USAMRIID is advancing the next generation of pathogen detection | Article | The United States Army
FORT DETRICK, Md. – Researchers at the U.S. Army Medical Research Institute of Infectious Diseases are testing a promising new biological threat detection method in high-fidelity training scenarios, demonstrating its suitability for use in the field so that it can someday fill a crucial biosurveillance capability gap.
The detection method works by adding molecular inversion probes, or MIPs, to samples of suspected pathogens collected in the field. MIPs are single strands of nucleic acid that quickly “capture” the DNA patterns of organisms they are exposed to. Researchers then use a laboratory process called polymerase chain reaction to multiply the captured DNA patterns until there is enough for researchers to compare against a master library of pathogens, called a panel. MIPs have the advantage of enabling researchers to detect pathogens in complex samples, such as tissues, using currently available detection equipment.
“Molecular inversion probes can be highly multiplexed, which means that thousands of genetic targets in a single sample can be amplified,” explains Dr. Christopher Stefan, chief of the Developmental Diagnostics Branch in USAMRIID’s Diagnostic Systems Division. “And because of the unique nature of the technology, you can add and remove targets from the panel without destroying the efficacy of the entire platform.”
In laboratory experiments that have led to numerous papers published in scientific journals, Stefan and his colleagues demonstrated that MIPs can be used to accurately identify the genetic markers for antibiotic resistance as well as for various types of highly contagious viruses such as chikungunya. More recently, they also demonstrated the efficacy of the MIPs technique in the field, through participation in a U.S. Army Combat Capabilities Development Command research and development program called Far-Forward Advanced Sequencing Technologies.
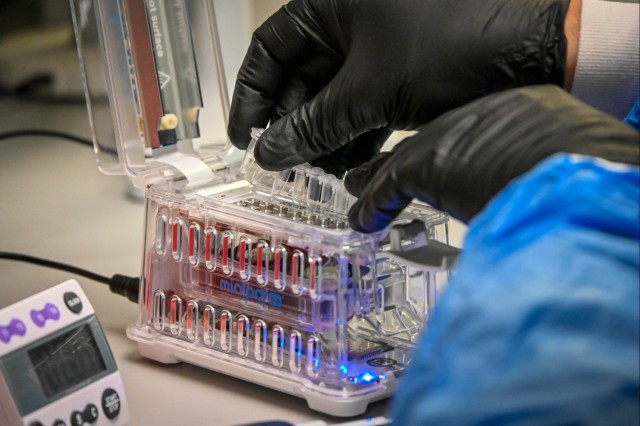
Sponsored by the Defense Threat Reduction Agency and led by the Naval Research Laboratory, F-FAST was a multi-year project to field test methods for rapidly detecting, analyzing, and sequencing biological agents at the point of exposure, rather than by sending samples to laboratories far behind the lines. In addition to the MIPs technique developed at USAMRIID, F-FAST also evaluated an “agnostic” sequencing method for pathogen identification – that is, one that tests whether a sample contains any pathogens at all, rather than testing for the presence of a specific one.
DEVCOM is pursuing development of agnostic sequencing methods under the auspices of the new Far-Forward Biological Sequencing program of record, while USAMRIID and NRL will continue to pursue the targeted sequencing method that uses MIPs plus PCR. The use of complementary detection methods will ensure Warfighters are able to maintain operational effectiveness in chemical and biologically contaminated environments.
USAMRIID’s unique capabilities – including the largest Biosafety Level 3 and 4 containment laboratories in the DOD – enable researchers to test the MIPs technique on a wide variety of threats. Furthermore, its engagement with the Defense Health Agency’s Global Emerging Infections Surveillance program enables it to conduct tests in the field, in the hands of actual Warfighters, under real-world conditions.
One recent field test involved providing a molecular sequencing tool and clinical specimens to the Level III hospital facility at Camp Arifjan, Kuwait, to test the efficacy and feasibility of using the technique for biosurveillance in a deployed environment. The mission also provided USAMRIID with valuable experience with the logistics of transporting equipment and samples to and from the camp.
Lt. Col. M. Kelly Hourihan, director of the division’s Special Pathogens Clinical Diagnostic Laboratory, said the project successfully demonstrated the value of next-generation sequencing capabilities in preserving force health protection, and also revealed gaps that will drive improvements to the technologies and procedures in the future.
“The purpose of the project was to show that you can rapidly move to a new location and set up and begin testing within a few hours,” says Hourihan. “As far as showing that the technology worked, it went really well. The preliminary results were promising, but we will know more when the device comes back to USAMRIID and Chris can analyze all the data.”
Recently, Diagnostic Systems Division personnel participated in African Lion 2025, U.S. Africa Command’s largest annual exercise. There, they put the sequencing tool into the hands of Warfighters to test in a joint, all-domain, multinational environment. Hourihan says that the experience gained from testing the MIPs method in realistic training exercises is vitally important for determining its efficacy in a wide range of conditions and scenarios, in order to ensure its effectiveness in protecting Warfighters from dangerous biological threats.
“During exercises, we can simulate a biological threat event with a simulated target as a way to test the process of collecting and analyzing samples, how that mitigates force health protection, and what the commander's decisions would be regarding whether to quarantine people or implement preventive medicine procedures,” says Hourihan. “These exercises inform us not only about capability gaps, but also about what types of technology we need to apply to next-generation sequencing to make this a truly far-forward capability.”










