BMC Medicine volume 23, Article number: 341 (2025) Cite this article
Immune checkpoint inhibitors (ICIs) are a cornerstone of modern cancer treatment, but their effectiveness is limited. Fecal microbiota transplantation (FMT), which alters the gut microbiome, has shown promise in enhancing ICIs’ therapeutic effects.
We conducted a comprehensive search of relevant studies available up to September 30, 2024, to analyze the clinical efficacy and safety of combining FMT with ICIs in cancer treatment. The primary endpoint was the objective response rate (ORR), with secondary evaluations of survival outcomes and safety.
A total of 10 studies involving 164 patients with solid tumors were included. The pooled ORR was 43% (95% CI: 0.35–0.51). Subgroup analysis revealed that the combination of anti-PD-1 and anti-CTLA-4 therapies was associated with a significantly higher ORR (60%) compared to anti-PD-1 monotherapy (37%; P = 0.01). The incidence of grade 1–2 adverse events (AEs) was 42% (95% CI: 0.32–0.52), while grade 3–4 AEs occurred in 37% of patients (95% CI: 0.28–0.46).
This meta-analysis provides preliminary evidence supporting the use of FMT as a strategy to enhance the efficacy of ICIs in patients with advanced or refractory solid tumors. However, larger-scale randomized controlled trials with long-term follow-up are required to confirm and optimize treatment protocols.
Over the past decade, immunotherapy, particularly immune checkpoint inhibitors (ICIs), has become a cornerstone of modern oncology treatment (1–4). These agents function by counteracting the immune suppression imposed by tumor cells, thereby reactivating the body’s antitumor immune response (5–7). ICIs have demonstrated significant clinical efficacy in treating various advanced malignancies, particularly in melanoma, non-small cell lung cancer (NSCLC), and renal cell carcinoma (RCC) (8–10). However, despite the durable antitumor effects ICIs can induce in some patients, a substantial proportion of individuals do not benefit from these therapies. This heterogeneity in treatment response has become a key focus in current research. Numerous clinical studies have demonstrated that the response rates to ICI therapy vary by tumor type, typically ranging from 20 to 40% (11). This variability is likely influenced by factors such as the tumor microenvironment (TME), patient-specific heterogeneity, and the immunogenicity of the tumor. Furthermore, ICI therapy faces several challenges, including primary and acquired resistance, immune-related adverse events (irAEs), and high treatment costs, all of which considerably limit the wider clinical implementation and long-term efficacy of ICIs (12–18). Therefore, enhancing the efficacy and safety of ICI therapy, while broadening the patient population that can benefit, remains a priority in the field of immunotherapy.
In recent years, a growing body of research has emphasized the strong relationship between the microbiome and the response to ICI therapy in cancer patients (19–25). Clinical studies suggest that the composition and diversity of the gut microbiome can significantly influence the therapeutic outcomes of ICIs (23,24,26–28). For instance, a study involving melanoma patients found that patients who responded well to ICI therapy exhibited significantly higher baseline gut microbial diversity compared to non-responders (28). Another study focusing on patients with NSCLC and RCC identified a positive correlation between gut microbial alpha diversity and the efficacy of ICI treatment (26,27). Additionally, specific bacterial species have been associated with ICI response in many clinical cohorts. For example, the presence of Akkermansia muciniphila has been associated with positive responses to ICIs in patients with melanoma, hepatocellular carcinoma, RCC, and NSCLC(29–31). Moreover, another study found that a high abundance of Faecalibacterium in the gut was associated with improved prognosis in RCC and small cell lung cancer patients receiving anti-PD-1 therapy (32,33). These findings have generated interest in modulating the gut microbiome to enhance the efficacy of ICIs. Among the various strategies explored, fecal microbiota transplantation (FMT), a method of directly reshaping the gut microbiome, has attracted significant attention in the field of immunotherapy (32). Originally developed for the treatment of refractory Clostridioides difficile infections, FMT has also demonstrated promising outcomes in conditions such as inflammatory bowel disease and metabolic disorders. In the realm of immunotherapy, FMT is considered a highly promising adjunct strategy to enhance ICI efficacy by modulating the gut microbiome in some large-scale clinical trials, thereby improving clinical outcomes (34,35).
Although FMT has shown initial promise in immunotherapy, its clinical application remains in the early exploratory stages, with several controversies and uncertainties. First, there is considerable heterogeneity in the current clinical research on the impact of FMT on the efficacy of ICIs (34–36). Some studies have reported that FMT can substantially improve ICI response rates. For instance, a clinical trial involving melanoma patients demonstrated that 40% (6/15) of patients experienced benefit when treated with FMT in combination with PD-1 inhibitors (35). Another trial on melanoma similarly demonstrated positive outcomes with the combination of FMT and ICIs (34). However, other studies did not observe significant benefits from FMT (36). This variability in outcomes may be attributed to several key factors, including donor dietary habits and gut microbiota diversity, recipient immune status and tumor immunogenicity, microbiota screening and functional analysis criteria, as well as the optimization of transplantation dosage and frequency. However, the optimal protocol for implementing FMT has yet to be established. Critical parameters, such as large-scale randomized controlled trials, interdisciplinary research, and long-term efficacy assessments, require further optimization (37). Clinical trials must rigorously standardize microbiota management and protocol implementation, establishing stringent inclusion and exclusion criteria for different tumor types and stages across diverse ethnic and geographical patient populations. Given individual patient variations and microbiota heterogeneity, coupled with complex interactions between microbial physicochemical properties and multiple factors including tumors, immunotherapeutic agents, and diet, scientifically sound yet personalized FMT protocols must be developed. Furthermore, optimizing FMT production and storage processes, developing safe and effective delivery methods, and establishing risk warning and intervention mechanisms are essential to construct a comprehensive and standardized therapeutic system from preparation to implementation. Third, safety concerns surrounding FMT remain a concern. While most studies report good tolerability, potential risks such as microbial translocation, immune-related adverse events, and long-term safety remain unresolved. Moreover, the precise mechanisms by which FMT influences the TME, modulates immune cell function, and synergizes with ICIs remain poorly understood, requiring further basic and translational research to clarify these molecular mechanisms. These controversies and uncertainties highlight the need for systematic evaluation and meta-analysis of FMT’s role in immunotherapy to synthesize existing evidence and guide future research directions.
In light of the potential and challenges surrounding the combination of FMT and ICIs in tumor immunotherapy, this study aims to comprehensively evaluate the clinical efficacy, safety, and potential predictive factors of this combination therapy through a systematic literature review and meta-analysis. Specifically, this study will focus on the following key areas: Efficacy: Assess the objective response rate (ORR), complete response (CR) rate, and partial response (PR) rate of FMT combined with ICIs. Survival outcomes: Analyze the impact of FMT combined with ICIs on progression-free survival (PFS) and overall survival (OS). Safety: Evaluate the safety of FMT combined with ICIs, including the incidence and severity of adverse events. Subgroup analysis: Investigate how different factors—such as ICI strategies (e.g., anti-PD-1 therapy, anti-PD-1 combined with anti-CTLA-4 therapy, mixed therapy), FMT donor sources (e.g., processed microbes, ICI responders, healthy individuals), FMT administration routes (e.g., stool capsules, colonoscopy, specific capsules), and tumor types that influence treatment outcomes. Through this comprehensive analysis, we aim to provide high-quality, evidence-based insights into the application of FMT in immunotherapy to support clinical decision-making, future research design, and the development of individualized treatment strategies.
In this meta-analysis, we systematically gathered published trial data on patients treated with ICIs and FMT, with the goal of comprehensively evaluating the clinical efficacy and safety of this combined therapy. The study was designed and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. As only de-identified patient data were used, this study did not require approval from an ethics committee or informed consent from individual patients, according to relevant regulations.
This study included all trials that applied ICIs in combination with FMT as the intervention. Studies focusing on patients with irAEs and those where ICI re-challenge was the primary focus were excluded. The target population for this analysis consisted of patients with advanced or refractory cancers, including patients with metastatic disease, who were either receiving or planning to receive ICI therapy. Eligible ICI regimens included anti-PD-1/PD-L1 monotherapy or combination therapy with anti-CTLA-4 agents. To be included, studies had to report at least one of the following efficacy outcomes, based on internationally recognized oncology evaluation criteria: ORR, CR, PR, PFS, or OS. In addition, studies had to provide safety data, including the incidence of adverse events (AEs) of any grade.
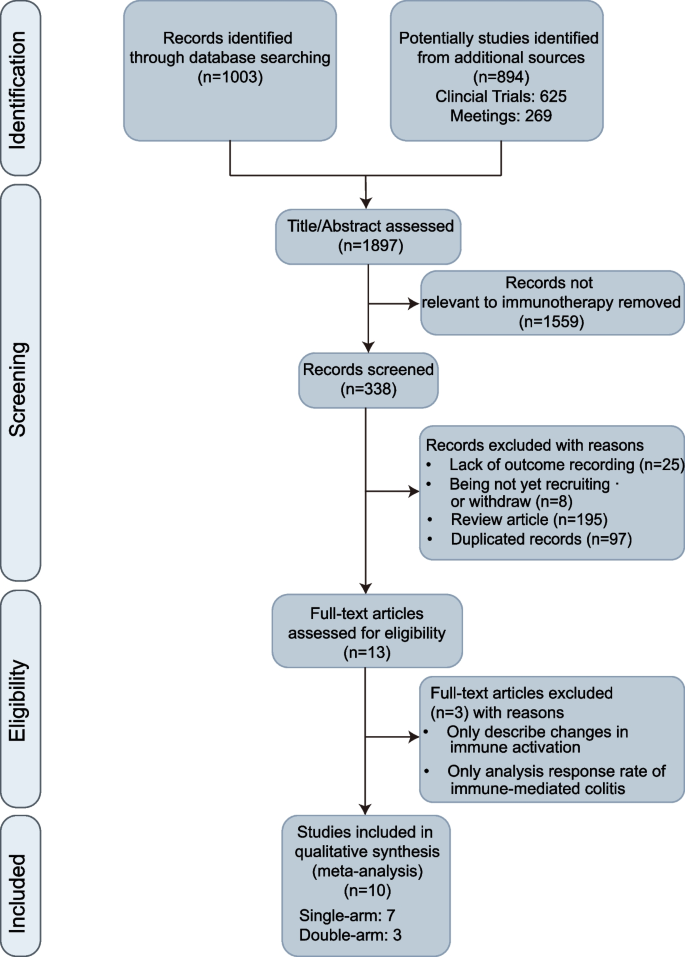
For published literature, we conducted a systematic search of the PubMed database for articles published online up to September 30, 2024. The search strategy was as follows: ("fecal microbiota transplantation"[MeSH] OR "fecal microbiota transplantation" OR "FMT" OR "fecal transplant") AND (tumor[tiab] OR cancer[tiab] OR neoplasm[tiab] OR malignan*[tiab] OR carcinoma[tiab] OR lymphoma[tiab] OR sarcoma[tiab] OR melanoma[tiab] OR leukemia[tiab]). No filters were applied regarding language or publication type, and the initial search yielded 1003 articles. Additionally, we performed a comprehensive search in ClinicalTrials.gov, chictr.org, and major oncology conference abstracts (including the European Society for Medical Oncology (ESMO), the American Society of Clinical Oncology (ASCO), and the Society for Immunotherapy of Cancer (SITC)), using “Fecal” and cancer-related terms as subjects or keywords, retrieving an additional 894 records. The study selection process is illustrated in Fig. 1.
Based on the predefined inclusion criteria, we screened relevant studies by reviewing titles and abstracts, excluding studies that did not involve ICI-based immunotherapy. The types of studies considered for inclusion were randomized controlled trials (RCTs), single-arm studies, and prospective cohort studies with control groups. To be eligible, the studies had to report both efficacy and safety outcomes during the follow-up period. Exclusion criteria included studies focused solely on immune microenvironment changes or alterations in the microbiome, reviews, posters, and conference records that did not provide specific data. The selection process was conducted independently by two researchers (LHYH and AQL), who conducted initial screening and data extraction to identify eligible studies. Any discrepancies in study selection were resolved through discussion to reach a consensus. Where there was a lack of consensus between the two reviewers, the secondary author (TW) acted as a senior reviewer to make a final decision on whether the study met inclusion criteria.To minimize selection bias, the final set of included studies underwent blinded review by an independent third-party evaluator (PL), who confirmed the eligibility of the studies without knowledge of the initial researchers’ decisions.
Two investigators (YL and CYZ) conducted risk of bias assessment according to the study design type, utilizing the revised Cochrane Risk of Bias tool for randomized trials (ROB 2.0) (38) for randomized controlled trials, and the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool (39) for quality assessment of non-randomized intervention studies. Additionally, to comprehensively evaluate potential publication bias, we performed Egger’s test using R (Version 4.4.1), while simultaneously calculating the Luis-Furuya-Kanamori (LFK) index and generating Doi plots through Stata 18 (StataCorp, TX, USA). According to established criteria, publication bias was considered absent when |LFK index|≤ 1, minor when 1 <|LFK index|≤ 2, and substantial when |LFK index|> 2 (40).
Meta-analyses were conducted using Stata 18 (StataCorp, TX, USA) and R (version 4.4.1) with packages such as meta (version 8.0.1), grid (version 4.4.1), forestplot (version 3.1.6), and metafor (version 4.6.0). The primary endpoint of this study was the ORR, defined as the proportion of patients achieving a CR or PR after receiving ICIs in combination with FMT therapy. Secondary endpoints included PFS and OS, which were used to assess long-term disease control. Safety was evaluated by focusing on the incidence of treatment-related AEs, graded according to the Common Terminology Criteria for Adverse Events (CTCAE). For the data synthesis, we conducted pre-specified subgroup analyses based on the type of ICIs, the source and mode of administration of FMT, and the tumor type. For single-arm studies, we extracted endpoint data, including their 95% confidence intervals (CI) and weights. For two-arm studies, we calculated the risk ratio (RR) and corresponding 95% CI. Heterogeneity was assessed using the Cochran’s Q and I2 statistic. To comprehensively evaluate the impact of FMT on both the efficacy and safety of ICI therapy, we employed both fixed-effect and random-effect models. P-value < 0.05 was considered statistically significant. Given the limitations in PFS and OS data, and the fact that most studies did not report hazard ratio (HR) and their 95% CI, we performed a weighted average analysis of the median PFS (mPFS) and median OS (mOS) to provide a preliminary assessment of the long-term efficacy of FMT in conjunction with ICIs.
Following a systematic literature search and screening, 10 studies(34–36,41–47) were included in the meta-analysis, involving a total of 164 patients with solid tumors. Among the included studies, 5 were phase I trials, 4 were phase II–III trials, and the single-arm interventional study by Sook et al. (NCT04264975) did not specify the study phase. The 10 studies comprised 1 observational study, 6 single-arm interventional studies, and 3 RCTs. The included studies targeted various tumor types: 4 studies focused on melanoma, 2 on metastatic clear cell RCC, 1 on metastatic microsatellite-stable colorectal cancer (MSS-CRC), and 3 had no tumor-type restrictions. For lines of immunotherapy, 2 studies focused on the ICI-resistant population, while 8 studies investigated the ICI-naive population. Geographically, most studies were conducted in Canada (4 studies) and the U.S. (3 studies), with the remaining studies based in China (1 study), South Korea (1 study), and Israel (1 study). Regarding ICIs, all studies employed anti-PD-1 antibodies, with 3 studies combining anti-CTLA-4 antibodies (ipilimumab). One study did not specify strict restrictions on monoclonal antibody choice. No special restrictions on gender or age were applied across the included studies (Table 1). All included non-randomized studies of interventions met at least moderate-quality standards according to ROBINS-I assessment, while the randomized controlled trials included reached the “some concerns” threshold based on ROB2.0 standards (Additional file 1: Table S1).
All studies were eligible for ORR analysis. The funnel plot shows that scatters are essentially symmetrical, and Egger’s test (t = − 1.21, p = 0.2625) suggested no significant publication bias in the included studies, but LFK index indicates minor asymmetry (LFK = − 1.04) (Additional file 2: Fig. S1A). The pooled estimate for ORR was 43% (95% CI: 35–51%), with moderate heterogeneity across studies (I2 = 59%, P < 0.01), indicating moderate variability between studies. In the three RCTs, heterogeneity increased (I2 = 64%, P = 0.06). The pooled RR was 1.31 (95% CI: 0.58–2.98), with the 95% CI crossing 1, suggesting the difference in ORR between the experimental and control groups was not statistically significant (Additional file 2: Fig. S1B).
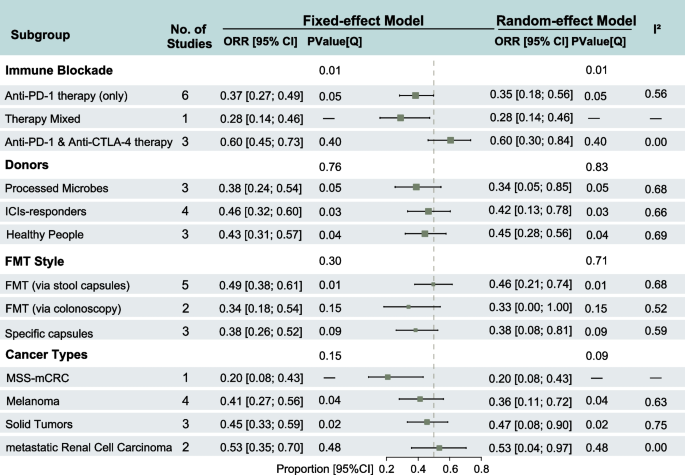
Subgroup analysis based on the choice of ICIs demonstrated that the combination of anti-PD-1 and anti-CTLA-4 therapy showed significantly better efficacy compared with anti-PD-1 inhibitor monotherapy (ORR: 60%, 95% CI: 45–73% vs. ORR: 37%, 95% CI: 27–49%). The heterogeneity across studies in the combination ICI group was low (I2 = 0), indicating high consistency. The difference in ORR between ICI strategies was statistically significant (P = 0.01). In the subgroup analysis based on the source of the FMT donor, studies that used ICI responders as donors reported an ORR of 46% (95% CI: 32–60%), which exceeded that of studies using healthy donors or commercial microbiota preparations. Regarding the route of FMT administration, oral fecal microbiota capsules resulted in an ORR of 49% (95% CI: 38–61%), surpassing endoscopic transplantation and special formulation capsules. However, significant heterogeneity was present within the subgroups (ICI-responder donor group: I2 = 66%; stool capsule: I2 = 68%). No statistically significant differences were observed between groups based on donor source or FMT administration method. However, significant heterogeneity was observed within studies using donors who were ICI responders (P = 0.03) or healthy populations (P = 0.04), as well as within studies that administered FMT via stool capsules (P = 0.01). In the subgroup analysis by tumor type, patients with metastatic clear cell renal carcinoma had an ORR of 53% (95% CI: 35–70%), with low heterogeneity (I2 = 0). There is no significant overall heterogeneity among the included studies, but significant heterogeneity was observed between studies focusing on melanoma (P = 0.04) and other solid tumors (P = 0.02) (Fig. 2).
Forest plots of subgroup analysis of clinical response of objective response rate (ORR) in the treatment of fecal microbiota transplantation (FMT) with immune checkpoints inhibitors (ICIs)
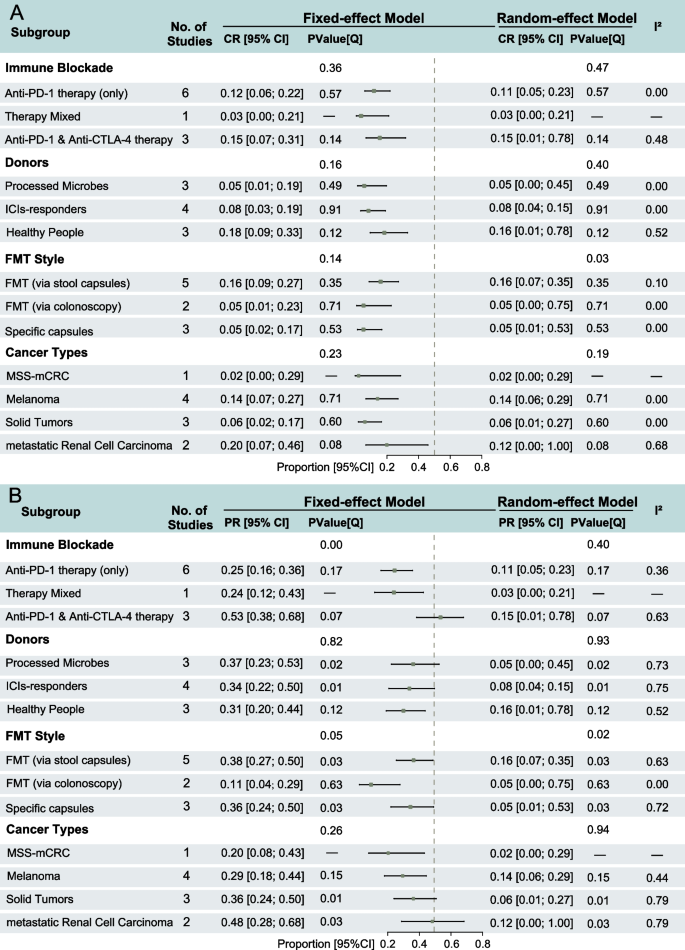
All included studies were eligible for CR rate analysis. The Egger’s test(t = − 4.36, p = 0.002, tau2 = 0.362) and LFK index of the Doi plot assessing studies reporting CR rates indicated minor publication bias (LFK = − 1.16) (Additional file 2: Fig. S2A). CR was achieved by few patients across most studies, and three studies did not report any CR cases. The pooled estimate for the CR rate was 12% (95% CI: 0.07–0.19). The heterogeneity test indicated low heterogeneity across studies (I2 = 8%, P = 0.37), indicating relative consistency in the results. Among the three two-arm randomized controlled trials, one study (Dizman et al., 2019) did not report any CR cases. The pooled RR for the two remaining trials was 0.79 (95% CI: 0.11–5.88), with the 95% CI crossing 1, suggesting no statistically significant difference in the CR rate between the treatment and control groups. Heterogeneity between these studies was low, although the heterogeneity test did not reach statistical significance (Additional file 2: Fig. S2B).
Subgroup analyses revealed that the CR rate comparisons across groups were consistent with those observed for ORR. None of the subgroup differences reached statistical significance, with FMT administration method using the random-effects model (P = 0.03, Fig. 3A).
Forest plots of subgroup analysis of clinical responses of complete response (CR) rate and partial response (PR) rate in the treatment of fecal microbiota transplantation (FMT) with immune checkpoint inhibitors (ICIs). A CR rate with 95% confidence intervals (CI); B PR rate with 95% CI
All included studies were eligible for PR rate analysis. The Egger’s test (t = − 2.99, p = 0.017, tau2 = 1.4117) and LFK index of the Doi plot assessing studies reporting PR rates indicated minor publication bias (LFK = − 1.16) (Additional file 2: Fig. S3A). The pooled PR rate estimate was 34% (95% CI: 0.26–0.42), with moderate heterogeneity across studies (I2 = 62%, P < 0.01), indicating considerable variability between studies. The PR rate ranged from 10% (Fernandes et al.) to 60% (Routy et al.). A meta-analysis of the three two-arm RCTs yielded a pooled RR of 1.54 (95% CI: 0.58–4.08), but demonstrated high heterogeneity (P = 0.04), indicating significant variability between studies (Additional file 2: Fig. S3B).
Subgroup analyses showed that PR rate comparisons between groups were largely consistent with those for ORR and CR rates. Compared to the fixed-effects model, the PR rates estimated under the random-effects model were more conservative. Significant heterogeneity existed between studies within each subgroup; notably, only the FMT administration method showed heterogeneity differences in both effect models, and the ICI treatment method showed heterogeneity differences only in the fixed-effects model (P = 0.00) (Fig. 3B).
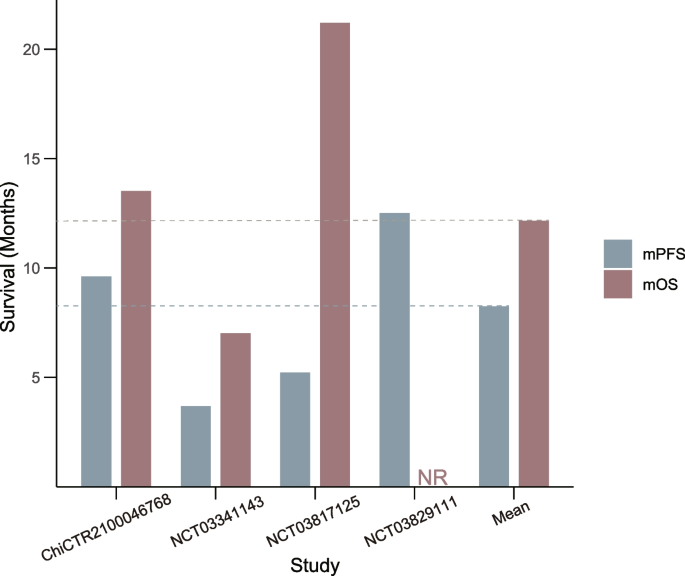
Of the studies included, four provided mPFS data, while three reported mOS data. All studies reported median follow-up times, although not all provided HR with corresponding 95% CI. The weighted average mPFS for 62 patients receiving FMT in combination with ICIs was 8.32 months. The weighted average mOS for 54 patients was 12.67 months (Fig. 4).
Weighted median progression-free survival (mPFS) and median overall survival (mOS) in studies combining fecal microbiota transplantation (FMT) with immune checkpoint inhibitors (ICIs)
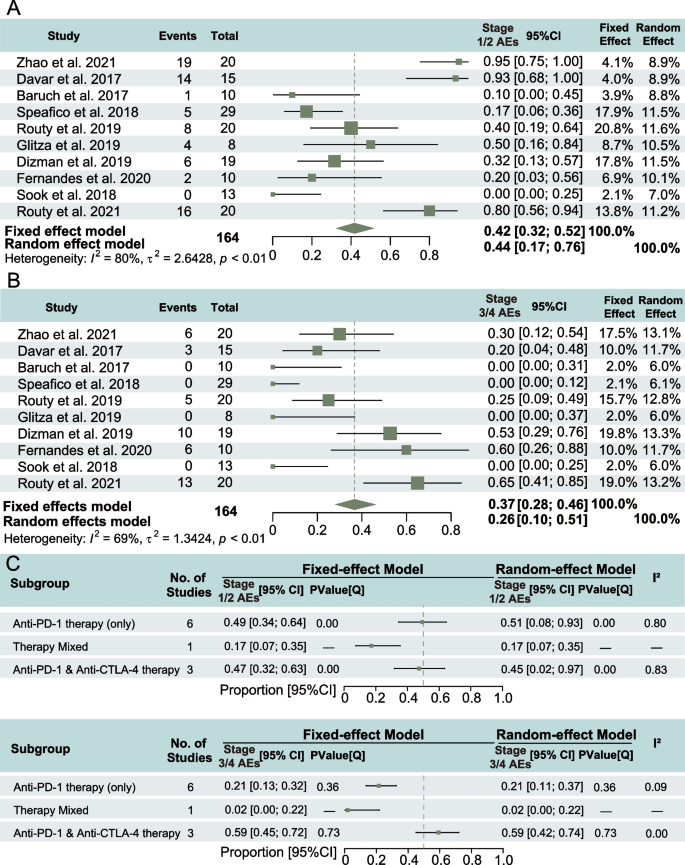
All studies reported on adverse events (AEs) of different grades, including clear descriptions of outcomes and resolutions. Four studies did not report any grade 3–4 AEs, while in three studies, more than half of the patients treated with FMT and ICIs experienced AEs. No fatal events related to FMT treatment were reported in any study. The pooled incidence of grade 1–2 AEs was 0.42 (95% CI: 0.32–0.52), and high heterogeneity was observed across studies (I2 = 80%, P < 0.01) (Fig. 5A). The pooled incidence of grade 3–4 AEs was 0.37 (95% CI: 0.28–0.46), with heterogeneity of I2 = 69%, P < 0.01 (Fig. 5B), suggesting a potential protective role of FMT against immune-induced adverse reactions.
Safety analysis of stages of adverse events (AEs) in the treatment of fecal microbiota transplantation (FMT) with immune checkpoint inhibitors (ICIs). A Incidence of stage 1/2 AEs with 95% confidence intervals (CI); B incidence of stage 3/4 AEs with 95% CI; C incidence of AEs with 95% CI in different immunotherapy
In the subgroup analysis of toxicity based on ICI strategy, the incidence of grade 1–2 AEs was higher for monotherapy compared to combination therapy, with a statistical significance of heterogeneity within each ICI approach (P = 0.00). Conversely, the incidence of grade 3–4 AEs was significantly higher in combination therapy compared to monotherapy. Notably, the heterogeneity within ICI approaches did not reach statistical significance (Fig. 5C).
This systematic meta-analysis comprehensively evaluated the clinical efficacy and safety of FMT combined with ICIs in patients with advanced and refractory solid tumors. The results demonstrated that FMT combined with ICI therapy provided significant clinical benefits, with an ORR of 43% (95% CI: 0.35–0.51), a CR rate of 12% (95% CI: 0.07–0.19), and a PR rate of 34% (95% CI: 0.26–0.42). Furthermore, subgroup analyses indicated that different ICI strategies (e.g., anti-PD-1 therapy, anti-PD-1 combined with anti-CTLA-4 therapy, and mixed therapies) had a significant impact on treatment outcomes of ORR and PR rate (P < 0.05). In terms of safety, FMT combined with ICIs showed a grade 1–2 AEs incidence of 42% (95% CI: 0.32–0.52) and a grade 3–4 AEs incidence of 37% (95% CI: 0.28–0.46). While adverse events were generally manageable, close monitoring of severe AEs is necessary. These findings offer valuable evidence for further exploring the role of FMT in immunotherapy and highlight areas for future research, including optimal FMT administration routes, personalized FMT strategies, and long-term efficacy and safety assessments.
The ORR of 43% observed in this meta-analysis for FMT combined with ICIs is notably higher than the 20–40% response rate reported in previous studies of monotherapy. This result supports the hypothesis that FMT may enhance the efficacy of ICIs by reshaping the gut microbiome(31), thus providing strong evidence for the efficacy of this combined treatment strategy. In recent years, several retrospective analyses have revealed a significant correlation between the gut microbiome and the efficacy of ICIs (48–51). For example, a study by Gopalakrishnan et al. involving melanoma patients found that gut microbial alpha diversity was significantly positively correlated with anti-PD-1 treatment response (P < 0.01). Moreover, a high abundance of Akkermansia muciniphila has been closely associated with better clinical outcomes in various cancers treated with ICIs (31). In a prospective study, the presence of Akkermansia muciniphila was associated with a significantly increased ORR (28% vs. 18%, P < 0.05) and median OS (18.8 vs. 15.4 months, P < 0.05) in NSCLC patients receiving ICIs compared to those without this bacterium(29). Additionally, gut microbial features associated with improved ICI efficacy or prognosis have been identified in melanoma, NSCLC, hepatocellular carcinoma, RCC, and gastrointestinal cancers(31,32). These findings offer a robust theoretical and biological basis for explaining how FMT might enhance ICI efficacy through the modulation of the gut microbiome. The higher CR rate (12%, 95% CI: 0.07–0.19) and PR rate (34%, 95% CI: 0.26–0.42) observed in this meta-analysis further support the potential advantages of FMT combined with ICIs in enhancing tumor response. Additionally, the mPFS and mOS for patients receiving FMT with ICIs were 8.32 months and 12.67 months, respectively. These significant clinical benefits may be attributed to the ability of FMT to reshape the gut microbiome, thus enhancing the anti-tumor immune response(31,32,52), which synergistically improves the efficacy of ICIs. Drawing from existing literature, we hypothesize that the mechanisms by which FMT enhances ICI efficacy may include the following (31,32,53–60): (1) increasing beneficial bacteria, such as Bifidobacterium longum, Enterococcus faecium, and Akkermansia muciniphila, which have been shown to enhance the anti-tumor effects of ICIs; (2) increasing gut microbial alpha diversity, thereby enhancing immune system function; (3) modulating the TME, promoting effector T cell infiltration and activation while inhibiting regulatory T cell function. These results are promising, but still highlight several critical knowledge gaps that warrant further investigation. Key questions remain regarding the optimal timing of FMT relative to ICI administration, the durability of microbiome changes following FMT, and the potential development of resistance mechanisms. Current research efforts are focusing on addressing these uncertainties through prospective trials incorporating longitudinal microbiome sampling and immune monitoring.
This study performed a detailed subgroup analysis to explore the potential impact of different ICI treatment regimens, FMT donor sources, administration methods, and tumor types on therapeutic outcomes. In terms of ICI strategies, the combination of anti-PD-1 and anti-CTLA-4 therapies achieved an ORR of 60%, which was significantly higher than the 37% for PD-1 inhibitors alone (P = 0.01). This result is consistent with previous studies and further supports the potential benefit of combination immunotherapy in clinical practice. This difference is likely attributable to the synergistic effects of PD-1 and CTLA-4 inhibitors, which activate a broader and more sustained anti-tumor immune response through distinct immune regulatory mechanisms (61). Moreover, FMT may enhance this synergistic effect by reshaping the gut microbiota, further enhancing the efficacy of ICIs. For example, a recent study found that specific gut microbes, including Prevotella copri, Bifidobacterium adolescentis, Faecalibacterium, Firmicutes bacterium, Barnesiella intestinihominis, Bacteroides eggerthii, Prevotella sp., Ruminococcus torques, and Odoribacter splanchnicus, may enhance the effects of both anti-PD-1 and anti-CTLA-4 therapies (62). These findings provide a solid theoretical foundation and potential clinical direction for FMT combined with dual immune checkpoint inhibition. Regarding FMT donor sources, using fecal material from responders to ICIs as donors appeared to result in a higher ORR (46%), although the difference was not statistically significant compared to other donor sources (P > 0.05). This trend aligns with a recent clinical study involving melanoma patients resistant to ICIs (NCT03341143), which found that FMT using fecal material from ICI responders significantly improved treatment outcomes in patients who initially did not respond, with 40% of patients achieving clinical benefit, including 1 CR, 2 PR, and 3 patients with stable disease (SD) for over a year (35). This phenomenon may be explained by the observation that the gut microbiota of ICI responders are enriched in features favorable for immunotherapy, such as higher alpha diversity, enrichment of beneficial bacteria (e.g., Akkermansia muciniphila and Bifidobacterium longum), and a metabolomic profile conducive to T-cell activation and anti-tumor immune responses. However, the possibility of significant differences in effect outcomes between studies using the same microbiota donor sources (such as from healthy populations, ICI responders, or processed microbial products) may be due to the lack of consistency in sampling, preparation, and preservation steps for microbiota donors across studies. Therefore, we need to emphasize the establishment of standardized protocols and management systems for the preparation of microbiota transplantation products. As for FMT methods, oral fecal microbiota capsules appeared to be more effective (ORR: 49%) compared to endoscopic delivery (ORR: 34%) or specially formulated capsules (ORR: 38%), although these differences were not statistically significant. This trend may be related to the sustained and uniform restructuring of the gut microbiota achieved through oral administration (63), as well as the potential for improved patient compliance and a lower incidence of AEs. Furthermore, differences in ORR across various tumor types were observed. For example, metastatic clear cell renal cell carcinoma had an ORR of 53%, melanoma had an ORR of 41%, solid tumors had an ORR of 45%, and MSS-mCRC had an ORR of 20%. Although these differences were not statistically significant, they suggest that the efficacy of FMT combined with ICIs may vary depending on the immunogenicity of the tumor, the characteristics of the TME (such as the abundance and functional status of tumor-infiltrating lymphocytes), and the tumor’s sensitivity to gut microbiome modulation (64). This finding emphasizes the need to optimize FMT protocols for specific tumor types in future research.
The safety profile of FMT combined with ICIs appeared generally acceptable, though careful monitoring of certain high-grade AEs remains essential. In this study, the incidence of grade 1–2 AEs was 42%, while grade 3–4 AEs occurred in 37% of patients. For comparison, in patients receiving ICI without restrictions of drug types, the incidence of any irAE is approximately 44.2%, with grade ≥ 3 irAEs seen in 15.7% of cases(65). The data on grade 1–2 AEs in this study are comparable to the safety profiles previously reported for ICIs alone, suggesting that FMT does not significantly raise the toxicity risk of ICI treatment. Interestingly, recent studies have suggested that FMT might not only avoid increasing the risk of AEs but may also reduce their occurrence in some cases. For instance, FMT has been shown to effectively treat ICI-induced colitis(66), indicating its potential to modulate immune responses and lower the risk of AEs. While FMT may reduce the risk of certain AEs (such as colitis), vigilance is still required regarding some high-risk adverse events, particularly microbial translocation leading to infections, primarily involving opportunistic bacterial and fungal infections in immunocompromised patients. While most gastrointestinal symptoms were self-limiting and manageable with supportive care, infection-related complications and metabolic disorders resulting from gut dysbiosis may necessitate treatment interruption and antimicrobial intervention. To prevent these high-risk AEs and fully leverage the protective potential of FMT, this study recommends several measures: strict donor screening to screen out potential pathogens, close monitoring of patients’ immune-related biomarkers and organ function, the development of personalized FMT protocols (including optimal dosing, frequency, and administration style), and the establishment of long-term follow-up mechanisms to assess the long-term safety of FMT and its impact on AE incidence. Additionally, this meta-analysis found that the incidence of grade 3–4 AEs in the combination ICIs group was higher compared to the monotherapy group. This finding underscores the need for enhanced safety monitoring in combination therapies and points to a promising avenue for exploring FMT as a potential strategy for AE prevention and management. Future studies should further investigate how FMT may reduce the incidence of AEs and explore how to optimize FMT protocols to maximize its protective effects while minimizing potential risks.
Despite providing valuable evidence regarding FMT combined with ICIs, several limitations of this meta-analysis should be noted. First, the limited number of included studies, with only 10 studies analyzed, most of which were early-phase clinical trials with small sample sizes, may have affected the robustness and generalizability of the findings. In addition, there was a lack of long-term follow-up data. This limitation prevented the meta-analysis from fully evaluating the long-term efficacy, safety, or potential delayed adverse events associated with FMT combined with ICIs due to this absence. Furthermore, the absence of randomized controlled trials was notable. The majority of the included studies were single-arm trials, lacking randomized control, which limits the ability to assess the true effect of FMT and increases the risk of selection bias and confounding factors. To address these limitations, future studies should focus on conducting large-scale, multicenter randomized controlled trials, using standardized FMT protocols and uniform efficacy assessment criteria to better establish the efficacy, safety, and optimal administration strategies of FMT combined with ICIs.
In conclusion, despite the aforementioned limitations, this meta-analysis provides preliminary evidence supporting the application of FMT combined with ICIs in the treatment of advanced or refractory tumors. The findings indicate that this combination therapy may offer significant clinical benefits, including improved objective response and disease control rates, with an acceptable safety profile. In the future, to further investigate the potential of FMT in the therapeutic application of ICIs, we should comprehensively examine the interactions among microbial specific properties, metabolites, colonization environments, immunopharmaceutical agents, tumorigenicity, and dietary strategies. Additionally, artificial intelligence algorithms should be leveraged to analyze human microbiome sequencing data, extracting key information for clinical applications. This interdisciplinary integration and cross-disciplinary collaboration will pave the way for significant breakthroughs in precision tumor therapy.
This meta-analysis provides a thorough evaluation of the clinical efficacy and safety of FMT combined with ICIs in the treatment of advanced or refractory solid tumors. The findings demonstrate significant clinical benefits, with an ORR of 43%, a CR rate of 12%, and a PR rate of 34%, and a generally manageable safety profile. Subgroup analyses revealed potential influences of ICI regimens, FMT donors, and administration methods on treatment outcomes, providing important insights for future research. Although this study is limited by small sample sizes and a lack of long-term follow-up data, it provides preliminary evidence supporting the application of FMT in immunotherapy, underscoring its potential as a strategy to enhance the efficacy of ICIs, particularly in patients resistant to ICIs. Large-scale, randomized controlled trials with long-term follow-up are necessary to further validate the efficacy and safety of FMT combined with ICIs. Additionally, these studies should aim to optimize personalized treatment protocols, including determining the optimal FMT timing, frequency, and duration, and exploring predictive biomarkers to offer new therapeutic options for patients with refractory tumors.
Supplementary information.
Additional file 1: Table.S1: Risk of bias in non-randomized studies of interventions and randomized controlled trials.
Additional file 2: Fig S1-S3. Fig. S1: A: Funnel plot and doi plot with LuisFuruya-Kanamori(LFK) index of studies in objective response rate (ORR) analysis for bias assessment. B: Forest plots of ORR in all studies and double-arm studies. Fig. S2: A: Funnel plot and doi plot with LuisFuruya-Kanamori(LFK) index of studies in complete response (CR) analysis for bias assessment. B: Forest plots of CR rate in all studies and double-arm studies. Fig. S3: A: Funnel plot and doi plot with LuisFuruya-Kanamori(LFK) index of studies in partial response (PR) analysis for bias assessment. B: Forest plots of PR rate in all studies and double-arm studies.
No datasets were generated or analysed during the current study.
- AEs:
-
Adverse events
- ASCO:
-
American Society of Clinical Oncology
- CI:
-
Confidence interval
- CR:
-
Complete response
- CTCAE:
-
Common Terminology Criteria for Adverse Events
- CTLA-4:
-
Cytotoxic T-lymphocyte-associated protein 4
- ESMO:
-
European Society for Medical Oncology
- FMT:
-
Fecal microbiota transplantation
- HR:
-
Hazard ratio
- ICIs:
-
Immune checkpoint inhibitors
- irAEs:
-
Immune-related adverse events
- LFK index:
-
Luis-Furuya-Kanamori index
- mOS:
-
Median overall survival
- mPFS:
-
Median progression-free survival
- MSS-CRC:
-
Microsatellite-stable colorectal cancer
- MSS-mCRC:
-
Microsatellite-stable metastatic colorectal cancer
- NSCLC:
-
Non-small cell lung cancer
- ORR:
-
Objective response rate
- OS:
-
Overall survival
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed death-ligand 1
- PFS:
-
Progression-free survival
- PR:
-
Partial response
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCC:
-
Renal cell carcinoma
- RCTs:
-
Randomized controlled trials
- ROB 2.0:
-
Revised Cochrane Risk of Bias tool
- ROBINS-I:
-
Risk of Bias in Non-randomized Studies of Interventions
- RR:
-
Risk ratio
- SD:
-
Stable disease
- SITC:
-
Society for Immunotherapy of Cancer
- TME:
-
Tumor microenvironment
This work was supported by grants from the Natural Science Foundation of Guangdong Province (2021A1515012593), the National Natural Science Foundation of China (82373129, 82172750, 82172811 and 82260546), the Guangdong Basic and Applied Basic Research Foundation (Guangdong‐Guangzhou Joint Funds) (2022A1515111212), the Science and Technology Program of Guangzhou (2023A04J1257), and Hunan Youth Science and Technology Talent Project (NO.2023RC3074).
Not applicable.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Lin, A., Huang, L., Jiang, A. et al. Microbiota boost immunotherapy? A meta-analysis dives into fecal microbiota transplantation and immune checkpoint inhibitors. BMC Med 23, 341 (2025). https://doi.org/10.1186/s12916-025-04183-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12916-025-04183-y