BMC Infectious Diseases volume 25, Article number: 783 (2025) Cite this article
Healthcare-associated infections (HAIs), which are associated with prolonged hospitalization and increased mortality, remain a global challenge. The COVID-19 pandemic paradoxically reduced some HAIs through enhanced hygiene measures but exacerbated others due to resource diversion, with effects after policy changes remaining unclear. Therefore, this study analyzed HAIs distribution, pathogenic microorganisms distribution, and antibiotic susceptibility capturing the period surrounding strict COVID-19 control measures in a southwest China tertiary hospital.
We conducted a retrospective study at a tertiary hospital in southwest China from 2019 to 2023. Data were extracted from real-time surveillance system, where HAIs were initially diagnosed by clinicians and subsequently verified by infection control personnel. The HAI incidence rate was calculated per 1000 hospital stays, and negative-binomial regression was used to compare incidence rates across years.
This study enrolled 2808 HAI cases, with 1665 males and 1143 females, averaging 61.37 years old. The incidence rates of HAIs from 2019 to 2023 were 1.75, 1.12, 0.98, 1.31, and 1.30 per 1000 hospital stays, respectively. Hematology (323, 11.50%), cardiology (309, 11.00%), and neurology (262, 9.33%) were the top three departments with the highest HAI rates. Lower respiratory tract (1198, 42.66%), bloodstream (419, 14.92%), and urinary tract (406, 14.46%) were the most common HAI sites. Klebsiella pneumoniae (173, 15.22%), Escherichia coli (155, 13.63%), and Acinetobacter baumannii (136, 11.96%) were the most frequent pathogenic microorganism. Acinetobacter baumannii was resistant to most antibiotics. Klebsiella pneumoniae was most resistant to cefuroxime Axetil, cefuroxime and ceftriaxone. Escherichia coli was most resistant to sulfamethoxazole and trimethoprim, ceftriaxone and cefuroxime. These three pathogenic microorganisms were all susceptible to tigecycline.
The incidence rates of HAIs fluctuated over the years, peaking in 2019, suggesting potential shifts in infection control dynamics. Klebsiella pneumoniae, Escherichia coli, and Acinetobacter baumannii were the predominant pathogens, and tigecycline may be considered as a potential option against these pathogens. The study highlights the importance of enhancing infection control measures in high-risk departments and sites, optimizing antibiotic stewardship, and continuously monitoring HAI trends to inform evidence-based infection control policies.
Not applicable.
Healthcare-associated/acquired infection (HAI), refers to an infection that occurs during the provision of medical care or treatment [1]. It is commonly associated with age, illness severity, comorbidity, duration of hospitalization and the use of invasive devices [2, 3]. The prevalence of HAIs varies significantly, with rates ranging from 3.5 to 12% in high-income countries and approximately 5.7–19.1% in low- and middle-income countries [4]. HAIs would lead to prolonged hospital stays, increased morbidity and mortality, and additional costs for patients [5, 6], posing a significant challenge to healthcare systems worldwide.
HAI surveillance serves as a crucial way for closely monitoring the occurrence and distribution of HAIs across high-risk specialties and sites [7, 8]. Surveillance data not only enables researchers to delve deeper into the causes and patterns of these infections, but also provides vital information for healthcare decision-makers to formulate effective prevention and control strategies [9]. The Chinese government has increasingly emphasized the significance of infection prevention program and has issued the ‘Administrative Measures of Healthcare Associated Infection’, mandating that all hospitals with over 100 beds in China must establish infection prevention teams in 2006 [10]. Furthermore, a number of standards and guidelines related to infection prevention have been issued, including hand hygiene, antimicrobial usage, disinfection and sterilization, as well as the surveillance of HAIs [11, 12]. As an integral component of healthcare quality assessment, the surveillance of HAIs is mandatory in Chinese hospitals.
The COVID-19 pandemic has introduced unprecedented complexities into the prevention and control of HAIs. On one hand, intensified infection control measures-such as enhanced hand hygiene, environmental disinfection, and widespread use of personal protective equipment (PPE)-may have reduced certain HAI risks. On the other hand, resource diversion, increased invasive procedures, and delayed infection reporting during the pandemic could have exacerbated specific infection types [13,14,15,16]. According to the National Health Commission of the People’s Republic of China, the Class A infectious disease control measures for COVID-19 were implemented on January 20, 2020 and lifted on January 8, 2023, after 1,084 days of enforcement [17]. This policy shift could profoundly reshape HAI epidemiology by altering hospital operations, patient flows, and resource allocation for infection control. However, no studies have systematically evaluated the variations in HAIs spanning both pre- and post-pandemic phases in Southwest China. Therefore, this study utilized surveillance data to assess variations in the incidence of HAIs across departments and sites, and to characterize the distribution of pathogenic microorganisms as well as their antibiotic susceptibility patterns capturing the period surrounding strict COVID-19 control measures.
This retrospective study was conducted at a tertiary hospital in southwest China from January 1, 2019 to December 31, 2023, a period spanning both pre- and post-pandemic. The hospital conducted real-time HAI surveillance by monitoring all inpatients (including healthcare-acquired COVID-19 cases) during their hospital stay, and the HAIs were initially confirmed by clinicians from each department, and infection management personnel conducted additional screenings to ensure no omissions were missed in the reporting process. Comprehensive clinical data were collected. Inpatients of any age discharged within the study period were eligible for the study, while outpatient cases were excluded.
The clinical data of HAIs were retrospectively collected by dedicated infection control personnel from a real-time nosocomial infection surveillance system (RT-NISS) [18]. The electronic surveillance system operates by utilizing routine process data gathered from various hospital databases. The RT-NISS system involves a three-step process for data collection: initially extracting data, followed by semi-automated screening, and ultimately confirming suspicious cases [1]. The RT-NISS gathers all data pertinent to inpatient infections from four hospital databases: the Hospital Information System (HIS), which includes demographics, antibiotic usage, and surgical data; the Laboratory Information System (LIS), providing microbiology and routine test outcomes; the Radiology Information System (RIS), offering radiology results; and the Electronic Medical Record (EMR), documenting diagnoses [2]. Suspicious infections are screened through multiple indices derived from microbiological reports, antibiotic administration records, serological and molecular test reports, and body temperature logs. Tailored screening strategies are employed for different types of HAIs [3]. The RT-NISS automatically generates daily alerts to clinicians about new suspicious HAIs (occurring at least 48 h after admission) and present on admission (POA, infections present within 48 h).
The HAIs initially confirmed by clinicians underwent a rigorous review process conducted by infection control specialists. Both infection control specialists and clinicians reviewed suspicious HAIs according to the established standard [19] and underwent regular training on infection control knowledge to enhance their prevention and control skills, as well as data management capabilities. This ensures the accuracy and objectivity of the data entered, which is vital for drawing meaningful study conclusions. Furthermore, the RT-NISS possess the capability to make logical corrections to the entered information and provide feedback on the pass rate of the completed data, ensuring its accuracy and reliability. For data that did not meet the quality standards, we conducted regular data validation checks by reviewing the patient’s medical records and laboratory reports.
The identification of bacterial strains was conducted using the microbial mass spectrometry detection system Autof ms1000 and its accompanying reagents. For antimicrobial susceptibility testing (AST), the VITEK2-compact system and its AST cards from bioMérieux S.A. (France), as well as the TDR-200 system and its AST cards from Hunan Changsha Tiandiren Biotechnology Co., Ltd., were employed. Breakpoint criteria were determined according to the antimicrobial susceptibility standard published by the Clinical and Laboratory Standards Institute (CLSI) [20]. Quality control strains included Staphylococcus aureus ATCC29213, Escherichia coli ATCC25922, and Pseudomonas aeruginosa ATCC27853, all sourced from the National Clinical Laboratory Quality Control Center under the Ministry of Health.
HAIs refer to infections acquired more than 48 h after admission, and not present or incubated at the time of admission [19]. In addition, if an infection with a definite incubation period occurs after the average incubation period following hospitalization, it is also classified as a HAI [19]. The Diagnosis and Treatment Plan for COVID-19 (Trial Version 10) indicates that the average incubation period is 3 days [21]. Therefore, if a patient develops symptoms or an asymptomatic patient tests positive for the first time on day 4 or later of the current hospitalization (with the day of admission counted as day 1), the patient is regarded as a suspected or confirmed case of hospital-acquired COVID-19. The underreporting rate of HAIs is the ratio of unreported HAIs to the total actual HAIs during the same period [22]. The delayed reporting rate of HAIs is the ratio of HAIs not reported within 24 h to the total HAIs [23]. The hospital infection diagnosis-related etiology test rate is the proportion of HAI cases who have undergone tests listed in the “Common Etiology Test Project Catalogue”, such as microbiological culture and susceptibility testing, microscopic examination, immunological testing, and molecular rapid diagnostic tests [24].
The research was conducted in accordance with the Declaration of Helsinki. Ethics approval was obtained from the Institutional Review Board of the Second Nanning People’s Hospital (No.Y2024228). As this retrospective analysis utilized routinely collected data, we submitted an application to our institutional review board for a waiver of informed consent and it was granted by the Institutional Review Board of the Second Nanning People’s Hospital.
Incidence rate of HAIs was defined as the number of new HAIs per 1000 hospital stays. The composition ratio of HAIs across departments or sites is the number of HAIs in each department or site divided by the total number of HAIs for the year. Statistical analysis was performed utilizing the SPSS 25.0 software (IBM, Chicago, IL, USA). Categorical variables were summarized using frequencies and percentages, and intergroup differences were compared using the Chi-squared test. Continuous variables with normal distribution were expressed as means (M) and standard deviations (SD), and were compared between groups with one-way ANOVA test. Non-normally distributed continuous variables were expressed as median and interquartile range, and were compared between groups using Kruskal-Wallis test. To compare annual differences in the incidence rates of HAIs, a negative-binomial regression model (incorporating overdispersion) was applied, with the log-transformed patient days as an offset and year as the sole covariate. Negative-binomial regression is widely used due to its ability to handle overdispersion, which allows for more accurate parameter estimation and reliable statistical inference [25]. A p value of less than 0.05 was statistically significant.
The demographic characteristics of the participants are shown in Table 1. A total of 2808 HAIs (1665 males and 1143 females) were enrolled in this study. The mean age of the patients was 61.37 ± 17.36 years. The average hospitalization time for HAIs was 8.00 (4.00–14.00). The age of patients with HAIs varied significantly between different years (P < 0.05).
Table 2 shows the comparison of incidence of HAIs from 2019 to 2023. The incidence rates of HAIs from 2019 to 2023 were 1.75, 1.12, 0.98, 1.31, and 1.30 per 1000 hospital stays (P < 0.05), respectively. The delayed reporting rates of HAIs from 2019 to 2023 were 19.76%, 36.07%, 47.16%, 51.74%, and 37.91% (P < 0.05), respectively. The hospital infection diagnosis-related etiology test rates from 2019 to 2023 were 69.50%, 82.77%, 57.99%, 73.87%, and 81.65%(P < 0.05).
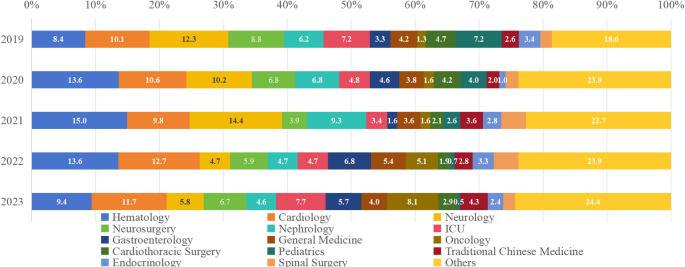
As shown in Fig. 1 and Supplement Table 1, the three departments with the highest HAI composition ratio from 2019 to 2023 was hematology (323/2808 = 11.50%, ranging from 8.38 to 14.95%), cardiology (309/2808 = 11.00%, ranging from 9.79 to 12.72%) and neurology (262/2808 = 9.33%, ranging from 4.70 to 14.43%).
Composition ratio of HAI departments from 2019 to 2023. The departments with the highest proportions of HAIs were hematology, cardiology, and neurology
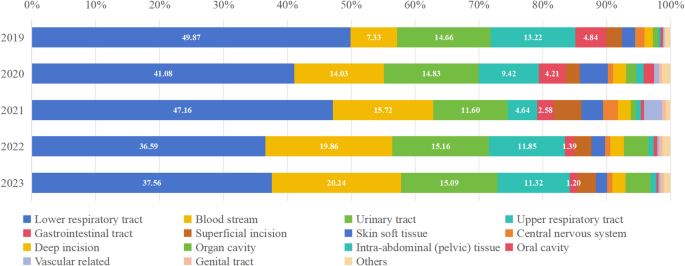
From 2019 to 2023, HAIs occurred frequently in the lower respiratory tract (1198/2808 = 42.66%, ranging from 36.59 to 49.87%) and bloodstream (419/2808 = 14.92%, ranging from 7.33 to 20.24%), with bloodstream infections exhibiting an increasing trend. Figure 2 and Supplement Table 2 detail the composition ratio of HAI sites.
Composition ratio of HAI sites from 2019 to 2023. HAIs occurred frequently in the lower respiratory tract, bloodstream and urinary tract. Bloodstream infections exhibited increase trend
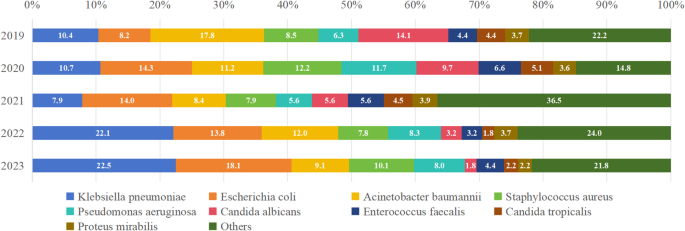
As depicted in Fig. 3 and Supplement Table 3, the most common pathogenic microorganisms included Klebsiella pneumoniae (173/1137 = 15.22%, ranging from 7.87 to 22.46%), Escherichia coli (155/1137 = 13.63%, ranging from 13.75 to 18.12%) and Acinetobacter baumannii (136/1137 = 11.96%, ranging from 2.81 to 30.00%).
Composition ratio of pathogenic microorganisms from 2019 to 2023. The most common pathogenic microorganisms included Klebsiella pneumoniae, Escherichia coli and Acinetobacter baumannii
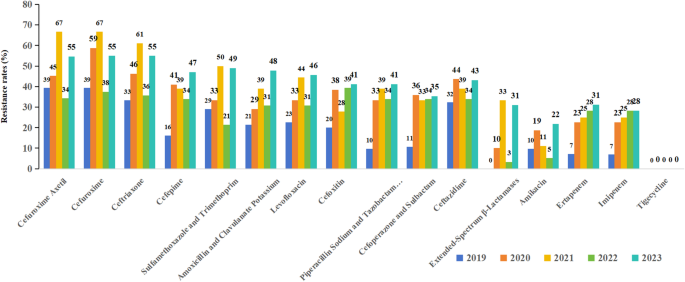
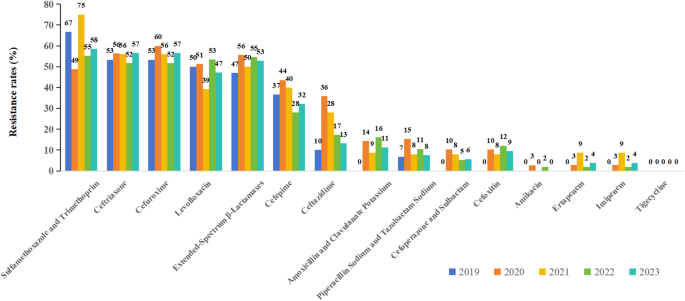
Figure 4 presents the results of AST for Klebsiella pneumoniae. The top three antibiotics with the highest resistant rates for Klebsiella pneumoniae were cefuroxime axetil, cefuroxime and ceftriaxone. The resistant rate of Klebsiella pneumoniae to extended-Spectrum β-Lactamases (ESBLs) showed a fluctuating trend over the study period, and rose from 0% in 2019 to 31% in 2023. From 2019 to 2023, resistance to ertapenem increased from 7 to 31%, while imipenem resistance rose from 7 to 28%. The resistance rate to tigecycline remained at 0% throughout the study period. The resistance rates to other antimicrobial agents all showed varying degrees of increase.
Resistance of Klebsiella pneumoniae to different antibiotics.The top three antibiotics with the highest resistant rates for Klebsiella pneumoniae were cefuroxime Axetil, cefuroxime and ceftriaxone
Figure 5 presents the results of AST for Escherichia coli. The top three antibiotics with the highest resistant rates for Escherichia coli were sulfamethoxazole and trimethoprim, ceftriaxone and cefuroxime. The resistance rates to several antimicrobial agents showed fluctuations, but remained at a relatively high level (over 30%) over the study period, including sulfamethoxazole and trimethoprim, ceftriaxone, cefuroxime, ESBLs, cefepime, and levofloxacin. Resistance rates to carbapenems (ertapenem, imipenem), β-lactam/β-lactamase inhibitor combinations, amikacin and tigecycline remained low.
Resistance of Escherichia coli to different antibiotics. The top three antibiotics with the highest resistant rates for Escherichia coli were sulfamethoxazole and trimethoprim, ceftriaxone and cefuroxime
Figure 6 presents the results of AST for Acinetobacter baumannii. Acinetobacter baumannii were resistant to most antibiotics (over 50%), including ceftazidime, piperacillin sodium and tazobactam Sodium, levofloxacin, cefepime, imipenem, meropenem, doxycycline, ticarcillin and clavulanate, ciprofloxacin, tobramycin, cefoperazone and sulbactam, sulfamethoxazole and trimethoprim. Moderate resistance rate was observed for minocycline, and low resistance rates were observed for polymyxin and tigecycline (lower than 10%). Klebsiella pneumoniae, Escherichia coli and Acinetobacter baumannii were all susceptible to tigecycline.
Resistance of Acinetobacter baumannii to different antibiotics. Acinetobacter baumannii was resistant to most antibiotics. Moderate resistance rate was observed for minocycline, and low resistance rates were observed for polymyxin and tigecycline
This study enrolled 2808 HAIs, with 1665 males and 1143 females, averaging 61.37 years old. The incidence rates of HAIs ranged from 0.98 to 1.75 HAIs per 1000 hospital stays, with 2019 being the highest incidence rate. Hematology, cardiology, and neurology were the top three departments with the highest incidence rates of HAIs. Lower respiratory tract, bloodstream, and urinary tract were the most common HAI sites. Klebsiella pneumoniae, Escherichia coli, and Acinetobacter baumannii were the most frequent pathogenic microorganisms. Among them, Acinetobacter baumannii were resistant to most antibiotics. Klebsiella pneumoniae was most resistant to cefuroxime axetil, cefuroxime and ceftriaxone. Escherichia coli was most resistant to sulfamethoxazole and trimethoprim, ceftriaxone and cefuroxime. The three pathogenic microorganisms were all susceptible to tigecycline.
According to data from U.S. Center for Disease Control and Prevention, the incidence rate of HAIs in 2019 was the highest in recent years [26]. The prevalence of HAIs was 1.17% (85/7,283) before the pandemic and 0.58% (43/7,437) during the pandemic in China [27]. A seven-year longitudinal study conducted in China from 2017 to 2023 has shown that the point prevalence of HAIs has been on a declining trend, while the incidence of community-acquired infections (CAIs) has been on the rise. Particularly during the COVID-19 pandemic, there has been a noticeable decrease in the point prevalence of HAIs, especially respiratory tract infections, and a significant increase in CAIs in 2023 compared to the period from 2020 to 2022 [28]. Another study conducted in China found that compared to the period of 2018–2019, the prevalence of HAIs decreased during 2020–2021, especially those related to respiratory infections [29]. Our study revealed the highest incidence rate of HAIs in 2019 and a declined trend during the pandemic, which aligned with previous studies. In 2019, hand hygiene compliance was significantly lower than in subsequent years, with a study reporting that compliance rates were only around 50% in many hospitals [30]. Additionally, environmental cleaning practices were not rigorous, especially for high-touch surfaces in patient rooms, resulting in inadequate cleaning and higher contamination rates [31]. Furthermore, the use of PPE was not as widespread as it became during the COVID-19 pandemic. Moreover, ongoing education and training programs for healthcare workers were insufficient, which may have contributed to suboptimal infection control practices. During the COVID-19 pandemic, these issues were significantly improved. Hand hygiene compliance rates increased dramatically, often exceeding 80% in many healthcare settings [30]. Enhanced environmental cleaning protocols were implemented, ensuring that high-touch surfaces were cleaned more frequently and thoroughly. The use of PPE became more widespread among healthcare workers and patients. Education and training programs for healthcare workers were expanded, focusing on infection control practices and patient safety. Furthermore, improved hand hygiene was also associated with reduced antimicrobial resistance rates in hospital environments [32]. These studies suggested that these interventions not only enhanced protocol adherence, but also reduced antimicrobial resistance rates, thereby contributing to a reduction in HAIs between 2020 and 2023 compared to 2019.
In addition to the improved infection prevention protocols mentioned above, a tiered and categorized regional admission policy was implemented within our hospital’s administrative region, where COVID-19 cases were centralized and treated at designated hospitals to optimize resource allocation and infection control [33]. Additionally, asymptomatic and mild cases were advised to self-quarantine at home under the guidance of local medical clinics or community health centers. Patients with moderate-to-severe or critical COVID-19, particularly elderly with comorbidities, were advised to seek immediate medical care [34]. Since our facility was not designated as an official COVID-19 treatment hospital, strict pre-admission nucleic acid testing was mandatory for all patients requiring hospitalization to thoroughly exclude COVID-19 infection. The redirection of high-risk patients towards designated hospitals, particularly those with comorbidities, could have influenced the risk of HAIs [13]. Furthermore, to accommodate patient demand, makeshift hospitals, gymnasiums, and nucleic acid sampling sites were repurposed into temporary fever clinics, diagnosis, and treatment centers. Concurrently, strict lockdown measures and fear of contracting COVID-19 within healthcare settings likely discouraged some patients, particularly those with less severe symptoms or chronic conditions requiring routine care, from seeking timely medical attention [35, 36]. These factors may contribute to decreased incidence rates of HAI by diverting patients away from healthcare settings. Notably, even after the relaxation of strict control measures, the rate of HAIs remained at a relatively low level in 2023. This suggests that the COVID-19 pandemic and associated containment policies may have altered public healthcare-seeking behaviors, with these effects persisting even after the relaxation of pandemic restrictions.
The underreporting rate of HAIs exhibited fluctuations between 2019 and 2023, despite the lack of statistical significance. The rate increased from 2.95% in 2019 to a peak of 5.95% in 2021, followed by a decline to 3.94% in 2023. The transient increase observed during 2020–2022 coincided with the COVID-19 pandemic period, suggesting that overwhelmed healthcare systems and diverted resources may have compromised routine HAI surveillance during this period. Furthermore, the delayed reporting rate continued to rise during the 3 years of strict control measures for COVID-19, and it decreased after the relaxation of the strict policies in 2023. The etiology testing rate for hospital infections peaked in 2020, but suddenly declined in 2021, and gradually increased in 2022 and 2023. The implementation of COVID-19 prevention strategies would affect the hospital infection prevention and control work [27, 37]. We speculate that this phenomenon may be attributed to the following reasons: (1) Strict COVID-19 measures overwhelmed hospitals, leading to increased delayed reporting of HAIs due to resource constraints and personnel overload [38]; (2) COVID-19 response prioritization diverted resources away from HAI prevention, contributing to reporting delays [39]; (3) COVID-19 altered patient flows, temporarily affecting HAI rates and reporting patterns [40]; (4) As hospitals resumed normal operations, increased attention to HAI prevention contributed to improved reporting rates [41]. The intense focus on managing COVID-19 often required hospitals to redirect resources and personnel towards this emergency, which may have compromised routine surveillance and management of HAIs. With staff overwhelmed by COVID-19-related tasks, the proactive identification and timely reporting of HAIs could have decreased. Additionally, as hospitals transitioned from pandemic response mode to normal operations in 2023, there was a renewed emphasis on reinforcing HAI prevention and control measures. During this transition, hospitals increased resources dedicated to HAI surveillance, improved training for healthcare workers, and raised awareness about the importance of accurate and timely reporting.
Our study revealed a notably high occurrence of HAIs within the departments of hematology (11.50%), cardiology (11.00%) and neurology (9.33%). The complexity of hematological conditions and the frequent use of invasive procedures may contribute to the high infection rates observed. Patients frequently experience compromised immune function as a result of high-dose chemotherapy treatment and the use of immunosuppressants [42, 43]. This immunocompromised state increases their vulnerability to both endogenous and exogenous infections. The combination of these factors poses a significant challenge in maintaining infection control in the hematology department. Cardiovascular surgeries and procedures often involve high-risk immunocompromised patient, making them particularly vulnerable to infections. Neurological procedures can be complex and invasive, often involving delicate tissue and prolonged recovery periods. A review on anti-COVID-19 measures [44] revealed divergent impacts across departments: HAI rates declined in cardiology and neurology departments, intensive care units demonstrated persistent high infection rates. Nonetheless, emerging evidence indicates these measures had no statistically significant impact on overall HAI rates [45], suggesting potential heterogeneity in infection distribution across departments. Future infection control policies should mandate hospitals to enhance infection control management in these high-risk departments across different regions. This can be achieved by increasing the frequency of targeted infection surveillance, allocating more resources to environmental cleaning and disinfection, and strictly enforcing aseptic operation protocols.
HAIs predominantly affected lower respiratory tract and bloodstream. This is similar with previous researches [46, 47]. Although the absolute number of lower respiratory tract infections decreased, the composition ratio remained stable. During the pandemic, hospitals strictly enforced the use of PPE and conducted nucleic acid testing for COVID-19 before and during hospitalization. These measures significantly reduced the incidence of HAIs, especially respiratory infections. Conversely, bloodstream infections exhibited significant increase. This may also be related to overwhelmed healthcare systems during peak pandemic phases. One possible explanation lies in the compromised adherence to aseptic protocols [48]. During periods of high patient volume, healthcare facilities often struggle to sustain optimal aseptic practices. Additionally, healthcare professionals have to manage a higher workload, which can lead to oversight in certain procedures. These factors may play a role in the rise of bloodstream infections [49]. To address this, healthcare facilities could increase staffing levels in high-risk departments by redistributing or recruiting personnel, ensuring sufficient manpower to maintain aseptic practices. Additionally, conducting regular infection control training and simulations would reinforce proper aseptic techniques. Implementing strict catheter management protocols and using electronic reminders to track catheter removal times can further reduce risks. Finally, establishing quality control teams to oversee and continuously improve infection control measures can help maintain high standards of aseptic practice.
The detection rate of pathogenic microorganisms in clinical settings remains a significant concern, with Klebsiella pneumoniae, Escherichia coli, and Acinetobacter baumannii being the most commonly encountered pathogens. The results reinforced previous studies [50,51,52]. Gram-negative bacteria are opportunistic pathogens that can cause infections in patients already compromised by severe underlying conditions, malignancies, or chronic inflammatory states. Consequently, there is a pressing need for robust control measures to prevent HAIs caused by these bacteria. It is encouraging to observe that all 3 pathogenic microorganisms exhibited the highest susceptibility to tigecycline. Tigecycline is a unique glycylcycline class of semisynthetic antimicrobial agents developed specifically for the treatment of polymicrobial infections caused by multidrug-resistant Gram-positive and Gram-negative pathogen [53]. Despite inherent or frequently reported resistance in some Gram-negative bacteria, tigecycline is effective against a broad range of multidrug-resistant nosocomial pathogens [54].
Acinetobacter baumannii was resistant to most antibiotics. It has emerged as a significant challenge in global public health due to its multidrug resistance to a wide range of antibiotics. This resistance is primarily achieved through mechanisms such as alterations in outer membrane porins, overexpression of efflux pumps, production of enzymes that hydrolyze antibiotics, and modifications of antibiotic targets, leading to extremely limited therapeutic options [55]. Epidemiological data from various regions worldwide indicate that Acinetobacter baumannii exhibits high resistance rates to commonly used antibiotics, including β-lactams, aminoglycosides, fluoroquinolones, and carbapenems. Therefore, there is an urgent need to strengthen infection control measures, such as hand hygiene, isolation, and environmental disinfection, to reduce its transmission [56]. Additionally, new drug development, antibiotic stewardship, and global collaboration are crucial for monitoring resistance patterns, sharing data, and coordinating response strategies [57].
The high resistance rates observed for Klebsiella pneumoniae to cefuroxime axetil, cefuroxime, and ceftriaxone reflect the increasing prevalence of multidrug-resistant strains. The fluctuating resistance rates to ESBLs and the significant increase in resistance to carbapenems (ertapenem and imipenem) from 2019 to 2023 highlight the evolving nature of resistance mechanisms in this pathogen [58]. The highest resistance rates for Escherichia coli were observed for sulfamethoxazole and trimethoprim, ceftriaxone, and cefuroxime. The significant fluctuations in resistance rates to these and other antimicrobial agents suggest dynamic changes in the circulating strains and potential shifts in resistance mechanisms. The low resistance rates to carbapenems, β-lactam/β-lactamase inhibitor combinations, amikacin, and tigecycline are promising, but ongoing surveillance is essential to monitor any emerging resistance. Acinetobacter baumannii exhibits high resistance rates to most antibiotics, reflecting its status as a challenging nosocomial pathogen [59]. The moderate resistance rate observed for minocycline and the low resistance rates for polymyxin and tigecycline indicate that these agents may remain effective treatment options. The data on resistance rates to commonly used antibiotics can help guide the selection of empiric therapy for infections caused by these pathogens. By choosing antibiotics that are more likely to be effective based on the resistance patterns observed in this study, healthcare providers can improve patient outcomes and reduce the risk of treatment failure. However, the increasing resistance to commonly used antibiotics, highlights the urgent need for standardized testing procedures and region-specific strategies to combat antibiotic resistance.
The limitations of this study included the following: (1) As the research was conducted in a single tertiary care hospital, the findings may not be generalizable to other healthcare settings; (2) Despite having rigorous data collection and review processes, the nature of retrospective research necessitated reliance on existing medical records; (3) Changes in hospital policies or staff training might have influenced HAI rates over the years, and the specific implementation, duration, and effectiveness evaluation of these policies or training may vary across hospitals and are challenging to quantify accurately; (4) Underreporting and detection bias could skew results, particularly in earlier years when reporting might have been less stringent; (5) Patient transfers between multiple departments could complicate the identification of infection sources and transmission pathways; 5) Antibiotic use is a critical factor in hospital infection control, but its administration is influenced by various factors such as doctors’ prescribing habits and patient compliance, which may influence the interpretation of study results to a certain extent.
This study provides insights into the variation of HAIs across departments and sites, pathogenic microorganisms distribution, and antibiotic susceptibility over a 5-year period (2019–2023). The incidence rates of HAIs fluctuated over the years, with 2019 being the highest rate, suggesting potential shifts in infection control dynamics. The lower respiratory tract, bloodstream, and urinary tract were the most common sites of HAIs. Hematology, cardiology, and neurology were the departments with the highest incidence. Klebsiella pneumoniae, Escherichia coli, and Acinetobacter baumannii were the predominant pathogens, and tigecycline may be considered as a potential option against these multidrug-resistant pathogens. The study highlights the importance of enhancing infection control measures in high-incidence departments and sites, optimizing antibiotic stewardship, and continuously monitoring HAI trends to inform evidence-based infection control policies.
The data used during the study are available from the corresponding author on reasonable requests.
We offer special gratitude to the participants of the study for their support.
The research was conducted in accordance with the Declaration of Helsinki. Ethics approval was obtained from the Institutional Review Board of the Second Nanning People’s Hospital (No.Y2024228). As this retrospective analysis utilized routinely collected data, we submitted an application to our institutional review board for a waiver of informed consent and it was granted by the Institutional Review Board of the Second Nanning People’s Hospital.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Liang, D., Liu, W., Zhong, Y. et al. Variation of healthcare associated infections at a tertiary hospital in Southwest China over a 5-year period (2019–2023): a retrospective observational study. BMC Infect Dis 25, 783 (2025). https://doi.org/10.1186/s12879-025-11173-1