BMC Geriatrics volume 25, Article number: 381 (2025) Cite this article
The prevalence of multimorbidity is increasing in aging populations globally. Multimorbidity involves various noncommunicable disease (NCD) combinations that extend beyond individual conditions. Identifying how multimorbidity patterns (MPs) configure is crucial for understanding the role of NCD patterns in health prognosis.
This study identified MPs and examined their associations with sociodemographic and economic factors in 23,452 participants aged ≥ 60 years from the Lifelines cohort in northern Netherlands (baseline: 2007–2013; follow-up: 2011–2019). Complete data on 14 NCDs at two time points were analyzed, with multimorbidity defined as ≥ 2 NCDs. Latent class and factor analyses identified clusters of NCDs, stratified into MPs based on multimorbidity presence. Multinomial logistic regression assessed the relationships between MPs and sociodemographic and economic traits.
Multimorbidity prevalence was 55% at baseline. Five MPs, consistent across assessments, were identified. The ‘Vascular’ MP included the fewest NCDs (2–4), while the ‘Complex-Treatment Spectrum’ had the most (5–11). Adjusted analyses revealed that lower education, not having a partner, and lower income significantly increased the relative-risk of belonging to high-risk MPs, such as ‘Metabolic Risk,’ ‘Major CVD-Vascular Conditions,’ and ‘Complex-Treatment Spectrum’, compared to participants without multimorbidity. These MPs reflect profiles with distinct risk factors and prognoses.
Multimorbidity manifests as stable patterns in this population. MPs derived from latent class analysis were more interpretable and consistent over time compared to correlation-based approaches. Income disparities influence MP profiles, highlighting the need for tailored interventions. Longitudinal studies are recommended to explore NCD contributions to MP dynamics and inform strategies addressing health and social inequities.
Chronic diseases, also known as non-communicable diseases (NCDs) [1], are leading contributors to global mortality and disability rates in adults over 50 years of age [2]. These conditions are complex and long-lasting, necessitating systematic and long-term treatment approaches. Sociodemographic and economic characteristics are population-specific factors that influence NCD pathogenesis and their aggregation over time [3]. Typically, NCDs take decades to become fully established in a population [4], posing significant burdens on governments, healthcare systems, and individuals [5].
Combined with factors such as lifestyle risk behaviors, genetics, and ageing [6, 7], physiological resilience and bodily system responses decline, making older people especially vulnerable to NCDs [8]. Since 1990, there have been increases in Disability-Adjusted Life Years (DALYs) for adults over 50, with ischemic heart disease, stroke, diabetes, and chronic obstructive pulmonary disease (COPD) seeing increases of 46.1%, 31.5%, 156.1%, and 12%, respectively. Older adults aged 75 and above show even greater increases, with percentage changes of 66.6%, 60.5%, 190.7%, and 63.6% for the same conditions [2]. Continuous improvements in NCD treatment ensure longer life and improved quality of life [9]. Consequently, chronic diseases accumulate with age, leading to multimorbidity [10].
Multimorbidity is typically defined as the presence of two or more NCDs in an individual [10], affecting around one-third of the global population [11], with 51.0% being older adults aged 60 and above [12]. However, merely accumulating chronic conditions does not capture the full phenomenon of multimorbidity, as the combination of diseases varies among individuals. Older adults rarely present with only one chronic condition [13]. The relationship among chronic disease accumulation, declining physiological homeostasis, and impaired stress responses suggests distinct patterns of NCDs underlying multimorbidity, influencing health outcomes at different rates [14]. Additionally, older adults experience progressive deterioration in neurological, physiological, and physical functions at varying paces, making individuals older than 60 particularly suitable for examining potential groupings of NCDs within multimorbidity [15].
Exploring potential multimorbidity patterns of NCDs is crucial, yet despite several attempts to uncover non-random clustering patterns of multiple diseases, no consensus has been established in the scientific community [16]. Previous research has shown that different patterns of NCDs can be identified using statistical techniques, and mortality risk varies depending on the NCD patterns are configured [17]. Other outcomes, such as frailty, are also influenced by NCD multimorbidity patterns [18]. To date, there is no consensus on the best algorithm to simplify and to capture the most variability of NCD combinations in multimorbid individuals [19]. To explore potential underlying groups of multimorbidity, techniques such as clustering, dimensionality reduction, or latent subgroup analysis can be useful [20, 21]. However, the range of parameters and considerations used to build these models is broad, underscoring the need to standardize procedures. First, it is essential to determine which method best fits specific circumstances, and second, to configure the parameters so that the results are comparable with those of other researchers and populations, particularly for epidemiological and clinical purposes [16, 20]. Factor Analysis (FA) and Latent Class Analysis (LCA) have emerged as promising statistical procedures for exploring NCD aggregation patterns in populations. Both techniques are suitable for exploring latent subgroups and share similar statistical principles, making them apt for identifying MPs.
The aim of this study was to identify different MPs by comparing solutions from FA and LCA and to examine their associations with sociodemographic and economic traits in older adults from the Northern Netherlands. To achieve this, MPs were first determined using both statistical techniques and compared them to assess the extent to which FA and LCA yield comparable results. Second, patterns were explored to evaluate if they exceed the simple addition of conditions by analyzing the distributions of NCD accumulation within the MPs. Third, the relationship between MPs with sociodemographic and economic traits was assessed using multinomial logistic regression to evaluate which of the MPs belong to these traits and to assess the differences.
In this study, the configuration of “MPs” refer to specific combinations of NCDs that may reflect distinct trajectories in health outcomes. MPs represent a spectrum of two or more NCDs that accumulate systematically over time, rather than occurring randomly. To identify MPs, we applied factor analysis (FA) and latent class analysis (LCA) to data on 14 chronic disease domains, extracting latent disease factors and classes, respectively. The resulting patterns from FA and LCA were compared, and the most stable and interpretable pattern across two time points was selected. This pattern was further stratified using the multimorbidity cutoff of having ≥ 2 NCDs. Finally, multinomial logistic regression models were employed to examine associations between MPs and sociodemographic and economic characteristics.
The Lifelines cohort is a population-based multidisciplinary prospective study aimed at exploring healthy ageing among 167,729 individuals aged 0 to 93 years, residing in the northern region of the Netherlands. Lifelines encompasses three generations and captures biomedical information as well as data on lifestyle, socio-demographic characteristics, behavior, physical health, and psychological factors. Baseline data collection took place between 2007 and 2013, with participants undergoing follow-up assessments every five years, from 2011 to 2019. Data collection involves administering self-report questionnaires to participants of all ages and conducting measurements on individuals aged 8 years and older. A diverse array of investigative protocols is employed to assess biomedical factors contributing to health and disease in the general population, with a particular focus on multimorbidity. More detailed information on the design, sample size, and structure of the questionnaires can be found elsewhere [22].
The present cross-sectional analysis with prospective measurements utilized data from adults aged 60 years and older at wave 1 (baseline; N = 23,452) who were followed approximately five years later at wave 2 (first follow-up). Data from living participants with complete observations were analyzed at baseline (n = 21,130) and during the first follow-up (n = 16,465).
To identify NCDs for analysis, 45 conditions were initially considered at baseline and first follow-up. Participants were classified as having a specific condition if they responded “Yes” to the question, “Have you ever been diagnosed with…”, reported receiving treatment or prescribed medication for the condition, met diagnostic criteria based on laboratory tests (e.g., blood, serum, and urine analyses), or had undergone procedures such as surgery (e.g., transplants, device implantation, bypass, or angioplasty) or dialysis. Additionally, diagnostic classification incorporated results from spirometry, blood pressure measurements, electrocardiograms (ECG), and anthropometric data, where applicable [23].
The NCDs were grouped into 14 domains following established classification criteria from previous studies relevant to this population, considering clinical significance, shared mechanisms, and affected systems. These domains included: cancer (bone, brain, breast, prostate, lung, colon and other type, “excluding for skin and cervical cancer [24]”); major CVD events (CVD) (heart surgery, myocardial infarct, heart failure and stroke) [25, 26]; other heart and peripheral vascular conditions (HPVC) (atrial fibrillation, arrythmia, heart valve, claudication, aneurysm, atherosclerosis and thrombosis); hypertension (HTN) (systolic/diastolic blood pressure ≥ 140 and/≥90 mmHg); arthritis; osteoporosis; digestive (liver disease, ulcerative colitis and Crohn’s disease); diabetes (DM) (type 1 and type 2); kidney (chronic kidney disease, disturbed kidney, kidney disorders and renal failure) [27, 28]; respiratory (asthma, breathing problems and COPD) [25, 26]; thyroid (Hypothyroidism, hyperthyroidism, and thyroid disease.); narrow degenerative diseases (NDD) (Alzheimer, dementia, Parkinson’s disease, epilepsy and multiple sclerosis); depression and obesity (body mass index ≥ 30 (BMI) [29]). All 14 NCDs were recorded as binary variables (No = 0, Yes = 1). Additional details are provided in Supplemental Table 1.
Anthropometric, sociodemographic and economic variables were assessed at baseline. The structure of these variables is described below:
Household equivalent income (€) was calculated by dividing the net household income (the midpoint of each participant’s income category) by the square root of the number of persons living in the household. Highest education attainment was categorized into: elementary education (no education or primary education), lower secondary education (lower or preparatory vocational education or lower general secondary education), upper secondary education (intermediate vocational education, apprenticeship, higher general secondary education, or pre-university secondary education), tertiary education (higher vocational education or university), and others. Employment was defined as “Retired”, “Part time job” (includes < 12 h a week job), “Full time job” (includes being a housewife) and “others”.
Age in years was evaluated as continuous and categorized by a quinquennial distribution (60–64, 65–69, 70–74 and 75+); sex categorized as “men or women”; marital status categorized as “cohabitating with a partner”, “not cohabitating with a partner”, “no partner” and, “other”.
Data from all participants were considered for descriptive tables and figures at baseline. Multimorbidity was defined as the accumulation of two or more NCDs simultaneously in the same subject [10]. Prevalence for NCDs and multimorbidity were graphically presented for each assessment period. Subjects with missing information in one or more NCDs were excluded from FA and LCA.
To assess potential selection bias, sociodemographic characteristics and the accumulation of NCDs were compared between participants who were excluded (7%) or lost to follow-up (14%) versus those who continued in the analysis (86%). The Wilcoxon signed-rank test and chi-squared distributional hypothesis tests were employed as appropriate (Supplemental Table 2).
The first step involved estimating latent disease patterns using FA and LCA. Previous analyses aimed at identifying chronic disease patterns indicated that among six statistical techniques, FA provided the most coherent groupings [17]. However, in the present study, FA is compared with LCA, considering the growing recognition of LCA as a robust algorithm for disease classification and the availability of a larger sample size from the Lifelines data [30].
For FA, tetrachoric correlations were estimated at each time point (Supplemental Fig. 1). Details of the feasibility and factor extraction can be found below Supplemental Table 3.
A confirmatory factor analysis (CFA) was conducted using the maximum likelihood approach with standardized values. Model testing parameters included a lower Bayesian Information Criterion (BIC), Chi-square for the user model (p < 0.05), degrees of freedom (df), a Comparative Fit Index (CFI) value ≥ 0.9, and an RMSEA value < 0.05 (Supplemental Table 4).
A maximum likelihood estimator was chosen to perform LCA. To assess quality and compare model fit performance, a sequence of models ranging from one to seven classes was conducted with 100 repetitions each. Model fit indices, including parsimony of the BIC, likelihood, and residual degrees of freedom, were used to select the best class model [31]. Additionally, Akaike’s BIC (ABIC), consistent Akaike information criterion (CAIC), and entropy were considered [32] (Supplemental Table 5).
The cut-off value for item-response probability classifications within classes was set to 0.3 or above to capture variability in the disease latent patterns and provide more flexibility in interpreting the latent classes [33]. A sensitivity analysis was performed by excluding HTN from the estimations to assess its potential impact on LCA estimations (Supplemental Table 6).
Although statistical guidance was carefully considered, the final selection and naming of the best class solution were based on its etiological explanatory value. The latent disease patterns derived from FA and LCA were then assigned to individuals. The results from both techniques were compared, and stability was assessed visually using tables and Sankey diagrams to track the migration of individuals between latent disease patterns from baseline to the first follow-up. The technique that demonstrated better stability was then used to stratify the results according to the multimorbidity cutoff point, leading to the identification of MPs.
To evaluate how the MPs relate to sociodemographic and economic characteristics, the relationship between MPs and population characteristics was assessed at baseline through independent multinomial logistic regression models for each sociodemographic and economic variable. The dependent variable for the analysis was the MPs (with the group of participants without multimorbidity as the reference), and populational characteristics were the exposure variables. Results were summarized with relative risk ratios (RRR) and presented in plots. The RRR can be interpreted as the relative risk of belonging to a particular MP compared to not having multimorbidity, based on each sociodemographic or economic trait. Age and gender were considered as confounding variables in all models. Only participants with two assessments over time were analyzed (n = 18,897).
A p-value < 0.05 was considered statistically significant for all analyses, and confidence intervals were estimated at 95%. All statistical procedures were performed using R software version 4.2.3 (2023-03-15 ucrt) [34,35,36].
In Fig. 1, the frequencies of the 14 NCD conditions are presented for both baseline and the first follow-up. The highest frequencies were observed for HTN and HPVC, with baseline frequencies of 41% and 36%, respectively, and first follow-up frequencies of 47% and 37%. Except for obesity and thyroid disease, the prevalence of NCDs increased over each assessment timeframe. The prevalence of NDD and digestive disorders remained below 2% in each assessment period.
Figure 2 presents a comparative visualization of the latent disease patterns identified using FA and LCA. The top section displays the results of the latent factors from FA, while the bottom section illustrates the LCA class solution. Prevalences are indicated above each latent disease pattern. In both panels of the figure, a dotted line marks the 0.3 threshold for disease inclusion (Fig. 2b).
Latent disease patterns from factor and latent class analysis at baseline (N = 21,130) *
* The figure presents solutions for latent NCD groups obtained via both FA and LCA. The upper section displays the FA solution, while the lower section shows the LCA solution. To the right, the NCDs used in the calculations are listed, and to the left, the corresponding factor loadings and class membership probabilities are provided. In the central area, the latent NCD groups are depicted with their respective prevalence rates shown above each group
Five similar solutions were obtained from both FA and LCA. For FA, the latent factors identified were ‘Risk conditions,’ ‘Heart and vascular conditions,’ ‘Metabolic risk,’ ‘Narrow degenerative diseases & digestive,’ and ‘Complex treatment conditions’ (Fig. 2a). The latent classes identified were ‘Vascular,’ ‘Metabolic risk,’ ‘Heart and vascular conditions,’ ‘Complex treatment spectrum,’ and ‘Major CVD and vascular conditions’ (Fig. 2b). The results suggest some consistency in disease patterns across both statistical methods, particularly for cardiovascular-related conditions, metabolic conditions, and complex spectrum groups of diseases (Fig. 2a and b).
FA provides solutions based on factor loadings, while LCA estimates the probability of presenting one disease given another. Thus, despite similarities between solutions in Fig. 2a and b, interpretations differ according to the technique employed. In FA (Fig. 2a), NCDs are grouped according to their maximum absolute loading across five latent factors, restricting participants to a specific set of diseases within each pattern. In contrast, in LCA (Fig. 2b), NCDs are not mutually exclusive, and membership in each pattern depends on the probability of presenting each disease. For instance, participants in the ‘Vascular’ latent class show probabilities below 30% for each of the 14 diseases of having any of the 14 NCDs, whereas those in the ‘Metabolic risk’ pattern have probabilities of 78%, 38%, 53%, and 32% for presenting HTN, HPVC, obesity, or diabetes, respectively, and below 30% for the remaining NCDs.
All solutions were named after the diseases characterizing them. In the ‘Vascular’ class, although probabilities for each NCD remain below 30%, HTN, HPVC, and respiratory diseases gather closely, with probabilities of 29%, 23%, and 17%, respectively. This class also represents participants with the lowest accumulation range of NCDs (range between 1.6 and 4), contrasting notably with the ‘Complex treatment spectrum’ latent pattern (range between 5 and 11) (Table 1).
Figure 3 is a Sankey diagram showing the distribution of participants within latent disease patterns at baseline and first follow-up. In general, participants maintained their belonging to the same latent disease pattern across follow-ups, with variations in disease accumulation and the prevalence of the groups.
Classes conformation by accumulation of NCDs, and flux of MPs between assessments
No NCDS: Baseline: 16.5%; 1st Follow-up: 13.9%
363 deaths are in the exclusion group. There are overlaps between the losses to follow-up and exclusions
Multimorbidity was present in 55% of participants at baseline. The prevalence of the MPs was estimated to be 42% in the ‘Vascular’ group (n = 5,057), 23% in the ‘Metabolic Risk’ group (n = 2,808), 18% in the ‘Heart & Vascular Conditions’ group (n = 2,208), 13% in the ‘Major Cardiovascular Disease (CVD) & Vascular Conditions’ group (n = 1,562), and 2.8% in the ‘Complex Treatment Spectrum’ group (n = 333). The only group that required stratification for making the MPs was the ‘Vascular’ latent disease pattern. Except for the ‘Vascular’ group, each latent disease pattern from LCA included two or more NCDs. The ‘Vascular’ group had the lowest complexity, with an NCD accumulation range of 1 to 4, while the ‘Complex Treatment Spectrum’ group had the highest range, with 5 to 11 NCDs. In contrast, all latent factors required stratification for multimorbidity, and the ranges in NCD accumulation were wider, indicating more complexity in their conformation (Table 1).
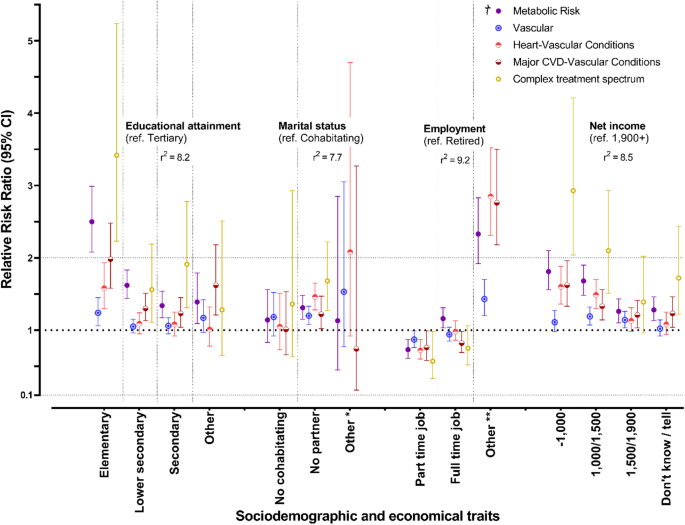
Results of a multinomial logistic regression are presented in Fig. 4. Participants with MPs were strongly associated with lower academic attainment, not having a partner, and lower net income. Comparing the ‘Vascular’ group as the least complex (and closest to a state without multimorbidity) with the ‘Complex Treatment Spectrum’ as the most complex, these groups represented two risk profiles distinctly differentiated by the RRRs of belonging to different sociodemographic or economic traits.
Baseline RRRs of belonging to a specific multimorbidity configuration by sociodemographic and economical characteristics
† Reference group for MPs: Without multimorbidity. * Complex treatment spectrum. - Marital status “Other”: RRR (95% CI) = 1.65 (0.22–12.49). ** Complex treatment spectrum. - Employment “Other”: RRR (95% CI) = 6.32 (4.22–9.46)
Compared with participants without multimorbidity, those in the ‘Vascular’ group had higher relative risk ratios (RRRs) for several sociodemographic factors: 1.24 (95% CI: 1.06–1.45) for having only elementary education, 1.20 (95% CI: 1.08–1.33) for not having a partner, and 1.19 (95% CI: 1.07–1.32) for a net income of €1,100–1,500. In the ‘Complex Treatment Spectrum’ group, these associations were even stronger: RRR 3.42 (95% CI: 2.23–5.24) for elementary education, 1.68 (95% CI: 1.27–2.22) for not having a partner, and 2.93 (95% CI: 2.04–4.21) for that same income bracket. Having a part-time job was linked to a lower likelihood of belonging to any multimorbidity profile, although this result should be interpreted with caution (Fig. 4).
Five MPs were identified: ‘Vascular’, ‘Metabolic Risk’, ‘Major CVD & Vascular Conditions’, ‘Heart & Vascular Conditions’, and ‘Complex Treatment Spectrum’. The average number of NCDs within the MPs varied, suggesting that disease accumulation is influenced by the probability of grouping diseases by etiology rather than adding NCDs. Among the techniques used, LCA showed the best performance in terms of interpretability and stability for grouping patterns. The risk of having MPs was higher in participants with lower income and among those without a life partner. The ‘Complex Treatment Spectrum’ MP was the most complex and had the highest risk, while the ‘Vascular’ MP was the least complex and had the lowest risk of association with sociodemographic and economic traits.
This study has some strengths. The classification of diseases was conducted using a robust database with a large number of participants and a well-planned, rigorous data collection methodology. The criteria for generating the list of 14 NCDs were based on previous publications using the same database, incorporating both self-reported criteria and objective clinical studies and measurements, ensuring strong classification accuracy. The analysis considered a large sample size, which reduces the likelihood of findings occurring by chance, enhances the representativeness of the MPs, and strengthens the overall findings. Additionally, the statistical techniques employed were powerful enough to generate precise estimates, and sensitivity analyses exhibited robustness.
Differences in our estimated patterns likely stem from statistical assumptions. FA, relying on variable correlations, captures linear relationships but may overlook complex patterns [37]. LCA, using a membership probability matrix, identifies distinct subgroups and better captures non-linear relationships and population heterogeneity [38], making it more robust for identifying stable MPs. While counting diseases to set a cutoff has practical value [39], visualizing NCDs grouped into MPs offers a more accurate perspective on multimorbidity [16]. These results highlight the complexity of multimorbidity and the need for an optimal approach to capture multiple disease associations [40].
Nevertheless, studies also show heterogeneity in findings when exploring disease combinations in multimorbidity [41]. The sources of this variation lie in four main domains: the design and sample size of the study (cross-sectional or longitudinal); the statistical techniques employed, including the parameters considered in the modelling; the set and number of diseases included in the models; and the type, definition, and magnitude of NCDs [19]. Despite this variability, there are consistently identified groups of multimorbidity across studies, such as musculoskeletal, metabolic, respiratory, cardiovascular/cardiometabolic, and mental disorders [33, 42, 43]. One limitation to address is the “naming fallacy,” which may lead to misinterpretation of latent groups. Although researchers commonly label the classes they identify, the complexity of these groups often means that the labels may not fully capture their underlying essence [31]. Also in our study, we encountered challenges in naming the classes, especially related to vascular diseases, so we cannot rule out that alternative labels might more precisely capture the characteristics of each multimorbidity pattern.
Sociodemographic determinants are strongly linked to multimorbidity [44]. A systematic review indicates that women are more prone to mental and musculoskeletal multimorbidity, while men are more affected by cardiometabolic, digestive, and genitourinary disorders. Not having a sentimental partner is a risk factor for mental health or complex multimorbidity clusters. Lower income or educational attainment increases the likelihood of developing cardiometabolic clusters and experiencing depression with arthritis, chronic pain, respiratory issues, or mental health problems [45]. Our results regarding sociodemographic and economic traits are consistent with findings from a meta-analysis, which reported that low educational attainment is associated with a 64% increase in the odds of multimorbidity (95%CI: 1.41–1.91) [46].
Potential sex differences may need to be considered in future analyses. Female patients often experience higher morbidity rates and more frequent adverse treatment reactions in various age-related conditions compared to males. Additionally, preclinical research often overlooks the long-term effects of pregnancy, childbirth, and breastfeeding [47]. Women in this population were more prevalent in the ‘Metabolic Risk’, ‘Heart & Vascular Conditions’, and ‘Complex Treatment Spectrum’ latent disease patterns (Supplemental Table 7). Further tests are needed to assess the impact of these differences.
In the present study, we used baseline measurements together with a single follow-up to identify how MPs are configured at two cross-sectional time points. Although we prospectively compared these periods using the same subjects, we recommend a longitudinal approach with repeated measurements like latent transition analysis. Such a design would enable a more precise evaluation of multimorbidity trajectories and a better understanding of how the classes evolve over time [48, 49].
The utility of latent multimorbidity groups may depend on the methods used to configure the MPs. Dimensionality reduction, distance-based clustering, and latent class identification all operate under different parameters and thus offer unique opportunities for characterizing MPs. In the case of latent classes, the probability of having one disease given the presence of another is estimated based on the potential combinations included in the calculations. Although LCA does not guarantee perfect class assignment, it is viewed as a more suitable approach because it relies on a model-based framework and provides fit indices, which enables statistical inference to determine the optimal number of classes for a population. For this reason, compared to traditional clustering methods, LCA is considered more statistically robust for grouping [21, 31].
Identifying the underlying patterns of multimorbidity creates significant possibilities for clinical care, as different combinations of 2, 3 or more diseases may confer more accurate prognosis than simply counting the number of diseases. In addition, by providing a real-life based outline for the grouping of diseases, configurations of MPs may offer insights that can better inform targeted interventions.
The findings reveal clear MPs in older adults, with LCA providing more interpretable and stable results than FA. A lower net income within MPs may mask the effects of environmental and behavioral risks, indicating that MPs are likely linked to social factors. This highlights the need to model MPs and to explore their relationships with lifestyles for more effective health interventions.
Access to Lifelines data is not publicly available but can be obtained from a third party. Researchers interested in the Lifelines data can submit an application. Further details on requesting Lifelines data and the terms of use can be found on their website. (https://www.lifelines.nl/researcher/how-to-apply).
- ABIC:
-
Akaike’s BIC
- CAIC:
-
Consistent Akaike information criterion
- KMO:
-
Bartlett’s sphericity test and Kaiser-Meyer-Olkin
- BIC:
-
Bayesian information criterion
- BMI:
-
Body mass index
- CFI:
-
Comparative Fit Index
- CFA:
-
Confirmatory factor analysis
- df:
-
Degrees of freedom
- DM:
-
Diabetes
- DALYs:
-
Disability-Adjusted Life Years
- ECG:
-
Electrocardiogram
- €:
-
Euro
- EFA:
-
Exploratory factor analysis
- FA:
-
Factor analysis
- HRs:
-
Hazard ratios
- HPVC:
-
Heart and peripheral vascular conditions
- HTN:
-
Hypertension
- LCA:
-
Latent class analysis
- CVD:
-
Major cardiovascular events
- MSA:
-
Measure of Sampling Adequacy
- MPs:
-
Multimorbidity patterns
- NCDs:
-
Noncommunicable diseases
- NDD:
-
Narrow degenerative diseases
- COPD:
-
Obstructive pulmonary disease
- RMSEA:
-
Root mean square error of approximation
- RRRs:
-
Relative Risk Ratios
The authors would like to thank the Lifelines cohort staff for making this study possible. Rafael Ogaz-González, a PhD student at the University of Groningen-UMCG, extends his gratitude to the Program in Medical, Dental, and Health Sciences at the National Autonomous University of Mexico (UNAM) for their support, and to the National Council of Humanities, Sciences, and Technology (Conahcyt) for the fellowship (CVU: 484055) awarded during the analysis and preparation of this manuscript.
The Lifelines initiative has been made possible through funding provided by the Dutch Ministry of Health, Welfare and Sport, the Dutch Ministry of Economic Affairs, the University Medical Center Groningen (UMCG), Groningen University, and the Provinces in the North of the Netherlands (Drenthe, Friesland, Groningen).
The Lifelines study adhered to the principles outlined in the Declaration of Helsinki and followed the research code of the University Medical Center Groningen. Approval for the study was obtained from the medical ethics committee of the University Medical Center Groningen under number 2007/152. All participants voluntarily agreed to participate in the study and provided written informed consent.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Ogaz-González, R., Zou, Q., Du, Y. et al. Multimorbidity patterns in older adults from Northern Netherlands: comparing factor analysis and latent class analysis solutions. BMC Geriatr 25, 381 (2025). https://doi.org/10.1186/s12877-025-06029-x