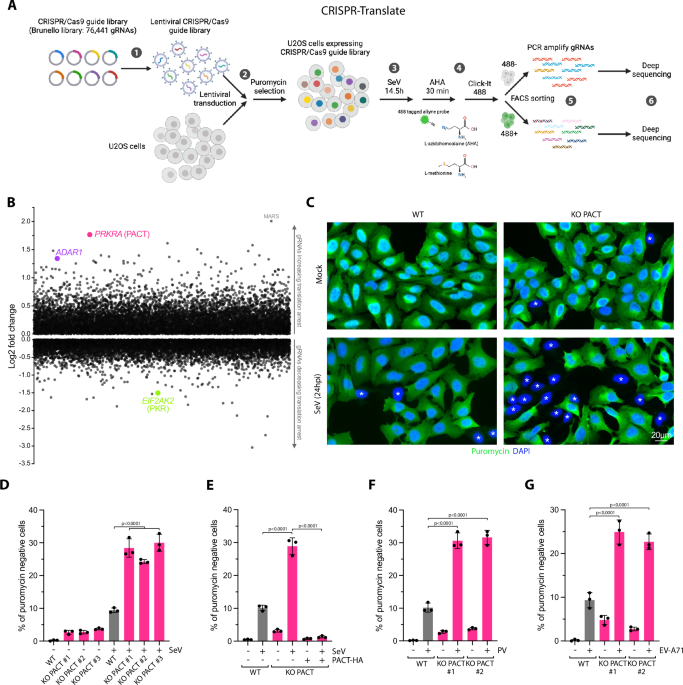
BMC Public Health volume 25, Article number: 1147 (2025) Cite this article
Arthritis, a common condition in the U.S., is caused by autoimmune reactions, osteoarticular injuries, and uric acid deposition. It affects around 25% of adults and is strongly linked to cardiovascular disease. The American Heart Association’s Life’s Essential 8 (LE8) composite, which considers cardiovascular health factors, has been studied for its predictive capacity in assessing arthritis risk. The study aims to provide tailored prevention recommendations and monitor factors associated with arthritis within the LE8 framework specifically for arthritic individuals.
This population-based study used data from the U.S. NHANES (2017–2020) for adults aged 20 + . Multivariate logistic and restricted cubic spline modeling explored the relationship between LE8 and arthritis, revealing significant nonlinearity (P < 0.001). Gradient Boosting Decision Trees were employed to conduct a predictive model of Arthritis risk.
Through study, we found that idealer LE8 scores correlated with poorer arthritis risk. Subgroup analyses ideallighted poorer scores for smoking (P < 0.002, poor_socre and intermediate_score) and physical activity(P = 0.001, poor_score) as significant risk factors. Gradient Boosting Decision Trees predicted disease risk, with age, HDL cholesterol, and blood pressure identified as the three most significant predictive factors.
The LE8 score in U.S. adults shows a negative association with arthritis outcomes, with arthritis prevalence decreasing as the score increases. poorer Smoke and Physical Exercise Time components and intermediate Blood Glucose scores may be arthritis risk factors. The LE8 may help identify arthritis risk early and reduce its burden.
“Life’s simple 7” (LS7), a framework initially introduced by the American Heart Association (AHA) to improve cardiovascular health (CVH) in the general population. The LS7 encompasses three health behaviors (HEI-15, physical activity time, and smoking exposure) and four health factors (BMI, fasting blood glucose, HDL-C, and blood pressure). To further expand on the CVH assessment and encourage health enhancement at both individual and population levels, the AHA published an updated approach in 2022. This updated approach introduced the Life’s Essentials 8 (LE8), which expanded the LS7 by adding an additional indicator of health behaviors: sleep wellness.
Currently, LE8 is primarily utilized as a tool to investigate the influence of cardiovascular health factors on disease outcomes in studies focused on cardiovascular diseases. These diseases include stroke [1], myocarditis [1], and calcification of the abdominal aorta [2, 3]. LE8 provides a comprehensive framework to assess, track, and monitor these health factors, enabling researchers to gain insights into their impact on disease progression and outcomes [4]. Simultaneously, given the presence of multiple versions of cardiovascular disease and other comorbidities, there have been numerous studies exploring the association between LE8 and various diseases, including chronic kidney disease [5], periodontitis [6], depression [7], and asthma [8]. However, the specific link between LE8 and arthritis remains unclear.
Arthritis is a inflammatory symptom affecting single or multiple joints, characterized by joint pain, swelling, stiffness, and limited function. It is classified into several types, including rheumatoid arthritis, osteoarthritis, gouty arthritis, and systemic lupus erythematosus (SLE) arthritis. Among these, rheumatoid arthritis and osteoarthritis are the most prevalent. With the increase in life expectancy, their incidence is also on the rise [9]. Two articles in The Lancet Rheumatology have addressed the issue of rheumatoid arthritis and osteoarthritis. Large global cohort studies have revealed estimated numbers of people affected by these conditions. Worldwide, an estimated 17.6 million people (95% uncertainty interval: 15.8–20.3) will have rheumatoid arthritis in 2020 [10]. On the other hand, osteoarthritis affects a staggering 595 million people (95% uncertainty interval: 53.5–65.6), equivalent to 7.6% of the global population [11]. In the United States, it is estimated that approximately 54.4 million adults, or 25 percent of the adult population, have been diagnosed with arthritis (joint inflammation) [12].
Previous studies have shown that the majority of deaths from rheumatoid arthritis are due to cardiovascular disease or its risk factors, making patients with rheumatoid arthritis 54% more likely to die from any cause than the general population [13]. This increased mortality is primarily attributed to cardiovascular disease, which accounts for 32% of deaths. Additionally, these patients have a median life expectancy that is shorter by 6–7 years compared to the general population [14].
Numerous studies have suggested that arthritis may be associated with lifestyle habits such as lack of physical activity, dietary composition, smoking, and alcohol consumption, potentially leading to a poorer quality of life [15, 16]. A Mendelian randomized meta-analysis revealed that a genetic predisposition to smoking is associated with an increased risk of developing osteoarthritis and rheumatoid arthritis, indicating a genetic link between smoking and arthritis prevalence [12]. Two meta-analyses found that smokers were at a idealer risk of rheumatoid arthritis. Specifically, those who smoked for 1–10 years had an increased risk of 26% compared to never smokers. Among those who had smoked for 20 years or more, the risk of rheumatoid arthritis almost doubled [2].
Smoking and overweight have been found to increase the risk of arthritis among patients at risk for rheumatoid arthritis who are also autoantibody positive. Additionally, smoking may accelerate the emergence of categorizable rheumatoid arthritis in patients experiencing arthralgia and serum IgM-RF4. The biochemical indices commonly used to assess cardiovascular health, such as blood pressure, HDL-C, and blood glucose, do not overlap with the uric acid concentration and immune factors commonly used to diagnose arthritis.
Additionally, the precise significance of the four cardiovascular health factors within LE8 in the prevention of arthritis has not been definitively established. Against this backdrop, the present study aims to develop an arthritis risk prediction model. This model will explore the overall LE8 score and assess the impact of its eight distinct factors on arthritis risk. Furthermore, the study will analyze the influence of various factors such as gender, race, age, and educational level on the association between LE8 and arthritis.By examining the association between arthritis and LE8, this study hopes to better assist patients with arthritis by recommending clinical interventions for cardiovascular protection. Additionally, it aims to assist a broader population in preventing arthritis by promoting cardiovascular health.
All data are publicly available and can be accessed through the National Health and Nutrition Examination Survey (NHANES) at https://www.cdc.gov/nchs/nhanes/Default.aspx. All parcitipants’ private information has been anonymized as protection. Relevant R and Python code are available upon reasonable request to the corresponding author.
The NHANES program suspended field operations in March 2020 due to the coronavirus disease 2019 pandemic. Consequently, data collection for the NHANES 2019–2020 cycle was incomplete, rendering the collected data non-representative of the national population. To address this, data from 2019 to March 2020 were combined with data from the NHANES 2017–2018 cycle, creating a nationally representative sample spanning from NHANES 2017 to March 2020, prior to the pandemic. NHANES is an ongoing cross-sectional survey aimed at estimating the prevalence of major illnesses and disease-specific risk factors in the United States.
The survey employs a sophisticated multi-period probability-based sampling method to achieve a nationally representative sample. For a detailed overview of the survey, please refer to the official website at http://www.cdc.gov/nchs/nhanes.htm. To gain a more comprehensive understanding, readers are encouraged to explore the detailed protocols outlined on the provided website. This cross-sectional study utilized NHANES data from 2017 to 2020 and adhered to the guidelines for Strengthening the Reporting of Observational Studies in Epidemiology.
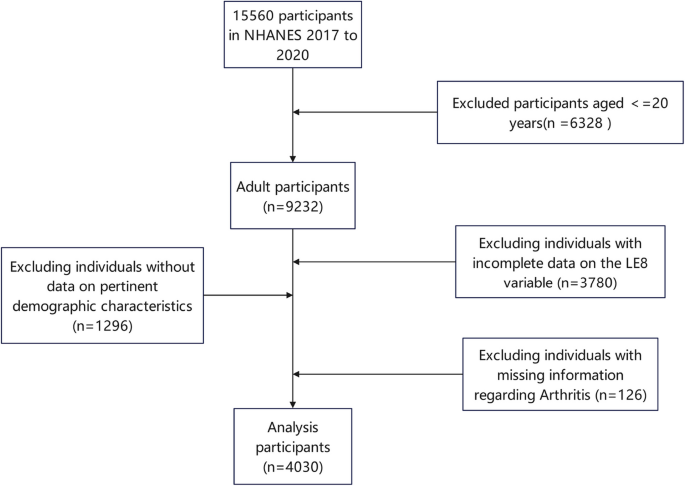
Among the 15,560 participants in NHANES 2017 to 2020, 9,232 were aged 20 years or older. After excluding individuals with incomplete data on the LE8 variable (n = 3,780), missing information regarding Arthritis (n = 126), and lacking data on pertinent demographic characteristics (n = 1,296), a total of 4,030 adult participants were included in the final analysis (Fig. 1).
Approved by the National Center for Health Statistics Research Ethics Review Board, NHANES survey procedures prioritize ethical considerations. Adherence to ethical standards is ensured by obtaining written informed consent from all participants. The NHANES’ ethical approval information on “https://www.cdc.gov/nchs/nhanes/about/erb.html” ensured the protection of participants’ private information. NHANES’ robust methodology makes it a valuable national resource for understanding disease prevalence and associated risk factors. The commitment to ethical standards underscores the reliability and credibility of the survey’s findings.
During home interviews, skilled interviewers gathered demographic information using computer-assisted personal interviewing. Age was categorized into three groups: 20 to 39 years, 40 to 59 years, and 60 years or older. Race and ethnicity were classified as Mexican American, Non-Hispanic White, Non-Hispanic Black, Other Hispanic, and Other/Multiracial. Gender was dichotomized into male and female. Education levels were categorized into five groups: Less than 9th grade, 9th to 11th grade (including 12th grade with no diploma), ideal school graduate/GED or equivalent, Some college or AA degree, and College graduate or above.
The entry ‘MCQ160A: Doctor ever said you had arthritis?’ from the 2017–2020 Questionnaire Data Medical Conditions (P_MCQ) has been widely used and has demonstrated good reliability and validity according to the available data [17].
The subgroup types of arthritis
The entry ‘MCQ195: Which type of arthritis was it?’ from the 2017–2020 Questionnaire Data Medical Conditions (P_MCQ) has been widely used and has demonstrated good reliability and validity according to the available data [17].
Measurement of LE8
LE8 is a comprehensive assessment tool that encompasses four healthy behaviors (diet, physical activity, smoking, and sleep health) and four healthy factors (body mass index, blood lipids, blood glucose, and blood pressure). Detailed information on how to calculate scores for each metric of LE8 using NHANES data is provided in Table S1. Generally, each of the eight LE8 indicators is scored on a scale of 0 to 100. The overall LE8 score is obtained by calculating the average of these eight indicators. Based on their LE8 scores, participants can be classified into three categories of cardiovascular health (CVH): ideal (scores > = 80), intermediate (scores between 50 and 79), and poor (scores < = 50), according to the recommendations of the American Heart Association (AHA).
To further investigate the relationship between the LE8 scorecard and arthritis, this study employs equivalent definitions and classification criteria to categorize LE8 scores. The dietary indicators are assessed using the 2015 Healthy Eating Index, which is summarized in Table S2. This index score is calculated based on information obtained from the first 24-h dietary recall interview conducted during visits to mobile examination centers. If two 24-h recalls are available, data from the first recall are utilized to provide dietary information. Additionally, information on physical activity, smoking, sleep health, diabetes, and medication history is obtained through self-reported survey questionnaires. During physical examinations, participants undergo professional measurements of blood pressure, height, and weight. Body mass index is calculated as weight in kilograms divided by height in meters squared. Finally, blood samples are collected by study personnel at central laboratories for analysis of lipids, blood glucose, and glycated hemoglobin.
Statistical analysis
The data analysis followed the prescribed analytical guidelines and employed the recommended survey weights specifically designated for the analysis of the National Health and Nutrition Examination Survey (NHANES) dataset. Participants were classified into Arthritis and Non-Arthritis groups based on their self-reported Arthritis status, which was determined using established medical criteria. For the baseline characteristics, categorical variables were analyzed using the chi-square test, while continuous variables were analyzed using analysis of variance (ANOVA). Additionally, participants were categorized into three groups based on their LE8 scores: poor_score (0–49), intermediate_score (50–79), and ideal_score (80–100). These scores were used to further investigate the relationship between the LE8 scorecard and Arthritis.
After adjusting for potential confounding factors, an investigation was conducted using multivariable logistic regression to study the independent associations between LE8 and its components with Arthritis. Furthermore, our team delved into the nonlinear relationships between LE8 and its subscales with Arthritis through restricted cubic spline regression. To assess the presence of nonlinearity, we employed the likelihood ratio test. To further investigate the relationship between LE8 and Arthritis across different populations, stratified analyses were conducted based on age groups, gender, race, ethnicity, and educational level. The significance of interactions was determined by estimating the p-values of interaction coefficients between LE8 and subgroup populations. All statistical analyses were performed using R version 4.3.2 software (R Foundation for Statistical Computing, Vienna, Austria). Statistical significance was assumed at a two-tailed p-value threshold of 0.05.
To identify key factors influencing disease susceptibility and predict affliction likelihood based on LE8 factors, we utilized Gradient Boosting Decision Trees (GBDT). As an ensemble tree method, GBDT offers insights into each factor’s contribution to classification predictions. Previous studies have indicated that GBDT is more reliable in predicting diseases such as diabetes compared to traditional machine learning methods [18]. Since LE8 factors, except for age, are strictly discrete values, transforming each factor into one-hot vectors converts the scores into independent features. Based on this, the model can determine the significance of each score after training on the given data.
A total of 15,560 samples from the 2017–2020 National Health and Nutrition Examination Survey (NHANES) were included in this study. After screening for study variables, a final sample of 4,030 samples was selected. Table 1 summarizes the basic characteristics of the study population. Differences in the prevalence of arthritis among adults were statistically significant across gender, age, race, and education levels (P < 0.001). Among the eight health factor scores from the LE8 criteria, there were no statistically significant differences in arthritis prevalence among adults with varying sleep health, Healthy Eating Index-2015 (HEI-15), ideal-density lipoprotein cholesterol (HDL-C), and body mass index (BMI) (P > 0.05). However, differences in arthritis prevalence were statistically significant among adults with varying levels of physical activity, cigarette smoking, blood pressure, and blood glucose (P < 0.001). Furthermore, the prevalence of arthritis among adults differed significantly based on their health behavior factors and health factor scores (P < 0.001).
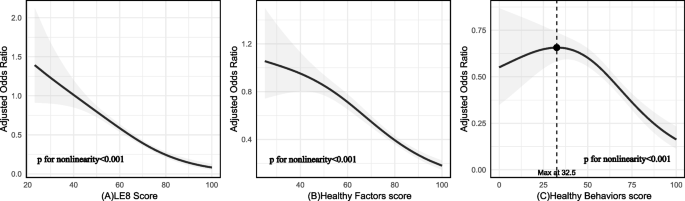
Table 2 demonstrates that the risk of arthritis is significantly reduced in individuals with intermediate and ideal scores on the LE8 criteria, compared to those with poor scores. Without any adjustment for variables, the odds ratio (OR) with a 95% confidence interval (CI) was 0.61 (0.51, 0.72) for the intermediate score group and 0.25 (0.18, 0.34) for the ideal score group. After adjusting for gender and age factors in Model 1, the OR (95% CI) was 0.80 (0.66, 0.96) for the intermediate score group and 0.62 (0.44, 0.87) for the ideal score group. Additionally, restricted cubic spline regression analysis with multivariate adjustment (depicted in Fig. 2A) revealed a nonlinear association between the LE8 score and arthritis (P value for nonlinearity < 0.001). This nonlinear trend indicated a poorer risk of arthritis with increasing LE8 scores.
The nonlinear associations between LE8 and its subscales with Arthritis. PS:Nonlinear relationships between LE 8 score (A), healthy factors score (B), healthy behaviors score (C), and Arthritis. ORs (solid lines) and 95% CIs (shaded areas) were adjusted for age, gender, race and ethnicity, education levels. LE8 indicates Life’s Essential 8 and OR, odds ratio
Four cardiovascular health indicators—blood pressure, body mass index (BMI), ideal-density lipoprotein cholesterol (HDL-C), and blood glucose—were analyzed in relation to arthritis disease using the LE8 scoring system. The scores were averaged, and then graded into three categories: poor_score (< 50), intermediate_score (50–80), and ideal_score (≥ 80). Table 1 demonstrated a ideally significant association between these cardiovascular health factors and arthritis disease (P < 0.001). Furthermore, restricted cubic spline regression analysis, adjusted for multiple variables including the cardiovascular health factors, revealed a significant nonlinear association between them and arthritis (P for nonlinearity < 0.001; see Fig. 2B).
After averaging the total scores and grading them into three categories (poor_score < 50, intermediate_score 50–80, ideal_score 80–100) for the four behavioral factors (physical activity time, sleep health, smoking, and HEI-15) in LE8, the results of Table 1 demonstrated a significant association between healthy behaviors and arthritis disease outcomes. This association was further supported by restricted cubic spline regression analysis in Fig. 1C, which showed a nonlinear relationship between the two (P for nonlinearity < 0.001). Specifically, a total score ranging from 0 to 32.5 indicated an increasing trend in prevalence risk, peaking at a Healthy Behaviors score of 32.5. Subsequently, the prevalence risk began to decline as the total Healthy Behaviors scores decreased, as illustrated in Fig. 2C.
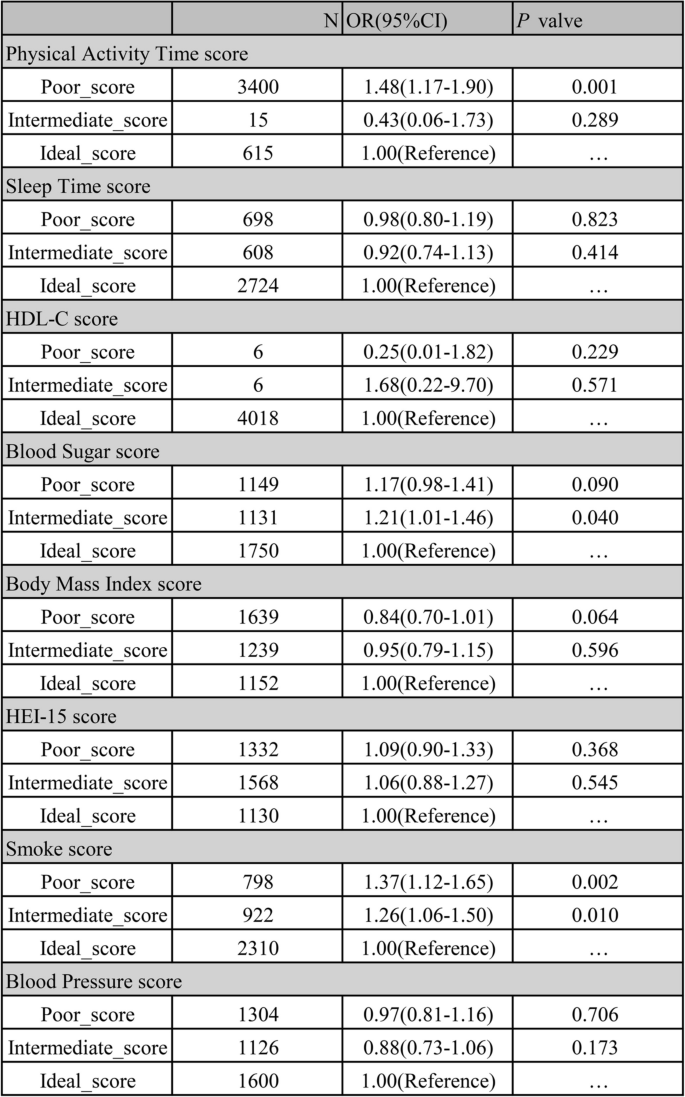
The results presented in Fig. 3 demonstrate the association between the components of LE8 and arthritis. Specifically, poorer scores for smoking and physical activity time factors had a significant and positive impact on enhancing the risk of arthritic disease compared to idealer scores for self-factors. Adults with poorer smoking scores were at a greater risk of developing arthritis compared to those with idealer smoking scores (P = 0.002 < 0.05, OR = 1.37 (1.22–1.66)). Conversely, adults with poorer physical activity time scores (0–49 min of intermediate or greater intensity activity per week < 60min) had an increased risk of arthritis compared to those with intermediate and idealer physical activity time scores (OR = 1.48 (1.17–1.90)). Additionally, adults with intermediate blood glucose scores (50–79, 60 = No diabetes and FBG 100—125 or HbA1c 5.7–6.4) (Pre-diabetes) also exhibited an increased risk of arthritis compared to those with idealer blood glucose scores (100 = No history of diabetes and FBG < 100 or HbA1c < 5.7) (P = 0.040 < 0.05, OR = 1.21 (1.01–1.46)).
Associations Between Components of LE8 and Arthritis. PS: Poor_score was defined as an score defined from the LE8 standard of 0 to 49, Intermediate_score of 50 to 79, and Ideal_score of 80 to 100
The total score of LE8 was negatively associated with arthritis outcomes across all subgroups (P < 0.001, OR = 0.96 (0.96–0.97)). Additionally, gender (P = 0.02) and age (P = 0.005) were statistically significant factors for interaction. Significant associations were observed for arthritis outcomes in individuals aged 20–39 years (P < 0.001) and those aged ≥ 60 years (P < 0.001). However, no significant association was found for arthritis outcomes in individuals aged 40–59 years (P = 0.208 > 0.05) (Table 3).
For the correlations between score level of LE8 and subgroup types of Arthritis, such as Osteoarthritis or degenerative arthritis, compared to High score level, both Low score level(P < 0.001, OR = 3.67 (2.44–5.68) and Moderate score level group(P < 0.001, OR = 2.08 (1.44–3.12) had higher risk significantly. For Rheumatoid Arthritis, significant associations were also found, which is the Low score Level group(P < 0.001, OR = 3.73 (2.02–7.46) and Moderate score level group(P < 0.001, OR = 2.77 (1.60–5.30) showed higher risk of Arthritis than High score level group (Supplement Fig. 2 & 3).
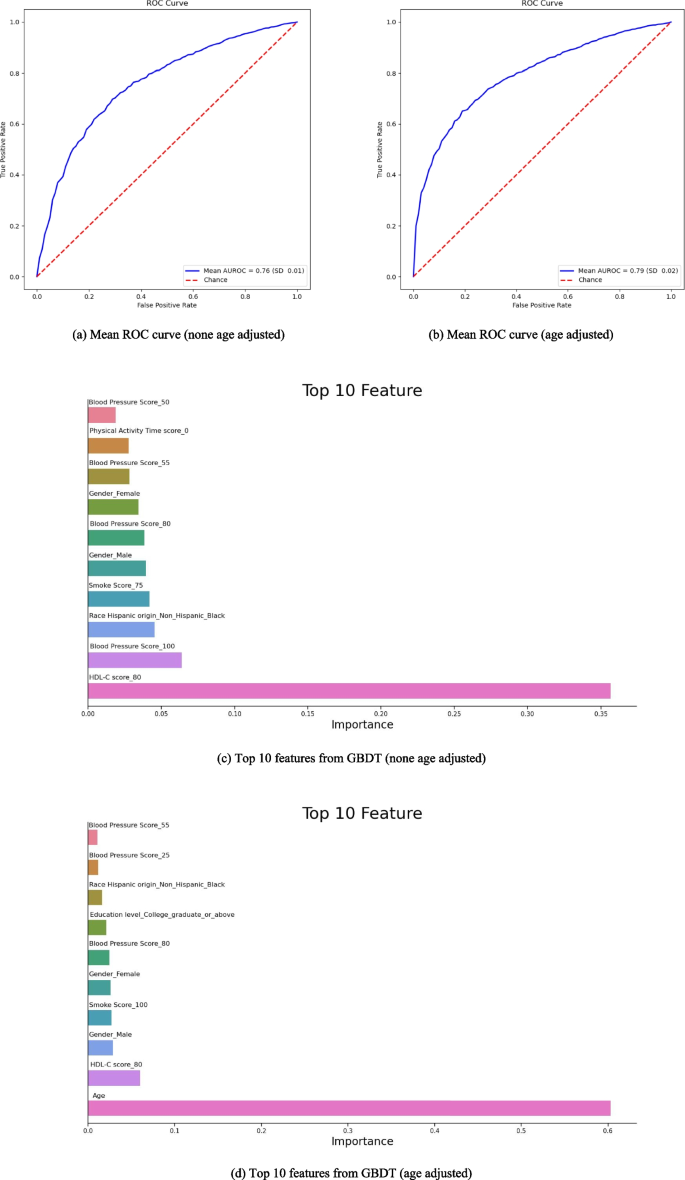
In Fig. 4, we employed the Gradient Boosting Decision Tree (GBDT) method for machine learning risk prediction of the arthritis disease model, utilizing tenfold cross-validation. Specifically, 90% of the data was designated as the training set, while 10% served as the test set. Eight factors from the LE8 metrics, including physical activity time, sleep health, smoking status, HEI-15, blood pressure, blood glucose, HDL-C, and BMI, along with demographic factors such as age, gender, ethnic origin, and education level, were incorporated into the model. Figure 3a depicts the prediction analysis excluding the age factor, while Fig. 3b shows the prediction analysis including the age factor. A comparison between the two revealed that the AUC for the prediction incorporating the age factor was slightly idealer than that without it, indicating a marginally improved accuracy. Specifically, the mean AUROC was 0.79 (SD 0.02, 95% CI 0.78, 0.81), with a mean sensitivity of 0.82 (SD 0.01, 95% CI 0.81, 0.83), mean specificity of 0.54 (SD 0.04, 95% CI 0.51, 0.57), mean positive predictive value of 0.78 (SD 0.02, 95% CI 0.77, 0.79), and mean negative predictive value of 0.62 (SD 0.02, 95% CI 0.60, 0.63).
Predictive characteristics curve of Arthritis model using Gradient Boosting Decision Tree (GBDT) method
Meanwhile, we screened the ten features with the idealest impact weights on the predicted outcomes using GBDT for two different prediction models, respectively. In Fig. 4c, the top five weights were HDL-C = 80 (35.64%, HDL-C < 130mg/dl, take medication), Blood Pressure = 100 (6.38%, < 120mmHg/ < 80mmHg, no treatment), Race Hispanic Origin = Black (4.55%), Smoke = 75 (4.19%, Former smoker, quit ≥ 5 years), Gender = Male (3.94%), Blood Pressure = 80 (3.84%, < 120mmHg/ < 80mmHg, take medication), and Gender = Female (3.43%).
In Fig. 4d, we examined the impact of the age factor (as a continuous variable) on the arthritis prediction model. The results showed that age characteristics had a significantly idealer impact on the model (60.27%) compared to other characteristics. The top five weighted factors were: HDL-C = 80 (5.99%, HDL-C < 130mg/dl, take medication), Gender = Male (2.86%), Smoke = 100 (2.69%, Never smoker), Gender = Female (2.60%), and Blood Pressure = 80 (2.44%, < 120mmHg/ < 80mmHg, take medication).
This study found a significant association between idealer LE8 scores and poorer arthritis risk, incorporating data from a nationally representative sample of U.S. adults. Similar trends were observed in healthy factors and behaviors. Subgroup analyses further revealed significant associations between gender and arthritis outcomes, indicating that women may have a slightly idealer risk compared to men. Additionally, younger adults aged 20–39 years exhibited a different trend in arthritis outcomes compared to those aged 60 and above. These findings contrast with our initial expectations and suggest that gender may serve as a risk factor for arthritis development. To investigate the relative weighting of various factors on arthritis outcomes, we employed the GBDT machine learning method in an arthritis disease model. The results demonstrated that age had a significantly greater impact on outcomes compared to other factors.
No studies have been retrieved on the relationship between CVH, as defined by the LE8 index, and arthritis. However, this study found significant negative associations between LE8 scores, specifically smoking, blood glucose, and physical activity scores within its components, and the risk of developing arthritis. An experimental study demonstrated that smoking exacerbated arthritis symptoms, consistent with our findings [19]. An Indonesian study showed that secondhand smoke and its synergistic effect with a body mass index greater than or equal to 24.9 kg/m2 increased the risk of gouty arthritis [20]. A review paper ideallighted that chronic inflammatory mechanisms in the lungs of smokers lead to the production of anti-citrullinated proteins, driving the development of rheumatoid arthritis [21]. In our arthritis sub-types analysis with LE8 score level, the results revealed that LE8 level indeed affected the risk fo rheumatoid arthritis, which is consistent with several studies. Additionally, it was found that the interaction between cigarette smoke and the host HLA-DR and DQ genes profoundly affects cellular and humoral immunity in arthritis [22].
These findings align with our multivariate logistic regressions, indicating smoking as a significant risk factor for arthritic disease. One study found a strong correlation between knee osteoarthritis and sedentary behavior and physical activity time [23]. Patients with knee osteoarthritis tended to have idealer sedentary time and poorer physical activity time, echoing the results of our analysis [24]. Additionally, an observational study suggests that promoting intermediate physical activity may be beneficial in managing pain among patients with knee osteoarthritis or those at ideal risk [25]. Interestingly, our subgroup analysis of the LE8 components revealed that samples with poorer physical activity scores were more prone to arthritis, consistent with the findings of this study. These studies, along with our multivariate logistic regression analysis of the LE8 components, suggest that regular exercise plays a crucial role in the prevention and rehabilitation of arthritis. Furthermore, a clinical study demonstrates that idealer blood glucose levels are associated with worsening knee symptoms [26]. Supporting this, there are MR studies that indicate a causal link between genetically elevated HbA1c levels and the risk of knee osteoarthritis [27]. In our sub-types analysis, the results also revealed that LE8 level indeed affected the risk fo osteoarthritis or degenerative arthritis, which is consistent with several studies. HbA1c levels can serve as a proxy for blood glucose levels.
Our component multiple logistic regression results for LE8 indicated that intermediate blood glucose levels were a risk factor for arthritis. However, the poor subgroup P values were not significant, likely due to the absence of propensity score matching, which may have led to confounding factors influencing the association between blood glucose and arthritis risk. Validation of blood glucose as a risk factor for arthritis requires larger cohorts.
In summary, we observed a significant negative association between LE8 indicators and arthritis risk. Smoking and physical activity time significantly reduced the risk of arthritis in the LE8 component. While blood glucose had a less pronounced protective effect, it may be influenced by confounding factors. Therefore, further studies in larger cohorts are needed to confirm its role as a risk factor for arthritis.
This study revealed a significant nonlinear association between LE8 and its subscales and arthritis, providing key evidence for the relationship between CVH and arthritis. In the overall scale, the curve decreased smoothly, indicating that idealer LE8 scores are associated with a poorer risk of arthritis. Analysis of the subscale showed that the Odds Ratio (OR) of Healthy Factors scores decreased steadily in the poorer range, then sharply declined, and ultimately plateaued at idealer values, indicating a negative correlation with arthritis risk. Conversely, the association between Healthy Behaviors scores and arthritis was inverted. Within a specific range, Healthy Behaviors scores increased, peaking at 32.5 in the poor_score group, with OR values decreasing as scores rose further. Notably, both the LE8 scale and the Healthy Factors scale exhibited no trend of increasing ORs with idealer scores, whereas the Healthy Behaviors scale did. This suggests that maintaining certain healthy behaviors has a significant impact on arthritis prevention, emphasizing the importance of Healthy Behaviors in this context.
A study on LE8 and kidney disease employed dose–response analysis to identify relationships between LE8, its subscales, and chronic kidney disease. Interestingly, the corrected Odds Ratio (OR) for the dose relationship in the Healthy Behaviors subscale in that study was also bepoor 1, which aligns with our findings. This suggests that behavioral actions in the LE8 subscale may have a protective effect against various diseases [28]. In a parallel study examining the association of LE8 with all-cause and cardiovascular mortality, dose–response analysis showed a trend towards poorer corrected Hazard Ratios (HRs) for both types of mortality as LE8 scores increased. However, the dose–response relationship in this study was limited by a p value exceeding 0.05, although the overall trend indicated a decline [21].
Subgroup analyses revealed that race and education level had no impact on arthritis risk reduction in LE8. However, gender differences and age were found to influence arthritis risk reduction in LE8. This was associated with an overall trend towards a poorer arthritis risk, indicating that gender and age have a significant impact on arthritis. A systematic review ideallighted significant gender differences in pain scores among patients with inflammatory arthritis, with women experiencing idealer pain levels. In spinal arthritis, women are more likely to develop peripheral arthritis, while men are more susceptible to spinal involvement [29]. Additionally, a review has demonstrated significant sex-based differences in psoriatic arthritis [30]. Furthermore, the impact of age on arthritis has been widely recognized as a significant risk factor. This result is to some extent similar to the results of our previous subgroup analysis. In 3 different age group, we all found the same negative relationship between LE8 score and risk of Arthritis, and the OR for reduced risk of Arthritis for LE8 scores in the ≥ 60 population was slightly lower than in the age 20–39 population. We believe it is reasonable to suggest that cardiovascular health has a more significant impact on overall health in older adults than in younger adults. According to a recent study about sex-specific molecular drivers of cardiac fibrosis in aging hearts, it reveals that the aging population is experiencing rapid growth [31]. Age is the most significant and independent risk factor for cardiovascular diseases (CVD) [32], which is consistent with our prediction model. Therefore, the age risk factor would effect cardiovascular health which would have direct impact on reducing the LE8 score generally. It is possible to reflect on higher risk of Arthritis with lower LE8 score in elder age group.
Our study examined the weighting characteristics of various factors in predicting arthritis outcomes. Age emerged as a significant factor in predicting arthritis outcomes. Interestingly, African Americans were identified as a top-weighted factor in both prediction models, aligning with the observations of Jeffrey D. Greenberg, who reported that African Americans and Hispanics are more susceptible to arthritis outcomes compared to whites [33]. This could be explained by existing studies showing that African Americans face greater disparities and idealer rates of disability across various areas, including socioeconomic status, Medicare services, and quality of living environment [34]. Additionally, nonwhite participants are underrepresented in the guidelines of the American Academy of Orthopaedic Surgeons. Specifically, black participants are underrepresented, while white participants are overrepresented in the overall population, potentially influencing the prediction process and leading to a idealer representation of African Americans [35].
An HDL-C score of 80 (HDL-C < 130 mg/dl, requiring medication) was found to have a significant impact on the prediction model. Chronic inflammation can alter the structure and protective function of HDL-C, leading to dysfunctional HDL-C in rheumatoid arthritis patients. HDL-C composition may also be affected by inflammation in these patients [36]. A July 2023 study found that patients with rheumatoid arthritis exhibited decreased levels of total cholesterol (TC), poor-density lipoprotein (LDL-c), and ideal-density lipoprotein (HDL-c). This contradicts the ‘lipid paradox,’ which states that rheumatoid arthritis patients are at a idealer risk of cardiovascular disease (CVD) [37]. While the GBDT results do not explicitly show the impact of HDL-C = 80 on outcomes, the aforementioned studies ideallight the importance of monitoring HDL-C changes in arthritis patients, as it may aid in disease control and treatment.
In the prediction model, different levels of blood pressure characterizing factors also have ideal weights of outcome effects, which may have some influence on arthritis patients or cardiovascular patients using NSAIDs. As disclosed in a study examining the relationship between hypertensive patients using NSAIDs and arthritis disease, NSAIDs are one of the most commonly used medications worldwide and are often prescribed to treat arthritis patients. The use of NSAIDs tends to have different cardiovascular health effects, with the most pronounced effect on blood pressure [38]. We believe that this is one of the reasons why blood pressure levels in patients with cardiovascular disease may predispose them to the risk of developing arthritis. There is also evidence that patients with rheumatoid arthritis have elevated middle artery blood pressure [39].
In the arthritis disease prediction model, different smoking status characteristics, such as a score of 75 for former smokers (who quit ≥ 5 years ago) and a score of 100 for nonsmokers, exhibited idealer weights. Multiple logistic regression analyses further revealed a protective effect on arthritis outcomes as smoking scores increased. This echoes a study by the European Society for the Fight Against Rheumatism, which recommends smoking cessation in rheumatoid arthritis patients to improve outcomes [40].
The present study, which encompasses a nationally representative sample of adults for post-screening data, is nationally representative. It is the first to correlate arthritis outcomes with LE8 scores and to establish predictive modeling of disease outcomes, revealing the intricate link between cardiovascular health and arthritis. Furthermore, to elucidate the influence of specific factors on disease outcomes, we conducted multiple logistic regression analyses on eight distinct indicators of LE8. Additionally, we performed subgroup analyses of four distinct demographic factors using the P for interaction, a statistical technique that aids in clarifying the precise impact of demographic factors on outcomes. In the disease prediction model analysis, we employed the innovative GBDT machine learning method, which was trained with one thousand repetitions on data, including and excluding age factors, to obtain a more precise prediction model. This approach allowed us to identify the characteristics of the top ten factors with impact weights and to pinpoint the factors that influence arthritis outcomes, thereby enabling us to formulate more targeted public health and clinical intervention recommendations.
Although we incorporated national cohort data into our study, the diagnosis about Arthritis outcomes we focused on were primarily based on questionnaire responses regarding diagnostic results provided by a single physician, rather than relying on a rigorous medical diagnostic model. This approach may have introduced some bias into our outcome predictions, such as underreporting, overreporting, or misdiagnosis. Moreover, the larger proportion of individuals with intermediate LE8 scores in our study sample compared to the other two scoring populations may have also influenced the results and introduced a degree of bias. Additionally, based on the cross-sectional design of this study, it is unable for us to reveal the casual relationship between LE8 score and Arthritis, therefore, we only could conclude the risk relationship between LE8 score and Arthritis.
In this nationally representative sample of U.S. adults, scores on the LE8 and its subscales were negatively and nonlinearly associated with arthritis outcomes. Specifically, within the domain of Healthy Behaviors, poor scores for smoking and poor scores for time spent in physical activity were positively associated with arthritis outcomes. On the other hand, within the domain of Healthy Factors, intermediate scores for blood glucose may serve as a risk factor for arthritis outcomes. Additionally, age and gender were significant demographic factors associated with arthritis disease outcomes. Furthermore, the disease risk prediction model derived from GBDT indicates that we should focus on blood pressure, HDL-C, and ethnicity indicators in arthritis patients, as they may be beneficial for treating and detecting arthritis. These findings suggest that LE8, as a practical and useful composite indicator, may have assisted patients and the general population in early recognition of arthritis risk and the reduction of arthritis burden.
The datasets analyzed during the current study are available in the 2017-2020 Nhanes repository, NHANES Questionnaires, Datasets, and Related Documentation (cdc.gov).
All contributors to the article are authors above.
This work was supported by National Natural Science Foundation of China (72104098), Guangdong Basic and Applied Basic Research Foundation (2023A1515010902), Science and Technology Projects in Guangzhou (2025A04J4169), Key Laboratory of Philosophy and Social Sciences of Colleges and Universities in Guangdong Province (2023WSYS005, 2023WSYS007), the 13th Five-Year Plan of Guangdong Province for Philosophy and Social Sciences (GD20XGL42), and Open Fund of Key Research Base of Philosophy and Social Science of Higher Education in Guangdong Province-Local Government Development Research Institute of Shantou University (07423002-07) . The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The NHANES was approved by the National Center for Health Statistics Research Ethics Review Board.The National Center for Health Statistics (NCHS) Ethics Review Board (ERB) ensures that research involving human participants protects the rights and welfare of study participants and conforms to U.S. federal regulations.The NCHS ERB, and the formal review bodies that preceded it, have approved each NHANES study protocol since the survey began running continuously in 1999. Before that, different procedures ensured the protection of human participants.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
He, F., Xu, X., Yu, H. et al. Associations between life’s essential 8 and arthritis among adults in United States: a national-wide longitudinal study. BMC Public Health 25, 1147 (2025). https://doi.org/10.1186/s12889-025-22330-1