Radiation Oncology volume 20, Article number: 91 (2025) Cite this article
Breast cancer patients may use a bolus to increase the dose to skin in radiotherapy after radical mastectomy. The 3D printed bolus (3DPB) specifically customized based on individual conditions offers better conformity. This study aims to provide clinical insights by evaluating the dosimetric benefits and safety of 3DPB in radiotherapy following radical mastectomy for breast cancer.
The study included breast cancer patients who received post-mastectomy radiotherapy with 3DPB. Researchers retrospectively collected dose data from patients’ radiotherapy plans, including with and without 3DPB, and prospectively observed the acute and late side effects in the cohort of patients undergoing mixed plan radiotherapy. To compare the dosimetric differences between radiotherapy plans with and without 3DPB, such as the dose distribution data of CTV, PTV, and organs at risk, matched sample T-test was used for data conforming to normal distribution, and non-parametric test was used for data not conforming to normal distribution. P < 0.05 was considered statistically significant.
A total of 35 patients were included with a median follow-up time of 16 months. In terms of radiotherapy side-effects, no level 4 acute side-effects occurred. A total of 82.2% of the patients had no obvious side-effects. No late radiotherapy side-effects of level 2 or higher occurred. In terms of dosage, radiotherapy plans with 3DPB showed better conformance (P < 0.001) and dose homogeneity (P < 0.001) than plans without 3DPB. The results indicated that the V95% dose of CTV1, CTV2, P-CTV, P-CTV1, and P-CTV2 was higher in the plans with 3DPB than in those without 3DPB (all P < 0.001).
The use of 3DPB in breast cancer radiotherapy is effective, achieving higher and more uniform dose distribution and better target conformity compared to without 3DPB. Additionally, it is associated with a low incidence of acute and late side effects.
Radiotherapy is a mainstay of breast cancer treatment [1, 2]. Residual sub-clinical lesions after breast cancer surgery can lead to local and regional lymph node recurrence and may become the source of distant metastasis [3]. The purpose of adjuvant radiotherapy is to eliminate residual sub-clinical lesions in the chest wall and lymph node drainage areas following surgery, thereby reducing the incidence of local recurrence (LR) and distant metastasis.
In the era of two-dimensional radiotherapy, a certain thickness of bolus was typically applied during chest wall radiotherapy to increase the dose to the skin and subcutaneous tissues [4]. Over the past decade, the technique of conformal intensity-modulated radiotherapy (IMRT) has matured, reducing the irradiation dose to the lungs and heart, and has gradually been adopted in the clinical practice of post-mastectomy radiotherapy (PMRT) for breast cancer [5,6,7,8]. As a result, some radiation oncologists believe that there is no need to place a bolus on the chest wall for PMRT of breast cancer. Nevertheless, others have reported that omitting bolus in chest wall may increase the risk of LR of breast cancer [9,10,11].
Recently, debate persists regarding the role of the skin as a target for radiotherapy following radical breast cancer treatment [12]. According to the European Society for Therapeutic Radiology and Oncology’s guidelines on target area delineation, the target area for the chest wall includes the outer edge of the skin, reduced inward by three millimeters [13, 14]. However, the Radiation Therapy Oncology Group (RTOG) guidelines expand the clinical target volume (CTV) of the chest wall by one centimeter to form the planning target volume (PTV). This adjustment primarily serves to accommodate the accumulation zone [15]. Therefore, in previous studies, a daily 3-mm thick bolus was applied to patients'chest walls to ensure adequate skin radiation dose [16].
Bolus is an auxiliary device utilized in radiotherapy to treat superficial lesions. It is typically employed to enhance the skin’s radiation dose and counteract the buildup effect [17, 18]. The buildup effect indicates that high-energy X-ray beams reach their maximum dose only after penetrating a certain depth into human tissue [19]. Nonetheless, achieving a perfect match between the bolus and the skin is challenging due to the chest wall’s uneven shape and surgical scars. The bolus is prone to deformation during radiation therapy, which often leads to air gaps between the bolus and the skin [20, 21]. According to some studies [22, 23], these gaps can cause uneven or insufficient skin radiation dosages, potentially reducing the effectiveness of post-mastectomy radiotherapy (PMRT).
The emerging field of 3D printing technology allows for the fabrication of patient-specific boluses, which can further maximize the efficacy of radiation therapy [24]. Previous studies have demonstrated that patient-specific boluses reduce unnecessary radiation to normal tissues and improve the conformance of radiation distribution for patients with uneven surfaces [25,26,27]. The results of the aforementioned studies indicate that individuals with uneven surface contours and varying target depths benefit from patient-specific boluses that optimize radiation dispersion conformance.
A limited number of studies have demonstrated the value of 3D-printed bolus (3DPB) in actual patient therapy, as the majority of publications have focused on phantom experiments or characterizing the dose characteristics of printable media [28,29,30,31]. Research on the application of 3DPB for PMRT for breast cancer remains scarce, and few studies have assessed whether the use of a bolus in conjunction with IMRT is still necessary.
This study aims to explore the dosimetric advantages and safety of 3DPB in PMRT for breast cancer, to evaluate the continued necessity of bolus use in the era of IMRT technology, and to provide valuable references for the radiotherapy of breast cancer patients.
Our study included patients with pathologically confirmed breast cancer diagnoses treated at our center between January 1, 2022, and April 30, 2023, who underwent radical mastectomy followed by adjuvant radiotherapy. The specific inclusion and exclusion criteria were as follows:
Inclusion criteria: (1) Women aged 18–80; (2) Patients who underwent radical mastectomy and were diagnosed with breast cancer by postoperative pathology; (3) Patients who received radiation therapy at our center, with 3DPB used in the process; (4) No evidence of distant metastatic lesions in the preoperative systemic examination; (5) Availability of complete clinical data.
Exclusion criteria: (1) Patients who underwent bilateral radical mastectomy for breast cancer; (2) Those who did not complete radiotherapy as planned; (3) Patients with other concurrent malignant tumors; (4) Patients who were lost to follow-up within 16 months after radiotherapy; (5) Patients with severe bone marrow suppression following chemotherapy; (6) Patients suffering from other systemic, uncontrolled diseases; (7) Patients with unhealed surgical incisions.
All patients received conventional fractionated radiotherapy in accordance with the standards for PMRT.The overall workflow and some details are illustrated in Fig. 1.
Positional immobilization
Patients with good upper limb function and well-healed surgical scars can be immobilized in a prone body position. The patient was positioned supine with the head slightly tilted towards the healthy side. A carbon fiber plate was used to secure the patient in a more comfortable position, and the position was further stabilized with a mold made from a styrofoam bag. The patient’s upper limbs were elevated to grasp the mouth of the styrofoam bag, ensuring that the chest wall remained essentially horizontal.
First computed tomography scanning
The patient underwent computed tomography (CT) scanning after the positional fixation mold was completed. A Siemens large-bore CT scanner with an 85-cm aperture was used for the parallel spiral CT scan. Patients were positioned supine. The scanning procedure consisted of two parts. First, a non-contrast CT scan was performed, with the approximate bolus boundaries marked using a lead wire: the inner boundary followed the paraspinal line of the affected chest wall, the outer boundary was at the midaxillary line, the upper boundary was the lower edge of the clavicular head, and the lower boundary was the skin crease at the lower edge of the contralateral breast. Subsequently, the patient’s body surface was marked with a marker pen, and a waterproof dressing was applied to protect the markings.
3DPB production process
After the completion of the scan, the CT images in Digital Imaging and Communications in Medicine format were exported as stereo lithography files and imported into 3D modeling software (Mimics 21.0). The patient’s body surface image was reconstructed within the system, and a rectangular bolus was fabricated based on the CT scan’s lead wire marking range. The raw material used is polylactic acid silicone, and the machine is Raise3D Shanghai Fuzzy's high-precision 3D printer Pro2 Plus.The thickness of the bolus was five millimeters. It takes about 6–8 h from reconstructing the 3D image to printing it. A portion of the 3DPB production process is depicted in Fig. 2.
2.1 Make body surface marks for 3D printed bolus. 2.2 3D printing system body surface reconstruction. 2.3 The placement of 3D printing bolus
Second CT scanning and 3DPB installation
Following the fabrication of the bolus, a second CT scan was performed, which included both contrast and non-contrast scans. The physician placed the bolus according to the markings, pressed it gently to eliminate air gaps, and then conducted the CT scan with a slice thickness of five millimeters. The physician assessed whether the bolus conformed to the patient’s body surface based on the CT images, made any necessary adjustments, and marked the bolus for proper repositioning. Once a good fit was achieved, the bolus was removed, the surgical scar was marked with a lead wire, and iodine contrast was injected to perform the contrast-enhanced CT scan with a slice thickness of five millimeters. The scanning range for the CT was from the lower edge of the mastoid process to the upper edge of the second lumbar vertebra.
When using a 3DPB conventionally, positional deviations and significant air gaps may occur. Therefore, it is crucial to apply lead wires for marking during patient immobilization and to instruct patients to carefully preserve these marks. When installing the bolus, strictly align it with the marked positions and use images from a second CT scan to verify and make timely adjustments. Finally, the position of the installed bolus should be marked again to facilitate precise placement during treatment.
Target outlining
The target zones were meticulously delineated following the 2022 Breast Cancer National Comprehensive Cancer Network (NCCN) [32] guidelines for the treatment of patients post-radical mastectomy. The CT images were transferred to the Monaco workstation for 3D reconstruction to define the clinical target volumes (CTV) and organs at risk (OAR) for radiotherapy planning. CTV1 encompassed the affected chest wall, CTV2 included the lymph node drainage areas above and below the clavicle, and CTV3 represented the lymph node drainage area in the internal mammary chain. Note that CTV3 was not delineated in some patients. The CTVs were refined based on the 3D reconstruction images to achieve a more regular and smoother shape.
The OARs were automatically contoured by an artificial intelligence system, including the thyroid, lungs, heart, spinal cord, and contralateral breast. These OAR contours could be manually adjusted to reflect the actual anatomical situation more accurately. A five-millimeter margin was added to the CTV in all directions to create the corresponding PTV, with the external part of P-CTV1 being reduced to three millimeters below the skin. Target delineation was performed on the contrast-enhanced CT images, and the contours were directly copied to the non-contrast CT images after verification. Any necessary modifications were made according to the actual situation, but efforts were made to maintain consistency in the target area between the images with a bolus and those without a bolus.
Plan design
The radiotherapy plan was designed based on the IMRT technique using the 6MV photon beam of the Elekta accelerator. Plan design and fabrication were conducted using the MediTech Elekta planning system. To ensure the accuracy of radiotherapy both with and without 3DPB, we designed two separate radiotherapy plans, two sets of treatment plans were developed: one with 3DPB and one without, based on CT scans with and without contrast images, respectively. The same physicist was responsible for designing and optimizing both plans. The dose prescription for 95% of the PTV was set to 50 Gy for the purpose of dose comparison and plan evaluation. The number of radiotherapy fractions was adjusted to achieve new actual radiotherapy plans: a 24 Gy/12 fraction plan with 3DPB and a 26 Gy/13 fraction plan without 3DPB. The PTV coverage of both plans was evaluated separately to ensure reasonableness. Subsequently, the two plans were integrated into a single 50 Gy/25 fraction plan for re-dose validation and critical organ assessment. The radiation field was designed as a tangential field. The total dose delivered was 50 Gy in 25 fractions, given five times per week.
Dose-volume histograms (DVHs) and Boolean algorithms were employed to determine the target doses at D2%, D50%, and D98%, as well as the volumes of interest: Vt,ref (the volume of the target area covered by the prescribed dose), Vt (the volume of the target area), and Vref (the volume of the total area covered by the prescribed dose). Additionally, the OARs, including the contralateral breast, affected lung, contralateral lung, both lungs, heart, spinal cord, thyroid, and esophagus, were assessed for both plans.
Program evaluation
Because the two plans had different doses and numbers of fractions, we combined the total dose of both plans to 50 Gy in 25 fractions during the radiotherapy plan design. This approach facilitated plan evaluation for clinicians and simplified the recording of dose distribution. The dose coverage of the PTV was assessed by radiation oncologist in both the 24 Gy in 12 fractions and the 26 Gy in 13 fractions plans, respectively. Ultimately, the PTV and the dose distribution to the OARs were evaluated for the cumulative 50 Gy in 25 fractions plan. In actual treatment, the two radiotherapy plans were executed sequentially. The prescribed dose for P-CTV was 50 Gy in 25 fractions. The plan was designed to meet the following guideline requirements: 95% of the PTV should receive the prescribed dose, with no more than 110% of the dose outside the target area. For the lungs, the plan aimed to achieve V20 < 30% and V5 < 60%; for the spinal cord, Dmax < 40 Gy; and for the contralateral breast, V5 < 55%, V10 < 35%, V20 < 20%, and V30 < 15%. Additional constraints included the heart’s V30 < 40 Gy and V40 < 30 Gy; the spinal cord’s Dmax < 45 Gy; the thyroid’s D50 < 45 Gy; and the esophagus’s D50 < 45 Gy.
Reset verification and radiotherapy implementation
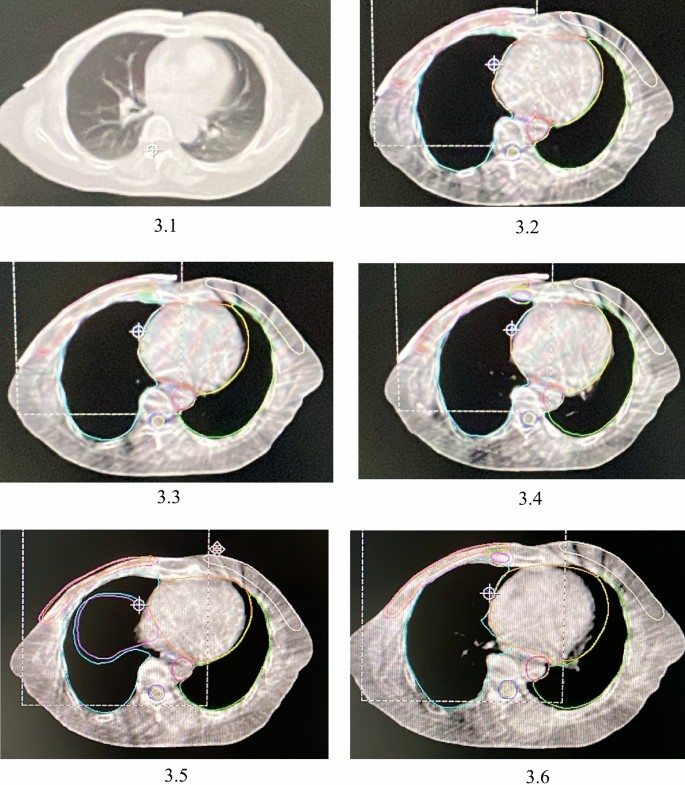
Prior to radiotherapy, setup verification was conducted using the emission field verification disk technique. Once the setup was verified, cone beam computed tomography (CBCT) was utilized for further confirmation. Additional surface verification was carried out using optical surface imaging and respiratory gating techniques, as appropriate for the equipment, before initiating radiotherapy. After these verification, radiotherapy was delivered using the Elekta Accelerator with a 6 MV X-ray photon IMRT technique. The initial 12 fractions included the use of a 3DPB, while the subsequent 13 fractions did not. Therefore, the first 12 fractions utilized the radiotherapy plan with the 3DPB, while the subsequent 13 fractions were conducted using the plan without the 3DPB. Patients were positioned in the supine position. The radiotherapist and radiotherapy technician were responsible for placing the bolus. Weekly CBCT scans were performed to verify the bolus fit, and the accuracy of bolus placement was assessed by comparing CBCT images with the planning CT scans, allowing for timely adjustments to be made. Patients were required to visit the radiotherapist’s clinic weekly for a myelogram review, skin reaction check, side-effect recording, and timely symptom management. The representative CT images used for the radiotherapy plan and the weekly CBCT images are presented in Fig. 3.
3.1 The representative CT image used to make the radiotherapy plan. 3.2 Representative CBCT image of the first week. 3.3 Representative CBCT image of the second week. 3.4 Representative CBCT image of the third week. 3.5 Representative CBCT image of the fourth week. 3.6 CBCT image of the fifth week
In this study, we created two sets of radiotherapy plans based on two CT scans: one with the 3DPB and one without. Both plans utilized the same target areas and planning methods. Our aim was to compare the dose distribution, target conformity, and dose uniformity of the two plans. The dosimetric parameters of the target area were evaluated using dose-volume histogram (DVH) analysis, which included the homogeneity index (HI) and conformity index (CI). The HI is defined as: HI = (D2-D98)/D50. where D2, D98, and D50 represent the minimum doses that cover 2%, 98%, and 50% of the target volume, respectively. The closer the HI value is to 0, the better the dose homogeneity within the target area.The CI is defined as: CI = (Vt, ref/Vt) × (Vt, ref/Vref), where Vt, ref is the volume of the target area covered by the prescribed dosage, Vt is the volume of the target area, and Vref is the total volume covered by the prescribed dosage, the closer the CI value is to 1, the better the conformity of the target area is.
In addition, D95 and D105 of PTV and the limiting doses for each OAR were also compared in this study. The two regimens were compared within the acceptable range of PTV doses for V5, V20, and V30 in lung, Dmean and V5 in the heart, Dmean in the contralateral breast, Dmax in the spinal cord, the thyroid, and the esophagus, respectively.
Measurements were expressed as mean ± standard deviation using SPSS27.0 statistical software. Matched Samples T-test was done to compare the doses of the two regimens, and P < 0.05 was statistically significant. Firstly, the normality test was performed for each measurement data by Shapiro–Wilk method, and the data conforming to normal distribution were expressed as x- ± s. The comparison of the data between the two groups was done by matched samples t-test, and the data not conforming to normal distribution was done by non-parametric test of the corresponding design. The test level α = 0.05 (two-tailed).
We utilized outpatient records, telephone calls, and the YIDU CLOUD Intelligent Follow-up Platform to conduct the follow-up. Demographic characteristics such as age, menstrual status, height, weight, and clinical data including tumor stage, pathology, and treatment were extracted from patients’ medical records. Simultaneously, after the completion of the plan design, we recorded the dose coverage, maximum dose, and average dose for each target volume. We also documented the dose distribution for each organ at risk to prepare for subsequent data analysis. The observational cohort included all patients recorded from the initiation of their first radiotherapy treatment, with a final follow-up date set for August 1, 2024. The follow-up assessments encompassed treatment-related adverse reactions, recurrence, and metastasis.
Throughout the radiotherapy process, patients were visited weekly to evaluate and document skin toxicity. To ensure consistency and accuracy in classifying acute skin side-effects, the skin condition (as detailed in Attachment 1) was assessed by two radiotherapists in accordance with the RTOG guidelines. In cases where the grading results were uncertain, a dermatologist was consulted to assess skin side-effects. All patients were briefed on the necessary precautions prior to commencing radiotherapy, and skin-protective medications were prescribed based on individual patient needs. Symptom management was also addressed during weekly follow-up visits.
Based on the inclusion and exclusion criteria for the cohort, a total of 35 patients who utilized 3DPB in PMRT for breast cancer were included in this study. The median follow-up time was 16 months. Within our observational cohort, 15 (42.9%) patients were non-menopausal, 18 (51.4%) had left-sided breast cancer, 16 (45.7%) had a body mass index (BMI) of 24 or higher, 33 (94.3%) underwent axillary lymph node dissection, and 32 (91.4%) had postoperative pathology revealing invasive ductal carcinoma. Additionally, 94.3% of patients had positive lymph nodes, and 71.4% of patients were classified as postoperative TNM stage III. Six patients exhibited skin or chest wall invasion. In terms of treatment, 10 (28.6%) patients received preoperative neoadjuvant chemotherapy, 74.3% received postoperative adjuvant chemotherapy, endocrine therapy was administered to 23 (65.7%) patients, and targeted or immunotherapy was utilized in 9 (25.7%) patients. The clinical and pathological characteristics of all patients are detailed in Table 1.
Due to the short follow-up period, there were no patients with recurrence or metastasis recorded during the follow-up in this cohort. Acute radiotherapy side-effects in patients are detailed in Table 2. Acute radiotherapy side-effects were defined as occurring during radiotherapy and within three months following the end of radiotherapy. Among the 35 patients, there were no instances of severe level 4 acute side-effects. No cardiac-related side-effects were observed, likely due to the widespread use of intensity-modulated radiation therapy.
Level 1 skin reactions were observed in 10 (28.6%) patients, while more severe level 3 skin reactions occurred in two (5.7%) patients. A majority of the patients, 65.7%, had no significant cutaneous symptoms. One (2.9%) patient experienced a level 1 mucosal reaction, and level 2 mucosal reactions were seen in two (5.7%) patients. Level 1 throat and esophageal side-effects were present in 34.3% of patients. Level 1 side-effects in the upper gastrointestinal tract and lungs were recorded in 8.6% and 5.7% of patients, respectively, with the remaining patients showing no significant reactions.
The incidence of hematologic side-effects was relatively high, particularly among patients with decreased white blood cell counts. Specifically, 11 (31.4%) patients experienced level 1 myelosuppression, three (8.6%) patients had level 2 myelosuppression, and two (5.7%) patients suffered from level 3 myelosuppression. There was one patient each with a 1 st, 2nd, and 3rd degree drop in platelet count. In summary, there were 42 (13.3%) events of level 1 acute side-effects, eight (2.5%) events of level 2 acute side-effects, and six (1.9%) events of level 3 acute side-effects in the patient cohort.
The incidence of late side-effects in the cohort was relatively low. Among the 35 patients, there were no adverse reactions of level 2 or higher. Specifically, four (11.4%) patients experienced level 1 late skin reactions, and three (8.6%) patients had adverse reactions affecting the subcutaneous tissue. No significant late side-effects were observed in any other tissues or organs. In total, there were seven events of late level 1 radiotherapy side-effects across the entire cohort (Table 3).
Additionally, we conducted a binary logistic regression analysis to assess the impact of clinical pathological characteristics and treatment conditions on radiation therapy side-effects. Given that skin reactions are the most common side effects during breast cancer radiotherapy, the correlation between skin reactions and other factors was analyzed.In addition to the axillary surgery potentially influencing the occurrence of acute side effects, other factors had no statistically significant impact on skin reactions. Patients who underwent axillary lymph node dissection (ALND) were more likely to experience early skin reactions. After reviewing the detailed patient follow-up records, it was observed that the skin reactions in the axillary region were more severe in these patients. This may be related to the disruption of subcutaneous lymphatic vessels following ALND, which leads to impaired lymphatic drainage and subsequently affects the skin barrier function. We have also analyzed the factors related to mucosal, esophageal, and lung side effects, and the results are temporarily included in the Attachment3. Interestingly, the axillary surgery appears to be an important predictor of radiation therapy side effects. Patients who underwent ALND had a higher incidence of acute mucosal and upper gastrointestinal side effects. Moreover, this surgical approach also had a certain impact on late subcutis side-effects. For more detailed statistical results, please refer to attachment 3 (Table 4).
In this study, dose data for the CTV, PTV, heart, lungs, esophagus, contralateral breast, thyroid, and spinal cord were collected for each set of plans. Both paired and normal distribution tests were conducted on the data from both plans. The Matched Samples T-test was applied to data that were normally distributed, while the Wilcoxon test was used for data that were not. The outcomes of the Matched Samples T-test are presented in Table 5.
It is understood that the closer the HI value is to 0, the better the dose homogeneity within the target area. The HI value for the plan with 3DPB was 0.0501, whereas the HI value for the plan without 3DPB was 0.0822 (P < 0.001), indicating a statistically significant difference between the two groups. No statistically significant differences were observed between the two plan groups regarding low-dose coverage, specifically V20 and V30 in the affected lung, V5 in the contralateral lung, V20 and V30 in both lungs, V5 in the heart, Dmean in the contralateral breast, and Dmax in the spinal cord.
The outcomes of the Wilcoxon test are presented in Table 6. Our analysis revealed that the V95% dose was significantly higher for the plans covering CTV1, CTV2, P-CTV, P-CTV1, and P-CTV2 with the 3DPB (all P < 0.001). Additionally, the Dmax to the P-CTV was higher in the plans that included the 3DPB compared to those that did not (P = 0.008). Furthermore, the CI was 0.883 for the plans with 3DPB, which was higher than the CI for the plans without 3DPB, and this difference was statistically significant (P < 0.001).
This study prospectively collected and followed a cohort of patients who underwent radiotherapy after total mastectomy. These patients utilized individually customized 3DPB. Given the relatively short follow-up duration, no recurrence or metastasis events were observed. Our objective is to compare the dose distribution, target conformity, and dose uniformity between the two plans. Since all 35 patients used the 3DPB, we did not make comparisons regarding safety; instead, we only recorded the incidence of acute and late side effects without comparing the two. The study found that patients using 3DPB did not experience any severe acute or late toxicities. Furthermore, the use of 3DPB allowed for an increased dose to the tumor target, achieving better dose homogeneity and conformity to the target volume.
Radiotherapy after radical mastectomy is known to reduce the incidence of locoregional recurrence and increase survival rates in certain individuals [33]. However, there is still no consensus on the use of boluses. When there is skin involvement, a tissue-equivalent bolus is recommended. Nonetheless, for patients without skin involvement, there is no agreement on the use of boluses during radiation therapy. While the American Society of Clinical Oncologists’ PMRT guidelines state that there is insufficient evidence to recommend bolus use, the current NCCN Breast guidelines suggest: ‘Special consideration should be given to the use of bolus material to ensure that the dose to the skin is adequate’ [34].
The debate over the use of boluses revolves around their safety and effectiveness. A study has shown that the use of a five-millimeter bolus increases the risk of level 2 acute skin reactions [35], and a 10-mm bolus raises the risk of level 3 acute skin reactions [36]. Besides exacerbating acute radiotherapy side-effects, the primary impact on late radiotherapy side-effects is the dilation of capillaries [37]. It has also been reported that the use of a bolus can increase the likelihood of reactions during mid-treatment [38]. Conversely, some studies [39, 40] have demonstrated that the use of a bolus does not increase the incidence of radiotherapy side-effects. In our study, the incidence of level 3 acute skin reactions was 5.7%, and late skin reactions occurred in only 11.4% of patients with level 1 skin reactions. Therefore, our analysis suggests that the incidence of radiation dermatitis is related to other factors as well. Chief among these is patients’ self-management, such as wearing tight clothes, frequent bathing, and the self-application of various medications, which may aggravate skin reactions. Additionally, body mass index, smoking, and the skin condition around surgical incisions are associated with the development of radiation dermatitis [41]. Timely assessment of patients’ subjective symptoms by clinicians also facilitates the prompt treatment of side-effects [42]. In summary, the occurrence of radiation dermatitis is related to a variety of factors, including the radiotherapy regimen, self-management, and individual skin conditions.
In addition to influencing radiotherapy side-effects, the purpose of the 3DPB is to increase the dose of skin or subcutaneous irradiation, thereby improving local control of the chest wall. For some individuals, PMRT reduces the risk of death from breast cancer and LR. The bolus ensures that superficial regions at risk of LR receive an appropriate dose by overcoming the skin-sparing effect of external-beam radiation [9]. However, a study by Nichol et al. [43] showed that the use of a 3DPB did not improve the local control rate. Moreover, interruptions in radiotherapy due to severe side-effects can also lead to LR [44]. Additionally, not using a bolus resulted in a lower incidence of level 2 and higher radiation dermatitis without increasing the LR rate [8]. Thus, the lack of a positive effect on local control rates may be the main reason why boluses are not routinely recommended.
Setting aside the controversy surrounding the use of 3DPB, we retrospectively examine its clinical application. As science and technology advance, 3D printing technology is being utilized in a variety of industries. Initially, 3D printing was mostly applied in various industrial production sectors, but it is now finding its way into medicine. Particularly in orthopedics and hepatobiliary departments, the application of 3D printing technology has led to more precise therapies, enhancing curative effects and advancing medical technology. There is considerable interest worldwide in using 3D printing in the radiation therapy process [45,46,47,48,49,50,51]. 3DPB has demonstrated promising applications in the radiotherapy of tumors at other sites. Hsu EJ et al. [52] discovered that a custom 3D printed integrated bolus/headrest provided a comfortable, consistent, and reproducible treatment setup, minimizing the risk of creating significant air gaps, and should be considered in the management of radiation therapy for patients with posterior scalp malignancies.
The shape of a conventional bolus is fixed, which often results in a large gap between it and the chest wall of patients who have undergone breast cancer surgery. This poor fit can affect the efficacy of radiotherapy. Currently, 3DPB has been applied in various superficial tissue radiotherapy, demonstrating good conformance and repeatability [53, 54]. Some studies have found that when 3DPB is used in postoperative radiotherapy for breast cancer, it fits more closely with the chest wall of patients and can improve the uniformity of dose distribution in the target area [55].
In a clinical trial, the dosage, application, and acute toxicity of conventional and 3DPB for PMRT with volumetric modulated arc therapy (VMAT) were compared [56]. The results suggested that 3DPB could potentially reduce cardiopulmonary exposure in patients undergoing PMRT with VMAT. Utilizing 3DPB allows for a comparison of dose buildup in the covered areas to that of commercially available boluses. A smaller field size is more susceptible to the effect of a larger surface-bolus distance. The higher conformity of 3DPB mitigates this effect, which may be particularly relevant for VMAT and IMRT techniques that involve a large number of smaller fields. The high conformity of 3DPB also reduces unintended dose buildup in healthy skin [57]. Additionally, 3DPB provides superior dose coverage for irregular superficial targets, enhances the conformance of radiotherapy plans, and reduces air gap volume in irregular superficial locations that may affect the delivery of superficial doses [58].
In terms of quality Assurance and workflow optimization, the key aspects that require precise management are the fabrication and fitting of the 3DPB. In our study, the Materials and Methods section details the process of 3DPB fabrication, which includes the strict demarcation of the 3DPB boundaries and size, as well as the proper marking of the bolus. The fabrication process of the 3DPB was carried out by professional 3D printing technicians. Furthermore, during the CT scanning process when the 3DPB is fitted, we strictly monitor and repeatedly adjust its placement. We also re-mark the attachment points of the 3DPB to ensure accuracy. Weekly CBCT scans are used to monitor whether the 3DPB is properly fitted and to make timely adjustments if necessary. As mentioned in reference [56], CBCT was daily used to verify the reproducibility of bolus placement and the 3D-printed bolus was remade if necessary.
At the same time, this study has some limitations. First, it only included patients using 3DPB, patients using conventional boluses were not part of the discussion, and the study lacked more robust control evidence. Second, although this was a prospective observational study, the short follow-up time meant that no recurrence or metastasis could be recorded, preventing a statistical analysis of the clinical benefits of bolus use. We will continue to follow up with this patient cohort in the future to explore the local control effects of 3DPB. Finally, the number of patients in this cohort was small, and the sample size was insufficient. Future prospective and retrospective studies with larger samples are needed to further verify the safety and effectiveness of 3DPB.
Overall, the use of 3DPB in PMRT for breast cancer allows for an increased dose to the chest wall and lymphatic drainage area without elevating the dose to normal organs. 3DPB contributes to a more uniform dose distribution within the target area and achieves better conformation to the target. The use of 3DPB is deemed safe and feasible, associated with a low incidence of both acute and late radiotherapy side-effects, and no severe complications have been observed. However, more rigorously designed prospective studies are necessary to confirm the feasibility and application value of 3DPB.
No datasets were generated or analysed during the current study.
The raw data used in this study were uploaded to the Research Data Deposit platform (www.researchdata.org.cn), with the approval RDD number: 2024300134. All data and tables can be viewed through the website.
- 3DPB:
-
3D printed bolus
- ALND:
-
Axillary lymph node dissection
- BMI:
-
Body mass index
- CBCT:
-
Cone beam computed tomography
- CT:
-
Computed tomography
- CI:
-
Conformity index
- CTV:
-
Clinical target volume
- DVH:
-
Dose and volume histogram
- HI:
-
Homogeneity index
- IMRT:
-
Intensity-modulated radiotherapy
- LR:
-
Local recurrence
- NCCN:
-
National comprehensive cancer network
- OAR:
-
Organ at risk
- PMRT:
-
Post-mastectomy radiotherapy
- PTV:
-
Planing target volume
- RTOG:
-
Radiation therapy oncology group
- VMAT:
-
Volumetric modulated arc therapy
There was no funding for the study.
The Sun Yat-sen University Cancer Center Ethics Committee approved this project (Ethical Consent Letter No.: B2023-284-01). This study was a retrospective collection of data and prospective observation of adverse effects in patients without human intervention factors, and according to the ethical requirements of our center, it was not necessary to obtain informed consent from patients.
The figures and tables included in this article are original and do not involve any conflict of interest on the part of others. The authors of this article agreed to publish it.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Attachment 1. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG).
Toxicity criteria of the Radiation Therapy Oncology Group (RTOG).pdf.
Attachment 2. Dosage abbreviations.
Dosoage abbreviations | Interpretation |
|---|---|
V5 | The volume covered by 5 Gy |
V20 | The volume covered by 20 Gy |
V30 | The volume covered by 30 Gy |
V95% | The volume covered by the 95% of the prescribed dosage |
V105% | The volume covered by the 105% of the prescribed dosage |
Dmean | The mean dose in the target area |
Dmax | The maximum dose in the target area |
Vt | The volume of the target area |
Vt, ref | The volume of the target area covered by the prescribed dosage |
Vref | The total volume covered by the prescribed dosage |
D2 | The minimum dose that surrounds 2% of the volume of the target area |
D98 | The minimum dose that surrounds 98% of the volume of the target area |
D50 | The minimum dose that surrounds 50% of the volume of the target area |
Attachment 3. The logistic regression results for acute and late side-effects.
Acute mucosa side-effects characteristic | Logistic regression analysis P value | Acute pharynx and esophagus side-effects characteristic | Logistic regression analysis P value |
|---|---|---|---|
Age | 0.383 | Age | 0.181 |
BMI | 0.446 | BMI | 0.288 |
Menstrual state | 0.727 | Menstrual state | 0.123 |
Tumor location | 0.512 | Tumor location | 0.122 |
Pathology | 0.096 | Pathology | 0.043* |
Histology state | 0.880 | Histology state | 0.491 |
Lymph node status | 0.152 | Lymph node status | 0.469 |
Tumor status | 0.523 | Tumor status | 0.785 |
TNM stage | 0.235 | TNM stage | 0.251 |
ER | 0.324 | ER | 0.529 |
PR | 0.886 | PR | 0.256 |
HER 2 | 0.164 | HER 2 | 0.736 |
Ki-67 | 0.410 | Ki-67 | 0.052 |
Endocrine therapy | 0.217 | Endocrine therapy | 0.506 |
Neoadjuvant chemotherapy | 0.849 | Neoadjuvant chemotherapy | 0.735 |
Postoperative chemotherapy | 0.752 | Postoperative chemotherapy | 0.944 |
Axillary surgery | 0.031* | Axillary surgery | 0.044* |
Targeted therapy | 0.139 | Targeted therapy | 0.884 |
Other antitumor drugs | 0.752 | Other antitumor drugs | 0.944 |
- *means the result is statistically significant
Acute upper gastrointestinal tract side-effects characteristic | Logistic regression analysis P value | Acute lung side-effects characteristic | Logistic regression analysis P value |
|---|---|---|---|
Age | 0.727 | Age | 0.093 |
BMI | 0.653 | BMI | 0.112 |
Menstrual state | 0.383 | Menstrual state | 0.207 |
Tumor location | 0.078 | Tumor location | 0.134 |
Pathology | 0.857 | Pathology | 0.905 |
Histology state | 0.673 | Histology state | 0.955 |
Lymph node status | 0.302 | Lymph node status | 0.827 |
Tumor status | 0.108 | Tumor status | 0.530 |
TNM stage | 0.915 | TNM stage | 0.711 |
ER | 0.324 | ER | 0.346 |
PR | 0.886 | PR | 0.698 |
HER 2 | 0.268 | HER 2 | 0.263 |
Ki-67 | 0.410 | Ki-67 | 0.204 |
Endocrine therapy | 0.971 | Endocrine therapy | 0.630 |
Neoadjuvant chemotherapy | 0.004* | Neoadjuvant chemotherapy | 0.357 |
Postoperative chemotherapy | 0.090 | Postoperative chemotherapy | 0.392 |
Axillary surgery | 0.031* | Axillary surgery | 0.720 |
Targeted therapy | 0.324 | Targeted therapy | 0.234 |
Other antitumor drugs | 0.752 | Other antitumor drugs | 0.013* |
Acute WBC side-effects characteristic | Logistic regression analysis P value | Acute PLT side-effects characteristic | Logistic regression analysis P value |
|---|---|---|---|
Age | 0.433 | Age | 0.727 |
BMI | 0.830 | BMI | 0.653 |
Menstrual state | 0.557 | Menstrual state | 0.383 |
Tumor location | 0.404 | Tumor location | 0.581 |
Pathology | 0.646 | Pathology | 0.096 |
Histology state | 0.648 | Histology state | 0.673 |
Lymph node status | 0.407 | Lymph node status | 0.893 |
Tumor status | 0.248 | Tumor status | 0.523 |
TNM stage | 0.155 | TNM stage | 0.915 |
ER | 0.032 | ER | 0.324 |
PR | 0.172 | PR | 0.164 |
HER 2 | 0.172 | HER 2 | 0.268 |
Ki-67 | 0.817 | Ki-67 | 0.410 |
Endocrine therapy | 0.728 | Endocrine therapy | 0.971 |
Neoadjuvant chemotherapy | 0.238 | Neoadjuvant chemotherapy | 0.849 |
Postoperative chemotherapy | 0.387 | Postoperative chemotherapy | 0.287 |
Axillary surgery | 0.900 | Axillary surgery | 0.656 |
Targeted therapy | 0.096 | Targeted therapy | 0.324 |
Other antitumor drugs | 0.929 | Other antitumor drugs | 0.752 |
Acute NEU side-effects characteristic | Logistic regression analysis P value | Late subcutis side-effects characteristic | Logistic regression analysis P value |
|---|---|---|---|
Age | 0.889 | Age | 0.383 |
BMI | 0.096 | BMI | 0.446 |
Menstrual state | 0.265 | Menstrual state | 0.727 |
Tumor location | 0.013* | Tumor location | 0.512 |
Pathology | 0.311 | Pathology | 0.096 |
Histology state | 0.324 | Histology state | 0.880 |
Lymph node status | 0.456 | Lymph node status | 0.152 |
Tumor status | 0.879 | Tumor status | 0.523 |
TNM stage | 0.692 | TNM stage | 0.235 |
ER | 0.189 | ER | 0.324 |
PR | 0.392 | PR | 0.886 |
HER 2 | 0.392 | HER 2 | 0.164 |
Ki-67 | 0.272 | Ki-67 | 0.410 |
Endocrine therapy | 0.467 | Endocrine therapy | 0.217 |
Neoadjuvant chemotherapy | 0.127 | Neoadjuvant chemotherapy | 0.849 |
Postoperative chemotherapy | 0.155 | Postoperative chemotherapy | 0.752 |
Axillary surgery | 0.552 | Axillary surgery | 0.031* |
Targeted therapy | 0.324 | Targeted therapy | 0.139 |
Other antitumor drugs | 0.752 | Other antitumor drugs | 0.752 |
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Wang, Y., Li, H., Hu, W. et al. The dosimetric value and safety evaluation of 3D printed bolus in adjuvant intensity-modulated radiotherapy after radical mastectomy for breast cancer: a prospective cohort study. Radiat Oncol 20, 91 (2025). https://doi.org/10.1186/s13014-025-02659-y