BMC Medical Informatics and Decision Making volume 25, Article number: 173 (2025) Cite this article
Polypharmacy can be both a public health and an economic issue. Medication reviews are structured interviews of the patient by the pharmacist, aiming at optimizing the drug treatment and deprescribing potentially inappropriate medications. However, they remain difficult to perform and time-consuming. Several clinical decision support systems were developed for helping clinicians to reduce inappropriate polypharmacy. However, most were limited to the implementation of clinical practice guidelines. In this work, our objective is to design an innovative clinical decision support system for medication reviews and polypharmacy management, named ABiMed.
ABiMed associates several approaches: guidelines implementation, but also the automatic extraction of patient data from the GP’s electronic health record and its transfer to the pharmacist, and the visual presentation of contextualized drug knowledge using visual analytics. We performed an ergonomic assessment and qualitative evaluations involving pharmacists and GPs during focus groups and workshops.
We describe the proposed architecture, which allows a collaborative multi-user usage. We present the various screens of ABiMed for entering or verifying patient data, for accessing drug knowledge (posology, adverse effects, interactions), for viewing STOPP/START rules and for suggesting modification to the treatment. Qualitative evaluations showed that health professionals were highly interested in our approach, associating the automatic guidelines execution with the visual presentation of drug knowledge.
The association of guidelines implementation with visual presentation of knowledge is a promising approach for managing polypharmacy. Future works will focus on the improvement and the evaluation of ABiMed.
Elderly often receive polypharmacy [1, 2], defined by French national health insurance as five drugs or more, prescribed for at least six months. Evidence shows it is a major problem in many countries, including Canada [3], Sweden [4] and France [5]. Inappropriate polypharmacy is both a public health, economic and ecological issue. It has been shown that each new drug administered in polypharmacy increases the risk of adverse events by 12–18% [6].
One solution for reducing inappropriate polypharmacy is medication review (MR), “a structured evaluation of a patient’s medicines with the aim of optimising medicines use and improving health outcomes [including] detecting drug-related problems and recommending interventions” [7]. In France, MR is carried out by the community pharmacist, in collaboration with the GP [8]: the pharmacist interviews the patient, assesses the treatment, and writes a synthesis with preconizations for the GP. Here, we will focus on “advanced” type 3 MR [7], which includes patient interview and requires clinical data. MR aims in particular at deprescribing inappropriate medications, such as drugs that are duplicated, no longer indicated (e.g., statins over 80 in primary prevention), or potentially dangerous for a given patient (e.g., contraindicated). Other drugs may see their dose changed, and drugs may also be added, e.g., to control adverse events. Clinical guidelines are available for MR, such as STOPP/START v3 [9].
Evidence shows that MR significantly reduces inappropriate polypharmacy [10] and emergency department (ED) visit [11], and can save 273 € per patient-year [12] without lowering the quality of care [10]. MR may also have a positive ecological impact, by reducing the consumption of drugs [13]. Health insurances pay pharmacists for performing MR in some countries, including France, Germany and Switzerland [14], some provinces of Canada [15], and in US with the Medicare Medication Therapy Management [16].
However, few pharmacists are engaged in MR, because of many barriers [17, 18]: poor motivation of the pharmacists, difficulties for communicating with patients and GPs, lack of time, lack of appropriate knowledge in geriatrics and/or lack of self-confidence, and limited remuneration. MR is a tedious task that requires to collect patient data [7], including drug orders but also clinical conditions that are often available only in the GP’s electronic health record (EHR) [19]. Pharmacists have to assess the interactions and adverse effects of 5–20 drugs, to identify inappropriate or missing drugs and to write the synthesis. Viewing the properties of 5–20 drugs is particularly tedious because drug databases have been designed to access the properties of a single drug at a time. Consequently, MR can take up to 2.5 h [18].
Clinical decision support systems (CDSSs) have been shown to be efficient in facilitating clinician work, increasing guidelines adherence, and improving healthcare [20]. CDSSs have been proposed for MR. In a literature review [21], we highlighted that most execute the rules found in guidelines, and sometimes automatically extract patient data from EHR. On the other hand, few CDSSs consist of the visual presentation of selected drug knowledge, e.g., for presenting the summed adverse effects of a drug order.
CDSSs based on the first, intelligent, approach include a knowledge base and an inference engine [22]. The knowledge base can be formalized in different ways, e.g., if/then rules or ontologies. The inference engine applies the rules to patient data and generates recommendations for clinicians. Recommendations can be provided in various ways, e.g., alerts or textual reports. For example, Medsafer [23] is an ontology-based system that goes beyond mere detection of potentially inappropriate drugs. It offers evidence-based strategies for deprescribing identified inappropriate drugs. N. A. Zwietering’s CDSS [24] implements the STOPP/START guidelines as if/then rules and generates alerts.
CDSSs based on the second, visual, approach rely on drug databases containing comprehensive drug information, e.g., adverse effects or interactions. The relevant drug knowledge can be displayed to clinicians, e.g., through graphs. It aims at providing efficient access to information through visual formats that synthesize complex information. For example, RXplore [25] focuses on adverse effects and presents the information graphically. Graphsaw [26] visualizes drug interactions and their associations with various entities, using a network-like structure.
Finally, a mixed approach combines both approaches. Few mixed approaches have been proposed [21]. For example, KALIS [27] integrates HTA guidelines and the Priscus inappropriate medication list, but also databases containing molecular and pharmacological information. KALIS integrates Graphsaw [26], offering both graphical representations and textual reports. The PRIMA-EDS system [28, 29] combines an inference engine with visual output to check for inappropriate drugs. It employs the PHARAO2 decision-support system as an inference engine, based on the EU(7) inappropriate medication list [30], as well as drug-oriented databases. The system presents the main adverse effects in tabular format, alongside detailed textual reports. It significantly improves the identification and management of inappropriate drugs.
In the ABiMed project [31], we aim at designing and evaluating a CDSS for helping pharmacist to perform MR and GPs to reduce inappropriate polypharmacy. ABiMed aims at going beyond state-of-the-art, by associating guidelines execution with visual approaches, and by supporting the communication between the pharmacist and the GP, including the transfer of patient data from the GP’s EHR to the pharmacist.
Most published papers on CDSSs for MR focused on evaluation [21], rather than describing the system design. On the contrary, the objective of this paper is to describe the ABiMed CDSS, including data exchange, ontological rule-based system, and original visual interfaces, and to focus on qualitative evaluations on the software aiming at testing how desirable are the functionalities we propose.
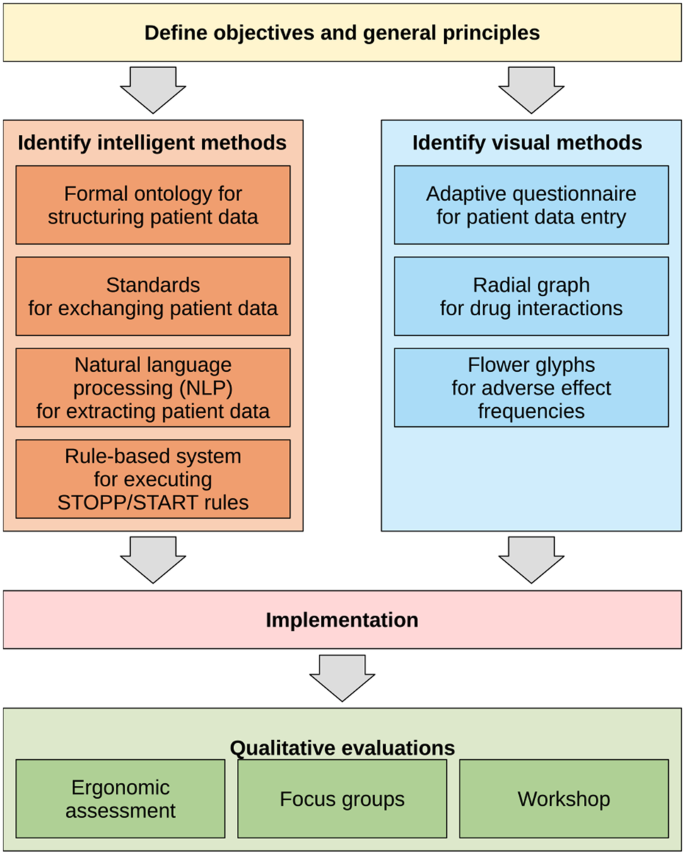
Figure 1 shows the main step of the methods followed for designing ABiMed.
The first general principle is to associate in the same CDSS an intelligent approach, implementing STOPP/START v3 rules, with a visual approach, consisting of the visual presentation of contextualized drug knowledge, adapted to the patient profile and treatment.
The second principle is to provide automatic patient data extraction, to prevent tedious data entry. Extraction is based on the reimbursement data of the French health insurance or the GP’s EHR, when the EHR software editor integrated support for ABiMed. An EHR editor, EIG Santé, is a partner of the project and its EHR, éO, will be used to demonstrate the feasibility of this approach and test whether it is accepted by GPs (who may be reluctant to share patient data with pharmacists).
The third principle is to display knowledge and recommendations on either a single drug treatment, but also on two drug treatments (i.e., current treatment vs post-MR, which we call the comparative mode). Indeed, most tools related to drug knowledge work at the drug level, or at the drug order level (for drug interactions). But the drug level is not appropriated for MR: when a patient takes 5 + drugs, it is too long for clinicians to read the 5 + corresponding drug pages. The drug order level is more convenient. However, when suggesting modifications to the treatment, it does not permit comparing the before-after MR treatments. For example, one may replace a drug involved in a serious interaction by another drug, involved in even more serious interactions.
The fourth principle is to permit a cooperative use of the CDSS, allowing the pharmacist and the GP to use it simultaneously and to exchange about the patient. This may turn MR as a more collaborative task, increasing the involvement of the GP.
Ontologies for structuring patient data
We previously translated the Observational Medical Outcomes Partnership - Common Data Model (OMOP-CDM), used to structure EHRs, into an OWL ontology [32]. It serves as the basis of the patient model in ABiMed, and facilitates the management of hierarchical relations in medical terminologies. The following terminologies were associated: ICD10 (International Classification of Disease, release 10), ATC (Anatomical Therapeutical Chemical classification of drugs), LOINC (Logical Observation Identifiers Names & Codes), and MedDRA (Medical Dictionary for Regulatory Activities).
Then, the ontology was enriched for polypharmacy management. Patient data was divided in 6 categories (OMOP-CDM providing the first three ones): (1) current drug treatment, including posologies and indications, (2) clinical conditions of the patient, (3) laboratory tests and exam results, (4) drug-related problems identified, either during the patient interview (e.g., a poor patient observance with regard to a given drug) or at the treatment analysis (without the patient, e.g., drug-drug interactions), (5) preconizations issued at the end of the MR (e.g., deprescription of a drug), and (6) chat messages exchanged by the clinicians (i.e., GPs and pharmacists).
Standards for exchanging patient data
We worked with the patient EHR system éO.Footnote 1 However, our aim is to be compatible with existing data flows and to encourage software publishers to endorse our approach. Thus, we used existing, standard and widely spread file formats for exchanging patient data. As the project takes place in France, we followed recommendations from French agencies, especially the CI-SIS specifications.Footnote 2 The standard we selected may not be the most recent ones, but are the most used today in France. Following these guidelines, ABiMed API uses JSON (ISO 21778) as an interchange format, as it is an open standard file format widely used for data interchange. Numerous tools are available to manage this file format, facilitating the integration of ABiMed API.
éO provides two categories of data: the data present in the EHR itself (including coded and free-text medical data), and the data éO extracts from reimbursement files from French social security (consisting in all drugs reimbursed for the patient, whatever the prescriber is), available from HRIFootnote 3 (Historique des Remboursements Intégrés). It is available in an XML format. It allows getting complementary information concerning the drugs taken by the patient, including those not prescribed by the GP but by other physicians, e.g., specialists.
Then, éO exports data for ABiMed using the VSM file formatFootnote 4 (Volet de Synthèse Médicale), based on the HL7 CDA R2 file format.Footnote 5 However, we had to make a few modifications to this format. We anonymised data by removing information such as patient and GP names, etc.
Natural language processing for extracting patient data from free text
Automatic extraction of patient data from text is performed using the Multi-Terminological Concept Extractor (MTCE) [33, 34]. This semantic annotator has been developed by the Department of Digital Health from the University Hospital of Rouen (France). It enables the annotation of texts using terminological and/or ontological concepts from the Healthcare Ontology and Terminology Portal (HeTOP) [35]. A large number of NLP (Natural Language Processing) tasks are involved (e.g., phrase and word detection, normalization, etc.).
For ABiMed, the primary purpose is to identify patients’ clinical characteristics and lab test results, as required for executing STOPP/START v3 rules. New functionalities were added to MTCE: the recognition of conditional and family history information, and negation support. In fact, many clinical data in consultation texts appear in negative form. Specific patterns were also designed to retrieves and extract measures (mostly numerical), e.g., lab test results. The aim was to enable MTCE to produce annotations in the form of (concept, value) pairs, where concept is a LOINC code and value is the numerical value associated, e.g., (8462-4, 95 mmHg), 8462–4 being the LOINC code for diastolic blood pressure.
Finally, MTCE’s internal algorithms were updated to improve recall. In ABiMed, MTCE is used with terminologies that are poorly adapted to information retrieval due to their complex labels, which are unlikely to appear in the texts (e.g., “Essential (primary) hypertension” in ICD10). To overcome this difficulty, it was made possible to exploit HeTOP’s inter-terminological semantic network by internally using more generic terminologies such as the controlled vocabulary thesaurus Medical Subject Headings (MeSH). The underlying idea is that MTCE matches the query not only with the terminologies required in the ABiMed project, but also with MeSH concepts. The MeSH concepts obtained are then transcoded into the required terminologies using exact match relations from HeTOP’s semantic network.
Rule-based system for executing STOPP/START
The integration of the STOPP/START v3 rules [9] involved the formalization and the validation of the rules through expert consensus. For more details, please refer to [36].
First, the STOPP/START v3 guidelines were analyzed. They include 191 rules in narrative text format. We identified the necessary logical, clinical, and attribute elements for detecting potentially inappropriate medications (PIMs). STOPP rules determine the potential inappropriateness of a prescription based on the presence or absence of specific clinical and therapeutic elements, while START rules indicate when a recommended prescription is absent from the current drug order and needs to be added. At the end of this step, we developed a formal rule model that supports all the logical, clinical, and attribute elements we identified. The rule model relies on the OMOP-CDM-based ontology model described previously. The general rule format is:
$$\begin{aligned} & if\,\, {E_1} \wedge {E_2} \wedge \ldots \wedge \left( {{U_1} \vee {U_2} \vee \ldots} \right) \wedge \left( {\ldots} \right) \wedge \neg {N_1} \wedge \neg {N_2} \wedge \ldots \\ & then\,\, (stop\,\, or\,\, start)\,\, prescription\,\, P \end{aligned}$$
(1)
where Ei, Ui and Ni are elements (i.e., clinical conditions, drug prescriptions or lab test results) and P is a drug prescription.
Second, the STOPP/START v3 rules were formalized using that model. For each rule, this was carried out in three sub-steps: (a) The declaration of the clinical elements necessary for expressing the rule: prescriptions, clinical conditions, and lab test results. Each element is associated with one or more codes in the corresponding terminology (ATC, ICD10 or LOINC, respectively) and can be completed by a set of attributes (e.g., indication or dose, for prescriptions), based on Huibers et al. [37]. (b) The writing of the rule logic, using the above rule format. (c) The writing of the rule alert text. French translation was based on Lang et al. [38]. Additionally, comments were added to a rule when the execution of the rule cannot be fully automatized. The initial draft of the formalized rules was written by three researchers in medical informatics that also hold a Pharm degree (AM, R Léguillon, JBL).
Third, the formalized rules were validated through expert reviews. Experts with diverse backgrounds were involved in the review: GP (HF), pharmacist (S Dubois), and geriatrician (JB).
Fourth, the structured rules were automatically translated into SPARQL queries by a Python program. Queries were then executed by the SPARQL engine in Owlready [39].
All STOPP/START v3 rules were considered, with the exception of the first three STOPP rules (A1, A2, and A3), which are too general and lack of specificity in terms of drugs.
Adaptive questionnaire for facilitating patient data entry
Automatic patient data extraction from EHR is not always possible (e.g., when the GP refuses, or the patient opposes), and the extracted data may contain errors and missing elements. In all these situations, manual patient data entry remains necessary, allowing the pharmacist to verify the data and complement it if needed. In ABiMed, we designed a questionnaire targeting the 108 clinical conditions considered in STOPP/START v3 rules. However, a 108-item questionnaire would be too tedious to fill. Thus, we developed an adaptive questionnaire that displays only the items strictly mandatory for executing STOPP/START rules for the current patient [40].
For example, rule STOPP J3 recommends to “Stop non-selective beta-blockers in diabetes mellitus with frequent hypoglycaemic episodes”. There are 3 conditions; one drug: a non-selective beta-blocker, and two clinical conditions: diabetes mellitus and hypoglycaemic episodes, related by a logical AND operator. In the questionnaire, diabetes and hypoglycaemia are not shown if the patient does not take a non-selective beta-blocker. If he/she does, only diabetes is shown in the questionnaire. If diabetes is checked, then hypoglycaemia is shown. Consequently, the questionnaire evolves with the patient data. We showed that this approach reduces the length of the questionnaire by about two thirds [40].
Radial graph visualization for presenting drug interactions
Drug-drug interactions can be modeled as an undirected labeled graph, each drug being a node and each drug-drug interaction being an edge between two nodes. Drug-disease interactions can be simply modeled as a label on the drug’s node. Many methods have been proposed for graph visualization [41]. We considered a radial graph disposition, in which the nodes, representing drugs, are organized on a circle [42]. Then, node and edge colors are used to represent drug-disease and drug-drug interactions and their associated level of gravity.
Flower glyphs and bar charts for presenting adverse effects
Adverse effects were described by: (1) nature (a Preferred Term from MedDRA), (2) frequency (as extracted from the SPCs, on a 5-level scale, very rare: 0.001–0.01%, rare: 0.01–0.1%, uncommon: 0.1–1%, frequent: 1–10%, very frequent: >10%), (3) seriousness (boolean, based on a list of serious MedDRA terms published by EMA, European Medical AgencyFootnote 6), (4) importance for the elderly (boolean, based on a list published by the French academy of medicineFootnote 7).
We designed two types of views for adverse effects. The first is an overview aggregating adverse effects in general anatomical categories. In a previous work [43], we designed flower glyphs for the visualization of adverse effect profiles extracted from clinical trial results. Flower glyphs are similar to bar charts, but bars are displayed circularly like the petals of a flower. Our glyph has 12 petals, corresponding to 12 general anatomical categories, plus a central region for a 13th category, unclassified effects (e.g., fatigue). Each petal and region has an inner, darker, region proportional to the frequency of serious adverse effects. Figure 2 shows a flower glyph example and describes the categories. Each category is associated with a specific color and direction, chosen to facilitate memorization. We adapted these flower glyphs to the visualization of the adverse effects described in the SPCs, for either a single drug or all drugs in the treatment. For each category, we summed up the frequencies of each drug.
The second view consists of horizontal bar charts. Bars use the same colors as flower glyphs. Various bar chart views are proposed (see results section).
We implemented the CDSS as a web application, in Python (for server) and Brython (a Javascript-compiled version of Python, for client). We used WebSockets for client-server communication, permitting the server to alert the client when patient data have been modified by another user. This allows several clinicians to collaboratively use the CDSS for the same patient at the same time, in the same spirit as what online office suites propose.
Ergonomic assessment
The interface of ABiMed was the subject of an ergonomic assessment, independently by two researchers (RT and JBL). We used two sets of criteria: the original set by JMC Bastien and DL Scapin [44], and those proposed by P Luzzardi et al. [45] for information visualization techniques. Problem severity was ranked on a five-value scale (very minor, minor, average, major, very major).
Focus groups on prototype
During two focus groups sessions, mixing GPs and pharmacists, we collected feedback on a first prototype of ABiMed. A session was organized in a rural area (the region of Bray and Bresle, in Normandy, France) and the other in an urban area (the 13th district of Paris). Clinicians were recruited on a voluntary basis, in the geographic regions mentioned above, but without any criteria related to their actual practice of medication reviews. During these two sessions, the initial ABiMed prototype was presented and a clinical case was analyzed collectively using ABiMed. The prototype and its interfaces were presented, and participants’ opinions were collected. The focus groups were recorded and the recordings were transcribed verbatim. Two team members, S Dubois and HF, analyzed the verbatim separately, and then jointly developed the synthesis by consensus. They aimed at staying as close as possible to the verbatim data, without interpretation, based on their previous expertise in focus group data analysis, and following the method known as qualitative description [46].
Workshop with GPs
We organized a workshop during the French Congress of General Medicine. Participants were mostly GPs, and the participation was anonymous. ABiMed was presented during the workshop, and then the participants were divided in small groups and asked to use ABiMed themselves, for analyzing a clinical case. Finally, they were asked to complete a qualitative evaluation questionnaire, developed specifically for the study (and available in supplementary file #2). The objective of the questionnaire was to assess participants’ satisfaction with the assistance provided by ABiMed after initial use on simulated cases. It included questions about the overall motivation for using ABiMed in their daily practice, on a scale ranging from 1 to 10, and the opinion about the usefulness and the presentation of the 4 main tabs of ABiMed (posology, adverse effects, interactions, STOPP/START rules), each expressed on a 5-level qualitative scale. Thus, the structure of the questionnaire was simple and followed the structure of ABiMed itself. The questionnaire was printed and was self-administered by participants in the last 10 min of the workshop.
Figure 3 shows the architecture of ABiMed. It is a client-server application. The ABiMed server is in relation with web browser clients, with a drug database (Thériaque), with an ontology quadstore that stores the data and execute STOPP/START rules, and with the éO EHR server. The éO server is connected to MTCE.
The CDSS interface includes 9 thematic tabs. A checkbox at the top of the screen allows switching between the display of drugs as trademarks or as International Normalized Name (INN). An interactive tutorial is also proposed. The interface uses colors, but a color-blind friendly version is available, which uses shades of grays. See Supplementary file #1 for additional screenshots.
Patient data
This tab displays the patient data. It contains three lists: the list of prescribed drugs, the list of clinical conditions, and the list of lab tests and exam results. For each item, the list indicates its source: manual entry by the pharmacist or the GP, or automatic extraction from EHR, reimbursement files or textual report. Buttons are proposed for adding, modifying or removing items. They permit entering patient data from scratch, or correcting possible errors.
In the drug list, the indications are automatically identified, by relating the drugs to the clinical conditions, according to the indications in the Theriaque drug database. The clinician may correct indications. When there is no indication for a drug, a red label “Indication???” is shown, alerting on a potential drug without indication.
Interview questionnaire
This tab displays an interview questionnaire that should be filled by the clinician with the patient. The first part lists the problems encountered by the patient with his treatment. Five categories of problem are proposed: (1) suspected adverse drug event, (2) drug intake difficulty, (3) drug dependency, (4) poor observance, (5) other (free text). These problems identified at patient interview are not to be confounded with problems identified at drug treatment analysis, later (such as drug interactions, and usually detected by the pharmacist in the absence of the patient).
The second part is focused on the patient lifestyle. It includes checkboxes related to car driving and addictions (tobacco, alcohol, etc.).
The third part is focused on clinical conditions. It is partly redundant with the clinical conditions in the previous tab, however, only the conditions relevant for the execution of STOPP/START rules are displayed, using checkboxes. The checkboxes are organized in 13 anatomical categories (the same as those for presenting adverse effects). When a checkbox is checked, a drop-down combo list appears, allowing the selection of a more specific ICD10 term (e.g., after checking “diabetes”, one may choose “type 1 diabetes” or “type 2 diabetes”). This questionnaire is synchronized with the clinical conditions in the first tab. Moreover, it is adaptive: the checkboxes shown depend on the drugs taken by the patient and the clinical conditions previously entered.
Interactions
This tab displays drug-disease and drug-drug interactions using radial graph visualization (Fig. 4). Each drug is represented by a small colored circle, and all circles are organized in a large circle. Drug-disease interactions are represented by the color of the drug circle: red if there is a contraindication, orange if there is a caution for use (but no contraindication), and green otherwise. Drug-drug interactions are represented by arcs relating the two drugs involved; the color of the arcs depends on the severity of the interaction, with four possible levels. Several arcs are displayed if there is more than one interaction between two drugs. This visualization gives an overview of all interactions in the treatment. In particular, it permits identifying drugs involved in a serious interaction, but also drugs involved in many interactions of lower seriousness.
By default, the right part of the tab displays the list of the most important interactions, as text. When the clinician clicks on a drug circle or an arc, the corresponding detailed information is shown on the right, including recommendation for taking the interaction into account and information about the mechanism of action. Buttons are provided for obtaining full references and for adding the interaction as a particular problem.
In comparative mode, two interaction circles are shown, one for the pre-MR treatment and the other for the post-MR treatment. To facilitate comparison, all drugs are present in both circles (including added drugs on the first circle, and removed drugs on the second), however, drugs absent in a treatment are grayed out and their interactions are not shown.
Adverse effects
This tab displays the adverse effects of the drug treatment (Fig. 5). On the left panel, an overview of the adverse effect profile of the entire drug treatment is shown, as a flower glyph (see section Flower glyphs and bar charts for presenting adverse effects). Smaller, per-drug, flower glyphs are displayed below, showing the contribution of each drug to the global profile. Flower glyphs provide, at a glance, an idea of the general categories of the most frequent adverse effects, for both serious and non-serious effects. When the mouse is over a petal or a region, or after a text search, a bubble shows the corresponding effects in a bar chart series. A triangle is used to mark serious effects. When an adverse effect is clicked, a per-drug frequency breakdown is shown. The “Add problem” button can be used to add the selected adverse effect as a particular problem identified during treatment analysis.
Screenshot of the adverse effect tab. The user preconized to deprescribe escitalopram, thus we are here in comparative mode
The right part of the tab displays a summary of the most frequent and important effects, using four series of bar charts, showing: (1) the adverse effects suspected in the patient (corresponding to those entered in the problem list of the previous tab, if any), (2) the five most frequent adverse effects (considering both serious and non-serious effects; a higher number of effects may be shown in case of equal frequency), (3) the five most frequent serious effects, and (4) the 13 effects that are of particular importance for the elderly. All bar chart series are sorted in decreasing order of frequency.
In comparative mode (i.e., when the pharmacist preconized modifications to the drug treatment), bar chart series display two bars for each adverse effect, one for the pre-MR treatment and the other for the post-MR treatment. In addition, a second flower glyph is displayed, presenting the adverse effect profile of the drug post-MR treatment. When mouse-hovering a flower glyph, its shape is drawn above the other glyph, facilitating the identification of small differences. The name of the added and removed drugs are displayed in blue and in red strikethrough, respectively.
Posologies
This tab displays the posologies of all drugs, in a table. The columns are the following: (1) the name of the drug, (2) the current posology (i.e., pre-MR), (3) the official posology, as found in the SPCs and the Theriaque drug database, (4) the posology preconized by the pharmacist (i.e., post-MR); it can be edited directly in the table and defaults to the current posology, and (5) the computed day dose for each active principle in the drug.
In order to reduce the text, the official posologies shown in column (3) are filtered according to drug indication, drug association, patient age, renal failure (including the stage and/or renal clearance) and hepatic failure. ABiMed uses patterns to recognize simple posologies, such as “1 morning noon and evening”, “1 tablet every two days” or “1 in case of pain max 6 per day”. If the dose is over the maximum dose, the maximum dose is highlighted in orange to alert the clinician. Similarly, when the drug should be taken at a specific moment (e.g., evening) and it is not mentioned in the posology, that part of the official posology is highlighted. In addition, an “Add problem” button allows adding the suspected dose error to the list of problems identified during treatment analysis. These problems will be displayed later in the synthesis of the MR. Finally, whenever an active principle is present in more than one drug, its total dose is shown in the fifth column, in addition to the per-drug dose.
STOPP/START rule-based alerts
This tab shows the STOPP/START v3 rules that match the patient profile. STOPP rules are shown at the top, ordered by drug. Red/orange/green traffic signs are used to indicate the three types of rules, respectively: STOPP rules that are fully automatized, STOPP rules that are not fully automatized and thus require some intervention of the clinician, and START rules. In comparative mode, an additional column on the right shows the rule triggered by the post-MR treatment. Similar drugs before and after MR are aligned, to facilitate the reading. It allows verifying that, after substituting a drug by another one, the new drug does not trigger the same STOPP rule, nor any other ones.
Buttons are proposed for deprescribing drugs matching STOPP rules, and for prescribing drugs recommended by START rules. These buttons will update preconizations issued from MR (see next section).
MR preconizations
This tab allows the pharmacist to enter the preconizations issued from MR. Preconizations on the drug treatment can be entered by modifying the list of prescribed drugs. Six buttons are proposed to preconize (1) the prescription of a new drug, (2) the deprescription of a drug, (3) the modification of a drug posology, (4) the replacement of a drug by another, (5) any other change (e.g., biological surveillance) and (6) to cancel a previous preconization.
The drug list displayed in this tab behaves differently than the one present in the first tab: whenever it is modified, removed drugs are not removed from the list but displayed in red and strike through, and added drugs are displayed in blue.
Chat
This tab contains a chat, accessible only to the GP and the pharmacist. It enables an asynchronous text-based communication, specifically devoted to the management of the current patient.
Synthesis
This tab helps the pharmacist to write the synthesis destined to be sent to the GP. The synthesis includes pre-filled salutations at the beginning and the end, and its content alternates free-text fields and generated tables. Four tables are proposed: the current drug treatment, the problems identified (including problems found at patient interview, problems found at treatment analysis, and STOPP/START rules detected), the pharmaceutical interventions proposed, and the proposed treatment after MR. Each table is organized by drug. Problems and interventions can be prioritized on a four-level scale (-, +, ++, and +++). Finally, a green button allows validating the MR and sending it to the GP.
Interdependence between tabs
Figure 6 shows the dependencies between the tabs and the patient data categories, illustrating the complexity and interdependence of ABiMed. Excepted chat, there is no one-to-one mapping. Many tabs need various patient data categories, sometimes for very specific items, e.g., the posology tab needs lab test results to find renal clearance.
Dependencies between the various tabs of the CDSS and the categories of patient data. One way arrows indicate that the tab only reads the data. Two-way arrows indicate that it reads the data and can modify it (for the sake of presentation, the second arrow is shown next to the first one)
Whenever patient data is modified, the CDSS automatically updates the information of all tabs as needed, by extracting the appropriate drug information and executing STOPP/START rules again. Tabs having new contents are highlighted with a yellow star, to alert the clinician, e.g., prescribing a new drug in the MR preconizations tab will add a yellow star to the STOPP/START tab if the prescribed drug triggers any STOPP rule.
Additionally, the CDSS can be used cooperatively by multiple users. A pharmacist and a GP can display the same patient. They can exchange via the chat, and both can perform modifications (e.g., correct patient data or modify the post-MR treatment). Other users will be warned of the changes by the apparition of yellow stars.
Ergonomic assessment
Fifty ergonomic problems were identified (48 from the criteria of JMC Bastien and DL Scapin, and 2 from the criteria of P. Luzzardi et al.), including 11 very minor, 24 minor, 14 average, 1 major and 0 very major. 30 have been corrected, including 8 very minor, 18 minor, 3 average and 1 major. Many problems were: (1) of very low severity, e.g., the absence of handling of the “escape” key to close dialog boxes, (2) not directly related to the decision support activity, e.g., the absence of a “password lost” functionality, or (3) not problematic for performing evaluations on a limited number of patients, e.g., the impossibility to sort the patient list by date. Other problems would require important developments, e.g., adding an “undo” functionality when modifying the patient data.
Focus groups on prototype
The rural focus group involved 4 pharmacists (gender: 2 males, 2 females, age from 35 to 45 years) and 4 GPs (all males, age 32–66). The urban focus group involved 4 pharmacists (3 males, 1 females, age 45–56) and 4 GPs (2 males, 2 females, 36–49). All participants considered that ABiMed could be useful in practice and that the interfaces were satisfactory. One said: “It’s practical, and visual too!”. The provision of contextualized drug knowledge from different sources, as well as the care taken in their visualization, were appreciated. One participant said: “Interesting, in particular, to identify drugs which do not have major interactions, but which participate in a large number of interactions”. The participants thought they could use it in their practice to analyze a prescription or before prescribing a new medication. A pharmacist internship supervisor intended to use it as an educational tool, saying: “For everyday practice, it will be a good tool, even just to check something”. ABiMed was found to be a good basis for promoting exchanges between doctors and pharmacists, but possibly also with patients. A point of vigilance concerned the notifications of messages between the GP and the pharmacist, multiplying messages being at the risk of losing responsiveness.
Workshop with GPs
The workshop included 13 participants (only 12 completed the questionnaire; supplementary file #3 gives the data collected via the questionnaire). Regarding gender, 6 were males and 6 females. Seven were practicing GPs (seniority ranging from 1 to 36 years), one was a retired GP, and three were working in agencies (Health Insurance and French National Health Agency). Participants were enthusiastic with regard to the proposed system. The average score for motivation to use ABiMed in daily practice was 9.1 on a scale of 1 to 10 (Fig. 7; three GPs, including those working in agencies, did not reply).
Opinion of the GP participating in the workshop on their motivation to use ABiMed (0: no motivation at all, 10: maximum motivation; 1 participant did not return the questionnaire and 3 others did not respond to this particular question)
Figure 8 shows the results for the questions on the 4 main tabs, posology, adverse effects, interactions and STOPP/START rules. All tabs were judged useful, but the opinion about their presentation is slightly more mixed. Surprisingly, the tabs that use complex visual analytics (i.e., adverse effects and interactions) were not perceived as less understandable.
Opinion on the main tabs of ABiMed of the 12 GPs participating to the workshop and returning the questionnaire
In this paper, we described the design of ABiMed, an intelligent and visual decision support system for medication reviews and polypharmacy management. In our previous publication [31], we only presented the project’s objectives, without any description of the resulting interfaces or the qualitative evaluations. ABiMed implements the rules from the STOPP/START v3 guidelines, and proposes contextualized drug knowledge with a visual presentation. The system also permits a collaborative multi-user usage, similar to online office suites. ABiMed was evaluated qualitatively by pharmacists and GPs during two focus groups and a workshop. The results show that health professionals are interested in the proposed system, and that, despite the use of complex visual analytics, it remains understandable.
We worked with EIG Santé as industrial partner, which develops the éO software for physicians. While it might have been more obvious to work with an editor of pharmacy management software, the amount of clinical data available in such software is very limited. Therefore, we chose to work with a vendor of medical practice management software, allowing the extraction of clinical data from the GP’s database. We currently worked with a single vendor, in order to establish a proof-of-concept, before considering the integration of ABiMed with additional software from other vendors. However, convincing all vendors is expected to be challenging.
The main limits of ABiMed are the lack of quantitative performance evaluation and clinical use in practice. It also requires a high degree of collaboration between pharmacists and GPs, which may be difficult to achieve. Regarding the adverse effects tab, we summed the frequencies of all adverse effects for each drug, however, in practice, it can lead to high percentages and the frequencies may not be additive. Nevertheless, those summed frequencies, although imperfect, gives an indication of the risk of adverse events. We performed an ergonomic assessment of ABiMed using various criteria and a 5-level severity scale, however, Bastien and Scapin defined the criteria but not the severity level, thus the ranking of the severity was performed intuitively by the inspectors and is not supported by any guideline. Additionally, the results of the focus groups and workshop studies should be addressed carefully, due to the modest number of clinicians involved, the possible selection bias (as all clinicians were volunteers), and the lack of strict methodology in the focus group organization and the development of the qualitative questionnaire.
In the literature [21], most CDSSs for MR were focused on the implementation of guidelines. ABiMed also proposes that, but goes beyond state-of-the-art, with the addition of visual tools for presenting contextualized drug knowledge. Moreover, in the literature, CDSSs devoted to community pharmacists were not connected to EHRs, because the pharmacist has no direct access to GP’s EHR. Consequently, the collaboration we propose between pharmacists and GPs, based on the transfer of patient data from the GP to the pharmacist, is innovative. We also proposed a comparative mode, showing both the analyses of the pre-MR and the post-MR treatment.
The main perspective is the evaluation of ABiMed. We are currently conducting a performance evaluation with pharmacists on clinical cases, under controlled conditions, aimed at showing that ABiMed leads to better MR. We also work on the evaluation of the rule-based system on retrospective patient data. In the next step, we plan to evaluate ABiMed in a clinical trial with real patients, associating both pharmacists and GPs.
In future works, we would like to explore the use of argumentation [47] as a way to structure communication between the pharmacist and the GP. Actually, effective communication between the pharmacist and the GP is important for MR. As both share a common goal, i.e., improving the health of the patient, exchanging arguments may resolve most of the disagreements and facilitate MR. In this context, drug interactions, adverse effects, STOPP/START rules, but also patient preferences, can be considered in the process of argumentation for justifying pharmaceutical interventions. Arguments may even be extracted from natural language messages in the chat. Another perspective is the addition of a “life line”, i.e., a chronological view showing the patient clinical conditions and drug prescriptions on a temporal axis [48]. Such a view might improve the understanding of the patient history. However, it requires temporal data, which may be difficult to obtain, especially for the pharmacist (e.g., when extracting drug treatment from health insurance reimbursement, only the drug delivered in the last months are present, thus the initial prescription date cannot be obtained).
In conclusion, we proposed an intelligent and visual clinical decision support system for medication review and polypharmacy management. It relies on (1) the automatic extraction of patient data from the GP’s EHR and its transfer to the pharmacist, (2) the implementation of the STOPP/START rules, and (3) the presentation of contextualized drug knowledge using visual analytics. Qualitative evaluations showed that clinicians were highly interested. Future works will focus on the evaluation of the system and its improvements.
Data is provided within the manuscript or supplementary information files.
- ATC:
-
Anatomical therapeutical chemical classification of drugs
- CDSS:
-
Clinical decision support system
- HER:
-
Electronic health record
- GP:
-
General practitioner
- HeTOP:
-
Healthcare ontology and terminology portal
- ICD10:
-
Iinternational classification of disease, release 10
- INN:
-
International normalized name
- LOINC:
-
Logical observation identifiers names & codes
- MedDRA:
-
Medical dictionary for regulatory activities
- MeSH:
-
Medical subject headings
- MR:
-
Medication review
- MTCE:
-
Multi-terminological concept extractor
- OMOP-CDM:
-
Observational medical outcomes partnership - common data model
- PIMs:
-
Potentially inappropriate medications
Not applicable
This work was funded by the French Research Agency (ANR) through the ABiMed project [Grant No. ANR-20-CE19-0017]. The funding bodies played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
The present research did not need an ethics approval or written consent to participate, according to French national regulations, as confirmed by the ethics evaluation committee of lnserm, the lnstitutional Review Board (1R800003888, 1ORG0003254, FWA00005831) of the French lnstitute of medical research and Health.
Not applicable
Stefan Darmoni and its team sold the MTCE software (which is used in the present work) via the Alicante company.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Abdelmalek, M., Romain, L., Nada, B. et al. ABiMed: An intelligent and visual clinical decision support system for medication reviews and polypharmacy management. BMC Med Inform Decis Mak 25, 173 (2025). https://doi.org/10.1186/s12911-025-03002-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12911-025-03002-x