BMC Pulmonary Medicine volume 25, Article number: 268 (2025) Cite this article
The impact of insulin resistance (IR) on chronic obstructive pulmonary disease (COPD) has caught increasing attention, and the triglyceride-glucose (TyG) index and related indices are deemed reliable indicators for evaluating IR. Nevertheless, the potential associations of TyG and obesity-related indexes with COPD are currently understudied. Hence, this paper was to inspect the links of TyG and obesity-related indices with COPD.
This was a cross-sectional study based on data from the NHANES 2013–2018. Weighted logistic regression (WLR), restricted cubic sample (RCS), and receiver operating characteristic (ROC) curves were leveraged to examine the links of the TyG index and obesity indices with COPD. The stability of the correlations was also assessed via subgroup analyses.
Data from 6383 participants were finally included, including 583 patients with COPD. WLR discovered positive associations of TyG, TyG-body mass index (TyG-BMI), TyG-waist circumference (TyG-WC), and TyG-waist height ratio (TyG-WHtR) with COPD regardless of covariate adjustment (p-value < 0.05, p for trend test < 0.05). After adjusting for all confounders, RCS analysis signaled notable linear links of TyG and obesity-related indices with COPD (p-value < 0.05, p for nonlinear > 0.05). TyG-WHtR showed the highest association with COPD among the indices tested, albeit with limited discriminative ability (AUC = 0.643, 95% CI: 0.619 ~ 0.665). Subgroup analyses further validated the stability and reliability of the results.
TyG and its combination with obesity-related indicators are associated with COPD. Among these, TyG-WHtR showed the strongest association with COPD, although causal relationships cannot be inferred and its discriminative ability remained modest. Further prospective studies are warranted to validate these findings.
Not applicable.
Chronic obstructive pulmonary disease (COPD), a prevalent chronic respiratory disease featured by persistent airflow limitation, is currently the third leading contributor to death worldwide [1]. The Global Burden of Disease survey predicts that COPD cases will increase to 592 million by 2050, a relative increase of 23% [2], posing an enormous challenge to public health worldwide. According to recent estimates, the global prevalence of COPD among adults aged over 40 years is approximately 10% [3], while in the United States, the prevalence among adults aged 18 years and older was about 6.2% during the 2017–2020 NHANES survey [4]. Thereby, identifying variables associated with COPD is essential for hypothesis generation and for guiding future longitudinal studies aimed at disease prevention.
Insulin resistance (IR) is featured by the reduced effectiveness of insulin in promoting glucose uptake and utilization. Previous articles have elicited a higher risk of metabolic syndrome in COPD patients [5, 6]. Another study shows higher IR in non-hypoxemic COPD patients than in healthy subjects [7]. A prospective cohort analysis in Korea shows that increased IR is related to decreased FEV1%-predicted and FVC%-predicted [8]. These observations imply the link of IR with COPD and the deterioration of lung function. The triglyceride-glucose (TyG) index, calculated from fasting triglyceride and blood glucose levels, is simple and reliable for assessing IR [9]. Previous reports have clarified the links of the TyG index with high risk and poor outcomes of metabolism-related diseases, like coronary heart disease, ischemic stroke, hypertension, and non-alcoholic fatty liver disease [10,11,12,13,14]. Recent evidence has illustrated that TyG combined with obesity-related indices, like TyG-body mass index (TyG-BMI), TyG-waist circumference (TyG-WC), and TyG-waist-to-height ratio (TyG-WHtR) may be superior to a single TyG index, more comprehensively assessing metabolic risk [15,16,17,18,19]. Nevertheless, the link between TyG index and obesity-related indices with COPD has not been clarified.
Hence, the present paper was based on data from the National Health and Nutrition Examination Survey (NHANES) database to explore the cross-sectional associations between TyG-related indices and COPD, providing a foundation for hypothesis generation and guiding future longitudinal studies.
This was a cross-sectional study based on the NHANES database, which is designed to estimate the nutritional health of Americans using stratified multistage sampling to obtain large, nationally representative samples with data on demographics, diets, physical examinations, laboratories, and questionnaires. This article included participants from NHANES 2013–2018, and the populations were excluded for (1) age < 20 years; (2) missing data on major key variables such as fasting glucose, triglycerides, weight, height, and waist circumference; (3) missing data on categorical covariates such as diabetes mellitus and hypertension; and (4) missing data on outcome variables. Ultimately, 6383 participants were included (Figure S1). For more detailed information about the NHANES database, please visit (https://www.cdc.gov/nchs/nhanes/).
COPD was determined by self-reported physician diagnosis during interviews using a standardized medical questionnaire. In the NHANES questionnaires (dataset of medical conditions), participants were defined as having COPD when they responded affirmatively to any of the following questions, “Has a doctor or other health professional ever told you that you have COPD/emphysema/chronic bronchitis?”; otherwise, they were defined as non-COPD patients. This method was utilized in multiple previous studies [20,21,22,23,24,25].
The primary independent variables encompassed TyG, TyG-BMI, TyG-WC, and TyG-WHtR. Baseline fasting glucose and triglyceride data were extracted from the laboratory data file of NHANES; while weight, height, and waist circumference data were harvested from the examination data file. The relevant indicators were computed as follows [15, 26, 27]:
Demographic data were acquired from the demographic data file, including age, gender, race, education, and marital status. Marital status was categorized into “Married” and “Other.” The “Other” group included individuals who were widowed, divorced, separated, never married, living with a partner, refused to answer, responded “don’t know,” or had missing data. Smoking was categorized into never smoking (< 100 cigarettes in your lifetime), current smoking (≥ 100 cigarettes in your lifetime and still smoking), and former smoking (≥ 100 cigarettes in your lifetime and quit smoking) according to the smoking questionnaire [23, 28]. Laboratory indicators such as serum alanine aminotransferase (ALT), serum creatinine (CR), serum uric acid (UA), serum potassium (K), and serum sodium (Na) were obtained directly from the laboratory data file [19]. Hypertension was diagnosed based on a self-reported physician diagnosis of hypertension or a mean of systolic blood pressure from three consecutive measurements ≥ 130 mmHg or a mean of diastolic blood pressure from three consecutive measurements ≥ 80 mmHg [25, 29]. Diabetes mellitus was diagnosed based on self-reported physician diagnosis of diabetes mellitus or fasting blood glucose ≥ 126 mg/dL [28, 29]. Information on congestive heart failure was obtained by self-report on a health questionnaire in which participants were asked, “Has a doctor or other health professional ever told you that you have congestive heart failure (CHF)?”. Those who answered “yes” were considered to have CHF. Coronary atherosclerotic heart disease, myocardial infarction, angina pectoris, malignant neoplasm, vigorous recreational activity, and moderate recreational activity were also defined according to the questions in the corresponding questionnaires [15, 30]. Additional NHANES variable codes and details of their use are listed in Table S1. More details on variables are available on the website (https://www.cdc.gov/nchs/nhanes/index.htm).
To account for the complex multistage sampling design of NHANES, we applied appropriate sample weights to ensure result validity. Specifically, MEC sample weights (WTMEC2YR) were used and divided by 3 to adjust for the 6-year combined survey cycle (2013–2018), following the official NHANES analytic guidelines (https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx).
The Kolmogorov-Smirnov test was adopted to check the normal distribution of continuous variables. Continuous variables were abnormally distributed in this paper, described as the median (P25, P75), and compared utilizing the Kruskal-Wallis test. Categorical variables were depicted as frequencies and proportions and analyzed utilizing the chi-square or Fisher’s exact test. Variables with > 20% missing data were excluded. For continuous variables with < 20% missing data, multiple interpolation was performed using the mice package. Outliers were dealt with using winsorization, with thresholds of 1% and 99%. Variables with covariance ≥ 5 were excluded using multicollinearity analysis. Weighted logistic regression (WLR) analysis was leveraged to assess the links of TyG and its combination with obesity-related indicators with COPD, with correlation results expressed as ORs and 95% confidence intervals (CIs). Between-group linear trends were assessed using quartiles of categorical variables as continuous variables. Model 1 was not adjusted for covariates; Model 2 included sex, age, race, education level, and marital status; Model 3 further added smoking status, laboratory indicators (ALT, CR, UA, K, Na), comorbidities (hypertension, diabetes mellitus, coronary atherosclerotic heart disease, myocardial infarction, angina pectoris, malignant tumors), and physical activity (moderate recreational activity, strenuous recreational activity). Restricted cubic spline (RCS) was employed to examine the dose-response links of TyG and obesity-related markers with COPD. Subgroup analyses were implemented to determine the associations in different populations stratified by age, gender, smoking, hypertension, and diabetes. Multiplicative interaction analysis was adopted to test differences between subgroups. All analyses were done in R statistical software 4.4.1. A two-sided p-value < 0.05 was deemed statistically significant.
Table 1 and Table S2 display the baseline traits of 6383 participants plotted based on quartiles of independent variables. The median age was 48 years, 3,093 (49%) were male, 1,622 (31%) graduated with a bachelor’s degree or higher, 3,924 (65%) were married, 3,569 (55%) never smoked, and 583 (8.4%), 1,290 (15.0%), and 3,511 (50%) suffered from COPD, diabetes mellitus, and hypertension, respectively; 1,452 (26%) and 2,652 (46%) participants accepted strenuous recreational activities and moderate recreational activities, respectively. Regardless of which indicator was used to categorize the group, the Q4 group with high TyG and obesity-related indicators had higher percentages of married participants and previous smokers, consistently showed higher prevalence of COPD, hypertension, diabetes mellitus, and CHF, had higher age, weight, BMI, waist circumference, fasting glucose, triglyceride, ALT, UA, and K levels, and lower educational and physical activity levels.
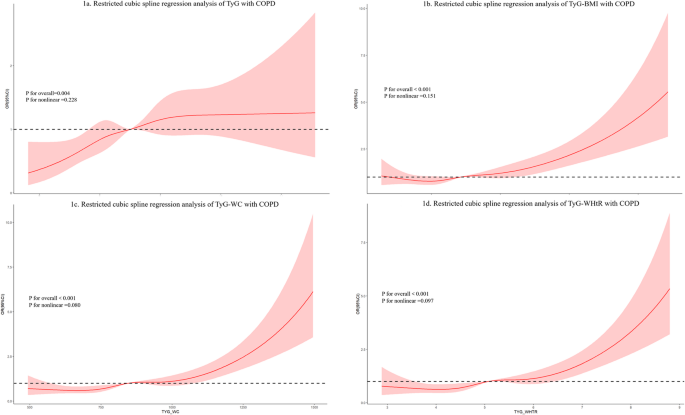
In Table 2, WLR analyses showed that when primary independent variables were considered continuous variables, there were independent positive correlations of TyG, TyG-WC, TyG-BMI, and TyG-WHtR with COPD regardless of whether the covariates were adjusted or not, in which the ORs for TyG and TyG-WHtR were higher at 1.348 (95% CI: 1.076–1.687) and 1.321 (95% CI: 1.135–1.538). In contrast, when they were considered categorical variables, all indicators were greatly and positively linked to COPD in the Q4 group relative to the Q1 group (p-value < 0.05, p-value for trend test < 0.05), regardless of covariate adjustment. After adjusting for all confounders, TyG-WC showed a more significant link to COPD (OR = 2.088, 95% CI: 1.399–3.116). RCS analysis signaled statistically notable linear links of these indexes with COPD after adjusting for all confounders (p-value < 0.05, P for nonlinear > 0.05) (Fig. 1).
Restricted cubic spline (RCS) plots showing the association between TyG-related indices and COPD. The black dashed line represents the reference value (OR = 1.00), the red solid line indicates the estimated odds ratio (OR), and the red shaded area indicates the 95% confidence interval (CI). The logistic regression models were adjusted for sex, age, race, education level, marital status, smoking status, laboratory indicators (alanine aminotransferase [ALT], creatinine, uric acid, potassium, sodium), comorbidities (hypertension, diabetes mellitus, coronary atherosclerotic heart disease, myocardial infarction, angina pectoris, malignant tumors), and physical activity (moderate and vigorous recreational activities)
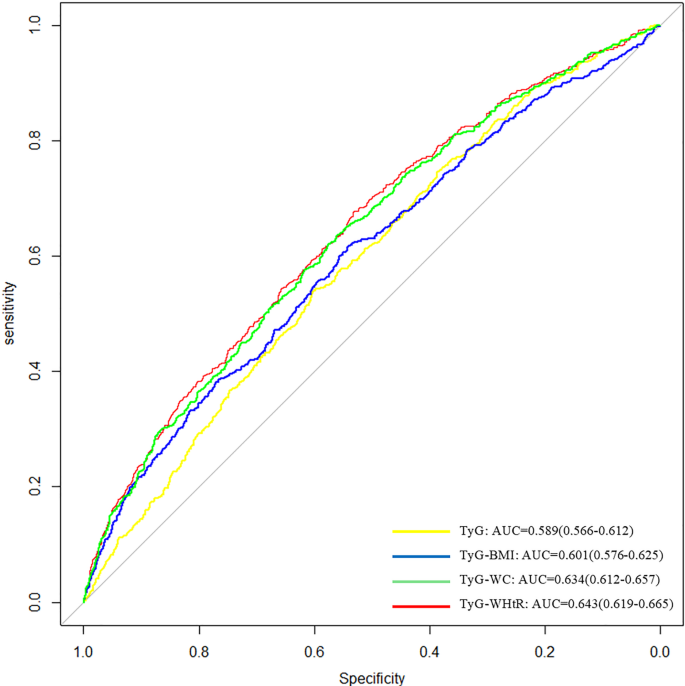
ROC curves showed that TyG-related indices had relatively higher AUCs compared to the TyG index alone, with TyG-WHtR yielding the highest value (AUC = 0.643). Nevertheless, the overall predictive performance remained limited. (Fig. 2).
Subgroup analyses are presented in forest plots (Figure S2), which visually illustrate the associations across various subgroups. No significant interactions were observed in the remaining subgroups, except for interactions observed in TyG-BMI with age and TyG-WHtR with hypertension and diabetes. More prominent links of these indexes with COPD were observed in the age < 60 years, current smoking, and no diabetes subgroups.
This cross-sectional paper explored the associations of TyG and its combination with obesity-related indices (TyG-BMI, TyG-WC, TyG-WHtR) with COPD. By analyzing 6,383 US adults from the NHANES database, our analyses uncovered that higher levels of these indexes were positively connected with COPD risk. RCS analyses disclosed linear positive correlations between these indices and COPD. TyG-WHtR had the highest AUC among the indices tested (AUC = 0.643); however, all indices demonstrated relatively poor discriminative performance (AUC < 0.7), indicating limited predictive utility. Our findings provide new evidence that these indices are associated with COPD prevalence; however, due to the cross-sectional nature of the study, predictive or causal inferences cannot be drawn.
The TyG index is a simple, feasible, and relatively inexpensive marker of IR. Recent studies have stated that TyG-related indices obtained by combining obesity indicators, such as TyG-BMI, TyG-WC, and TyG-WHtR, can visibly improve the validity of the TyG index for assessing IR [17, 31, 32]. Previous investigations on TyG and its related indexes have focused on metabolism-related diseases, like diabetes [33, 34], cardiovascular diseases [14, 35], ischemic stroke [36, 37], and nonalcoholic fatty liver [38,39,40]. The TyG index has also been more frequently introduced into the study of respiratory diseases in the context of growing interest in the effects of IR and metabolic disorders on respiratory diseases. Wu et al. found that a higher TyG index was related to an elevated risk of respiratory symptoms, including coughing, phlegm production, exertional dyspnea, wheezing, and chronic bronchitis [24]. Meng et al. evinced that TyG-BMI was positively connected with obstructive sleep apnea [41]. Staggers et al. constructed a 5-year retrospective cohort and unraveled that those with an augmented TyG had a 6% higher risk of severe asthma exacerbation (95% CI: 3-10%), suggesting TyG index as a risk predictor for severe asthma attacks [42].
Given the potential associations of IR with COPD and lung function deterioration, research is also now focusing on the application of TyG index in COPD. Zhou et al. manifested that TyG index was a potential predictor of all-cause mortality in critically ill COPD patients and may be useful for risk stratification and prognosis prediction [43]. Recent research has also used it as a potential risk marker for future COPD in the general cohort. A Swedish prospective study with a mean follow-up of 31.4 years illustrated that the risk of COPD was increased by 21% in high-TyG cohorts, suggesting that TyG index was a valid predictor of COPD [44]. While these prior studies were based on prospective designs, it is important to note that our study employed a cross-sectional approach, thus limiting interpretations to associations rather than predictions. Similarly, our study uncovered that TyG index was positively linked to COPD risk. RCS analysis proved linear connections between them. However, they only explored the link between TyG index and COPD. We introduced obesity-related indexes and found positive correlations of TyG-BMI, TyG-WC, and TyG-WHtR with COPD. RCS analysis also unveiled linear associations between these indexes and COPD. Overall, our study revealed cross-sectional associations between the TyG index and COPD and highlighted the potential contribution of obesity-related indicators. However, causality cannot be inferred, and further longitudinal studies are warranted to validate these findings.
ROC curve analysis revealed that TyG and obesity indexes had better predictive ability than a single TyG index, consistent with previous studies [15, 18, 39, 45]. Notably, our results demonstrated that TyG-WHtR had the highest AUC value among the indices (AUC = 0.643). Nonetheless, given that this value falls below the commonly accepted threshold for good discrimination (AUC < 0.7), its predictive capability remains limited. This finding was also supported by previous studies that compared the predictive accuracy of TyG and its derived metrics in different populations. A study pointed out that TyG-WHtR had the greatest predictive power in forecasting cardiovascular mortality and myocardial infarction [15]. Another study manifested that TyG-WHtR had the highest AUC for detecting fatty liver in a diabetes subgroup [46]. In addition, a prospective study in Chinese middle-aged and elderly populations with long-term follow-up found that TyG-WHtR was better than other TyG-related indices in predicting early diabetes in the subgroup with normal fasting glucose [45]. WHtR, an obesity-related indicator that considers waist circumference and height, identifies individuals with low body weight and ectopic fat accumulation, and is considered a favorable indicator for assessing visceral obesity [47]. Visceral obesity is superior to general obesity indicators as a critical risk factor for metabolic diseases. Therefore, TyG-WHtR showed better predictive ability.
Many articles have focused on the potential mechanisms of metabolic factors in COPD. IR may be associated with decreased lung function. A large prospective cohort study in Korea noted that for each unit increase in log-transformed HOMA-IR, the β-values of FEV1%-predicted and FVC%-predicted were − 0.23 and − 0.2, respectively [8]. Similarly, a longitudinal cohort study of US asthma patients showed markedly lower FEV1 and FVC values in patients with IR than those without IR [48]. The possible mechanism is that insulin mediates airway inflammation by activating airway immune cells and promoting the proliferation of airway smooth muscle cells, leading to the release of collagen, resulting in airway narrowing and airflow limitation [49]. TyG and obesity-related indices are reliable markers for IR, and their elevation suggests dyslipidemia and hyperglycemia. In addition to IR-associated decreased lung function, these metabolic abnormalities may also contribute to COPD through multiple pathological pathways. Under hyperglycemia, the increase of glycosylation end products, combined with the receptor for advanced glycosylation end products (AGEs), triggers inflammatory responses and oxidative stress, and the increased release of cytokines and ROS, thereby exacerbating airway inflammation [50, 51]. AGEs also activate the generation of pulmonary collagen and elastin through a non-receptor-dependent pathway, decreasing pulmonary elasticity and consequently affecting lung function [52]. Hyperglycemia may also disrupt normal immunity by suppressing the function of macrophages and dendritic cells, thereby increasing the risk of respiratory infections [53]. Dyslipidemia may provoke inflammation through excess circulating free fatty acids, leading to reduced lung function. Excessive circulating free fatty acids and hypertriglyceridemia may induce a lipotoxic state, promoting inflammatory responses and lung injury via activation of intracellular signaling and endoplasmic reticulum stress [54, 55]. On the other hand, surface active substances in lung tissues have a unique need for lipids, and lipid metabolism disorders may motivate airway hyperresponsiveness and collagen deposition, thus affecting pulmonary elastic recoil [56, 57]. Collectively, insulin resistance and associated metabolic dysfunction may contribute to COPD pathogenesis through several interrelated mechanisms. Chronic systemic inflammation and oxidative stress, commonly observed in insulin-resistant individuals, can lead to persistent activation of inflammatory pathways, including TNF-α and IL-6 signaling [58]. These inflammatory cascades are known to promote small airway fibrosis, epithelial cell apoptosis, and alveolar wall destruction, all of which are central pathological features of COPD [59]. Furthermore, metabolic dysregulation may impair lung tissue repair processes and exacerbate airway remodeling, thereby accelerating disease progression.
A previous article showcased that an augmented TyG index was connected with all-cause mortality in critically ill COPD patients, especially in younger individuals [43]. Our subgroup study also illuminated that TyG, TyG-WC, TyG-WHtR, and TyG-BMI were more prominently associated with COPD in the younger population. This difference may reflect age-related variability in metabolic indicators. However, the implications of these associations should be interpreted cautiously given the cross-sectional design. Subgroup analysis also uncovered that the correlation of these indexes with COPD appeared to be more significant in the non-diabetic population than in diabetic patients. Guo et al. [60] evinced that TyG did not show a correlation with COPD in diabetic populations, so did the studies of Zhou et al. [43] and Deng et al. [15]. This phenomenon may be related to the application of hypoglycemic drugs in diabetic patients, which regulate blood glucose and directly affect the TyG index. Nevertheless, these subgroup findings are exploratory in nature and should be interpreted with caution, given the cross-sectional design of our study and the reliance on self-reported diagnoses.
Notably, we observed stronger associations between TyG-related indices and COPD in the smoking population. This phenomenon may be attributed to the combined impact of smoking-induced oxidative stress and metabolic dysregulation. Recent transcriptomic and clinical studies have shown that cigarette smoke exposure leads to widespread immune dysfunction, increased oxidative DNA damage, and insulin resistance [61, 62]. These biological disturbances may amplify systemic inflammation and contribute to airway remodeling and lung tissue destruction, thereby increasing the susceptibility to COPD in smokers compared to non-smokers [59].
Our paper has several notable strengths. First, we utilized a large, nationally representative sample from the NHANES database, which enhances the generalizability and reliability of our findings. Covariate adjustments were implemented to minimize potential confounding. Second, this study comprehensively examined the individual and combined effects of TyG and obesity-related indices on COPD, addressing gaps in prior literature. Finally, the TyG index and its combination with obesity-related indicators are easy to obtain and may be useful for metabolic assessment in COPD-related research, providing insights for future risk models pending validation in longitudinal studies.
Nonetheless, some limitations warrant attention. First, COPD cases were identified based on self-reported physician diagnosis due to the inherent nature of the NHANES database, which may introduce recall bias and affect the accuracy of COPD classification. Self-reported COPD is known to have low sensitivity and may miss a substantial proportion of individuals with airflow limitation, leading to underestimation of COPD prevalence [63]. Conversely, overdiagnosis may also occur when respiratory symptoms are incorrectly attributed to COPD without objective verification. Such misclassification could bias the observed associations toward the null, potentially underestimating the true strength of the relationships between TyG-related indices and COPD [63]. Future studies employing spirometry-based diagnostic criteria are warranted to validate and refine these findings. Second, the cross-sectional design of the study limits our ability to infer temporal or causal relationships between insulin resistance (IR) and COPD. Future cohort studies are needed to establish temporality and causality. Third, due to limitations of the public database, certain variables and potential confounders were not available for inclusion in our analyses, which may affect the comprehensiveness of the study. These limitations highlight the need for further research to validate our findings and explore underlying mechanisms. Moreover, they underscore that the observed associations are correlational and not indicative of temporal or causal relationships.
TyG and obesity-related indicators demonstrated significant positive associations with COPD in this study. These findings provide preliminary evidence of associations between TyG, TyG-BMI, TyG-WC, and TyG-WHtR and COPD. Among these indices, TyG-WHtR showed the strongest association, although its discriminative capability was limited, and causal relationships remain to be determined in future longitudinal studies. Nevertheless, these results should be interpreted with caution due to the cross-sectional nature of the study, which precludes establishing causality. Additionally, the findings are based on a specific dataset and may not be generalizable to all populations. Future multicenter prospective studies are warranted to confirm these associations and elucidate the mechanisms linking TyG and obesity-related indices with COPD.
The NHANES datasets used in this study are publicly available from the Centers for Disease Control and Prevention (CDC) at https://www.cdc.gov/nchs/nhanes/index.htm. The code utilized for variable construction and statistical modeling during the current study is available from the corresponding author upon reasonable request.
- COPD:
-
Chronic obstructive pulmonary disease
- IR:
-
Insulin resistance
- TyG:
-
Triglyceride-glucose
- BMI:
-
Body mass index
- WC:
-
Waist circumference
- WHtR:
-
Waist height ratio
- TyG-BMI:
-
Triglyceride glucose-body mass index
- TyG-WC:
-
Triglyceride glucose-waist circumference
- TyG-WHtR:
-
Triglyceride glucose-waist height ratio
- NHANES:
-
National health and nutrition examination survey
- ALT:
-
Serum alanine aminotransferase
- CR:
-
Serum creatinine
- UA:
-
Serum uric acid
- K:
-
Serum potassium
- Na:
-
Serum sodium
- CHF:
-
Congestive heart failure
- WLR:
-
Weighted logistic regression
- Cis:
-
Confidence intervals
- RCS:
-
Restricted cubic spline
- ROC:
-
Receiver operating characteristic
- AUC:
-
Area under the curve
- AGEs:
-
Advanced glycosylation end products
Not applicable.
This work was supported by grants from Natural Science Foundation of Fujian province (No. 2023J011690), Medical and health guidance project of Xiamen science and technology bureau (No. 3502Z20224ZD1085).
The NHANES program was approved by the National Center for Health Statistics Ethics Review Board, and all participants provided written informed consent.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Below is the link to the electronic supplementary material.
: Table S1. NHANES variable codes and details of their use.
: Table S2. Baseline features stratified by quartiles of TyG-BMI, TyG-WC, and TyG-WHtR.
: Figure S1. Flowchart of participant selection.
: Figure S2. Forest plots illustrating subgroup analyses of the associations between TyG, TyG-BMI, TyG-WC, and TyG-WHtR and the prevalence of COPD across different population subgroups. Odds ratios and 95% confidence intervals were derived from fully adjusted logistic regression models (Model 3).
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Chen, J., Chen, A., Yang, S. et al. Association of triglyceride glucose and obesity indices with chronic obstructive pulmonary disease in US adults: data from 2013 to 2018 NHANES. BMC Pulm Med 25, 268 (2025). https://doi.org/10.1186/s12890-025-03738-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12890-025-03738-2