).
There was no difference in the distribution of age, sex, comorbidities, corticosteroid treatment and type of ventilation between responders who attended follow-up (i.e. performed pulmonary function test respectively questionnaires) and non-responders (Supplementary Table 2). Those with missing information about SAPS 3 had less often received mechanical ventilation (p < 0.05) (Supplementary Table 3).
In this study, patients who did receive corticosteroid treatment had a worse diffusion capacity for gas exchange (DLCO) at 6 months follow-up, although not meeting our definition of clinically relevant difference. For health-related quality of life outcomes, there was no statistically significant difference between the groups.
We excluded patients who had been on corticosteroids before disease onset, as they received corticosteroid treatment during the virus replication phase of COVID-19 and were believed to have a less good immune response, and they consisted of a higher proportion of women and were older. We also excluded those who had not received corticosteroids according to inclusion criteria since they were judged to have received it as “rescue treatment” early in the pandemic before corticosteroids were routinely given and a confounding indication, and they had been treated with invasive ventilation to a higher extent with a longer stay in the ICU.
The corticosteroid-treated group in this study had longer ICU stay. Since corticosteroid treatment has been associated with higher frequency of ventilator-associated pneumonia (VAP) this may be a possible explanation [29]. The participants not treated with corticosteroids were all from the first pandemic wave before treatment with corticosteroids was routinely given. They seemed to have been less severely ill at ICU admission as reflected by lower SAPS 3 values, used to predict hospital mortality. This may have contributed to the better recovery for the group not treated with corticosteroids. While the benefit of corticosteroid treatment on short-term mortality is well described [3], the knowledge of long-term outcomes is sparse and uncertain. One study by Menendéz et al., showed that one year after severe COVID-19 corticosteroid treatment on one hand was associated with incomplete radiological resolution but on the other hand not with impaired DLCO [30]. However, other studies at 3 months follow-up have opposing results. A randomized controlled trial showed that corticosteroid treatment improved forced vital capacity (FVC) in hospitalized patients with COVID-19 [31], which is in line with another study also showing better FVC for patients treated with corticosteroids [32]. Forced vital capacity on the other hand does not give information about the lungs’ capacity for gas exchange, here DLCO is a more sensitive variable for parenchymal damage [33]. Furthermore, vaccination against COVID-19 in the region started with the most fragile and oldest persons first and for adults ≥ 75 at the end of March 2021 [34]. Vaccination is therefore believed to have had little impact on this cohort.
There may be different explanations for the findings of worse pulmonary outcomes in this study for those treated with corticosteroids. Corticosteroids are used to suppress inflammation and prevent lung injury and guidelines now include corticosteroids for severe community-acquired pneumonia (sCAP) as it has showed to reduce short-term mortality but little is known about long-term effects [35]. Recent research on COVID-19 has shown that even if the etiology of ARDS is the same, subgroups might have different effects of corticosteroid treatment, which might be a contributing explanation to the heterogeneity in results of the few studies there are on corticosteroids and lung function after severe COVID-19 [36]. A plausible negative effect of corticosteroid treatment is more frequent VAP which also has been shown to increase the mortality rate [29]. Another possible negative effect of corticosteroid treatment may be delayed viral clearance, which previous studies of corticosteroid treatment in Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS) coronavirus outbreaks have shown [37, 38]. However, viral load has shown to be highest during the first week of disease onset and subsequently decline and corticosteroids are supposedly introduced after the viral replication has peaked [39]. These findings show the lack of knowledge of the long-term outcomes regarding lung function and therefore, further studies are warranted.
For health-related quality of life outcomes, there was no statistically significant difference between the groups. This may be due to that the ICU survivors in their daily life did not use the maximum of their lung capacity. These results may also suggest that factors other than the extent of lung function are involved in patients’ trajectory of recovery after severe COVID-19 infection, such as neuropsychiatric symptoms (memory and concentration), or mental health problems [40].
The main methodological strengths of the study lie in the comprehensive data collection together with the use of well-validated outcome tests and questionnaires. The study has some limitations. Firstly, this study only included survivors and it is possible that more severely ill patients survived later in the pandemic and there is the risk of selection bias as some COVID-19 survivors opted out of the follow-up program. However, patient characteristics and ICU-related data were similar between survivors who attended the follow-up and non-participants, as presented in a previous study [41]. Further, there were cases with missing information about SAPS 3, but when analyzing the cases with complete data with adjustment for SAPS 3, similar results for pulmonary outcomes where shown. The lack of baseline data prevents the identification of new-onset problems since data on pulmonary function and HRQL is difficult to obtain pre-ICU. The HRQL results were self-reported and may include a risk of response shift. Results were adjusted for known confounders. However, since data on virus strain were not available, we could not adjust for this parameter and residual confounding cannot be ruled out.
This study demonstrated poorer pulmonary outcomes for COVID-19 intensive care survivors who received corticosteroid treatment compared to those who did not. However, the impact on HRQL seemed to be minor. The results of this study need to be confirmed in future studies.
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
- ARDS:
-
Acute respiratory distress syndrome
- BMI:
-
Body mass index
- CI:
-
Confidence intervals
- DLCO:
-
Diffusion capacity for carbon monoxide
- FVC:
-
Forced vital capacity
- HFNO:
-
High-flow treatment nasal oxygen
- HRQL:
-
Health-related quality of life
- ICU:
-
Intensive care unit
- IQR:
-
Interquartile range
- Md:
-
Median
- MERS:
-
Middle East Respiratory Syndrome
- MSD:
-
Mean score differences
- NIV:
-
Non-invasive ventilation
- SAPS 3:
-
Simplified Acute Physiological Score III
- SARS:
-
Severe Acute Respiratory Syndrome
- sCAP:
-
severe community-acquired pneumonia
- IQR:
-
Interquartile range
- VAP:
-
Ventilator-associated pneumonia
We would like to thank all patients who participated in the study and caregivers involved in the follow-up.
Open access funding provided by Karolinska Institute.
Support was given through the regional agreement on medical training and clinical research (ALF) between Stockholm County Council and Karolinska Institutet awarded to AS, with grant number RS 2022 − 0674.
Study approval was granted from the Swedish Ethical Review Authority (Dnr 2020–03760, 2020–06106 and 2022-03531-02). The study adhered to the Declaration of Helsinki. Written informed consent was obtained from all included participants.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Roël, M., Schandl, A., Jonmarker, S. et al. Corticosteroids and long-term pulmonary function after critical illness due to COVID-19– a single-center cohort study. BMC Pulm Med 25, 201 (2025). https://doi.org/10.1186/s12890-025-03659-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-025-03659-0