Biological Research volume 58, Article number: 29 (2025) Cite this article
Lead is a ubiquitous environmental and industrial pollutant with worldwide health problems. Lead acetate toxicity induces both genotoxic effects and apoptosis. The present study aimed to investigate the usage of mesenchymal stem cells (MSCs) in the treatment of the genotoxic effect of lead acetate (LA) in the testis and its effect on the expression of the apoptosis marker caspase-3 and the proliferation marker Ki-67 in the injured testicular tissue.
Twenty-one adult male rats were used in this investigation (7 rats/group). Group I received saline and served as the control group (ctrl group); Group II received lead acetate (100 mg/kg) and was called the LA group; Group III received both lead acetate (100 mg/kg) and MSCs (1 × 106 cells/rat) and was called the LA-MSCs group. Body and testis weight, plus semen analysis, were performed in all groups. Reproductive hormones, serotonin, and cortisol were determined in sera. Additionally, oxidative/antioxidative status and lead acetate-induced genetic variations were investigated. Immunohistochemical staining for the proliferation marker Ki-67 and the apoptosis marker caspase-3 was also performed.
revealed that the weight of the body and testis and semen parameters (sperm count, viability, and motility) of the LA group exhibited significant reduction compared to the Ctrl and the LA-MSCs group. In addition, the LA group showed reproductive hormonal imbalance and an increase in oxidative stress biomarkers compared to the LA-MSCs group that showed a significant improvement in these parameters. Compared to the ctrl group, the LA group showed a highly genetic distance value (0.0031), while the LA-MSCs group showed a low genetic distance value (0.0019). This illustrated that the LA-MSCs group exhibited reduced genetic variation induced by LA compared to the LA group. Histological evaluation indicated the presence of severe diffuse degeneration and necrosis in the spermatocytes in the LA group compared to the control one, while co-treatment by MSCs induced significant reduction in these degenerative changes. Immunohistochemical investigation revealed increased expression of the caspase-3 antibody in the testicular tissue of the LA group, while it is significantly decreased in the LA- MSCs group. In contrast, the KI67 antibody revealed a significant decrease in its expression in the LA group, while it was significantly increased in the LA-MSCs group after treatment by MSCs.
It can be concluded that the MSCs are a potential therapeutic for the treatment of testicular dysfunction induced by LA through the reduction of oxidative stress, genotoxic effect, and apoptosis marker caspase-3, and an increase in the proliferation marker Ki-67 in the testicular tissue associated with restoration of hormonal imbalance.
The rapid growth of contemporary industry has coincided with a sharp rise in heavy metal pollution of the environment in recent years. Human activity frequently causes the high levels of heavy metals in animal feed and feed products, whether through industrial production, agriculture, or unintentional or intentional usage [1]. Lead (Pb) is one of these metals, and its levels in both urban and periurban areas have significantly increased in recent years [2]. Paints, eye cosmetics, gasoline, enamels, and water pipes are important commercial products that contain this harmful material [3]. Lead causes a wide spectrum of physiological and biochemical dysfunctions and has a profoundly complex effect on the health of both humans and animals [4]. For examples, the neurological, hematological, gastrointestinal, cardiovascular, renal and reproductive systems are among the many bodily systems that are essentially impacted by this cumulative toxicant after being consumed, inhaled, or absorbed via the skin [5,6,7,8].
The precise mechanism of action of lead, a strong neurotoxic toxin, has not been fully understood despite decades of research and requires further clarification. The induction of apoptosis is one among the most proposed mechanisms of lead [9]. Because lead is a multi-target toxicant, it disrupts the delicate pro- and antioxidant balance in human cells by producing excessive amounts of reactive free radicals [5].
To lessen the symptoms of lead toxicity, a number of antioxidant strategies have recently been put forth [10]. A recent research has demonstrated the antioxidant properties of several natural products against a wide range of harmful metals [11].
In the male reproductive system, the pituitary–testicular axis is the primary site of major unfavorable lead reactions, which can result in morphological changes and a reduction in sperm count, ultimately leading to infertility [12]. Low androgen levels, increased aberrant sperm morphology, degeneration of spermatogonia and spermatocytes, necrosis of spermatogonia and Sertoli cells [13], and lower sperm quality [14], have all been linked to lead exposure. Lead inhibits the activities of the steroidogenic enzymes, which hinders spermatogenesis and Leydig cell steroidogenesis [15]. Follicle stimulating hormone (FSH), luteinizing hormone (LH), and testosterone production are all affected that may reach to their breakdown [16, 17]. Lead changes the maturation of sperm via affecting the epididymis [18].
Male reproductive impairment is caused by the toxic effects of lead, which are deposited in the testis, vas deferens, seminal vesicle, and ejaculate. Male reproductive failure linked to lead exposure has been linked to two mechanisms: apoptosis and testicular oxidative stress. According to previous report, lead raises the expression of the caspase-3 protein in rats'testes [19]. Additionally, it has been shown to promote lipid peroxidation of cells and a decrease in antioxidant enzyme activity, which leads to an overabundance of reactive oxygen species (ROS) and oxidative stress [19, 20]. Excessive ROS production has been connected to sexual dysfunction. Overproduction of ROS damages sperm DNA, sperm function, testicular metabolism, and fertilization-related fusion processes, leading to infertility [21].
Mesenchymal stem cells have been proved to be effective in treatment of variant diseases such as lung injury, liver injury and kidney injury, and their mechanisms include the following aspects. First, they play an anti-apoptotic role, stimulate tissue repair, and produce nutritional healing factors. Both transforming growth factor (TGF-β) and hepatocyte growth factor (HGF) have the ability to repair liver damage and cirrhosis [22, 23]. Lung damage is positively impacted by Nrf2 (NF-E2-related factor 2) and Keap1 [24, 25]. The healing of acute kidney injury is significantly impacted by both epidermal growth factor (EGF) and insulin-like growth factor (IGF) [26, 27]. Second, it is possible to create exosomes, which work with cytokines and contain a variety of tiny molecules, including proteins and nucleic acids.
Despite the above mentioned deleterious effect of lead toxicity and the above mentioned pharmacological and therapeutic properties of stem cells, little research has been done on the potential protective function of stem cells against the negative effects of reproductive toxicity caused by lead acetate. Therefore, the present study aimed to determine whether MSCs can ameliorate Pb-induced reproductive damage through investigation of regenerative effect of MSCs on genetic variation induced by LA toxicity and its effect on the expression of the apoptosis marker caspase-3 and proliferation marker Ki-67 in the testicular tissue.
Lead acetate was purchased from El-Gomhouria Company for Chemicals and Laboratory Supplies, located in Assiut, Egypt. Kits of Malondialdehyde (MDA), Superoxide dismutase (SOD), Catalase (CAT) and Glutathione peroxidase (GPx) were purchased from Biodiagnostic company, 29 El Tahrir, Ad Doqi, El Omraniya, Giza Governorate 3,750,164. The MSCs were purchased from Lab of Prof. Dr. Heba M. saad Eldien (Assiut University, Assiut, Egypt).
Twenty one male Albino Wistar rats, weighing 180–200 g, were obtained from the Department of Zoology’s Animal House, Faculty of Science, South Valley University, Qena, Egypt. The animals were maintained at a controlled temperature of 24 ± 1 °C with a 12–12 h light–dark cycle, and were allowed free access to water and standard chow ad libitum.
Twenty one male rats were randomly allocated into three groups (seven for each). The 1 st group received saline and served as the normal control group (ctrl group), while the 2nd group injected intraperitoneally with lead acetate (LA) (100 mg/kg body weight for seven days) and served as the standard model of acute LA-toxicity group [28] and called LA group. The 3rd group injected intraperitoneally with lead acetate (100 mg/kg b.w.t for seven days) and treated with a single dosage of mesenchymal stem cells (1 × 106 cells/rat intravenous route) [29, 30] then left for 30 days and called LA-MSCs group.
A sensitive electronic weighing balance (Scout Pro, Ohaus Corporation, USA) was used to assess body weight every day. All of the rats were sacrificed at the end of the trial using an appropriate dosage of ethyl ether. After dissection, an absolute weight of the testis was measured using the same device during sacrifice. In addition, extraction testis tissues was performed followed by one was immediately placed on ice, and after that, it was placed in liquid nitrogen, where it was promptly frozen at − 80 °C until the tissue was homogenized for biochemical analysis. While the other one for a full of 24 h was fixed in 10% natural formalin. For histopathology, it was then stored in 70% ethyl alcohol. Blood was collected and serum was separated by centrifugation at 3000 rpm for 15 min and stored at (− 20 °C) for hormonal analysis.
Epididymal sperm counts was performed according to the previous research [31], was used to do epididymal sperm counts. This method involved chopping the caudal epididymis in 5 mL of 0.9% NaCl and thoroughly mixing each sample with a shaker for 10 min. For two minutes, the suspension of epididymal sperm was incubated at 20 °C. For the supernatant dilution (1:100), an alkaline solution including eosin and 35% formalin was utilized. Sperm were counted using a Neubauer hemocytometer under a 200 × light microscope. The count is reported in millions per milliliter of the epididymal solution. A high-magnification (400×) light microscope was used to calculate the percentage of total sperm motility [32, 33]. Evaluations of sperm count and morphology were conducted in compliance with WHO recommendations.
Regarding sperm motility, evaluation was classified as grade A (fast progressive sperms that swim quickly in a straight-forward direction), grade B (slow progressive sperms that move forward but in a haphazard line) and grade C (non-progressive sperms that can move their tails but can’t move forward).
Commercially available ELISA kits (supplied by Biomatik Co., Kitchener, Ontario N2 C 1 N6, Canada) were used for determination of serum hormone assays for follicle-stimulating hormone (FSH), testosterone, and gonadotropin-releasing hormone (GnRH) with catalogue numbers: EKL54319, EKF58719, EKF60674, respectively. ELISA kits for the detection of serotonin and cortisol were purchased from BioSource Co., with catalogue numbers MBS494156 and MBS843477, respectively.
A portion of testis was homogenized by manual technique. Homogenates of the tissues were prepared in 1.0 ml of phosphate buffer per 100 mg of tissue. The samples were spun at maximum speed at 4 °C for the biochemical examination, and the supernatant was used. Tetramethoxypropane was used as an external standard in measurement of malondialdehyde (MDA) in accordance with the methodology of Ohkawa et al. [34]. The ability of superoxide dismutase (SOD) to prevent epinephrine from autoxidizing in an alkaline solution was used to measure its activity [35]. A colorimetric kit was used to assay catalase (CAT) activity in accordance with Lück’s [36]. Glutathione peroxidase (GPx) was determined according to Flohé and Günzler [37]. The supernatants from the tissue homogenates were used for colorimetric assays of (MDA, SOD, CAT & GPx in the tissues (using commercial kits supplied by Biodiagnostics, Egypt) with a spectrophotometer (Chem-7, Erba Diagnostics Mannheim GmbH, Germany).
By using the DNA extraction method of QIAamp DNA Mini kit (Qiagen, Hidden, Germany) DNA was isolated from the tissues following the manufacturer’s instructions.
Primers that were previously described by Simon et al. [38] were used in the amplification of genomic DNA using a total volume of 40 μL. Including 1 μL of DNA, 1 μL of both forward and reverse primers, 17 μL of nuclease-free water, and 20 μL of master mix. In the PCR amplification process, an initial denaturation at 95 °C for three minutes was followed by thirty cycles of denaturation, annealing, and extension for one minute at 94 °C, 48 °C, and 72 °C, respectively. At 72 °C, cycling was finalized with a 10-min extension. Electrophoresis was used to separate and visualize the PCR products on an agarose gel stained with 1.5% ethidium bromide. Each group produced a single band from the PCR amplification. To obtain the accession numbers, the sequences were sent to GenBank/NCBI.
Sequence alignment was performed using MUSCLE [39] with default settings. Phylogenetic analyses among the three groups and Rattus norvegicus MZ782915.1from GenBank based on partial sequences of (16S rRNA) gene were performed with MEGA version 11.0.11 [40] using Neighbour Joining phylogenetic method. The bootstrap analysis was determined with 1000 replicates [41]. The sequence divergences were calculated using Kimura2-parameter distances [42].
Rats were slaughtered at the end of the study, and the left and right testes were removed, weighed, and prepared. Fixation in 10% Neutral Buffered Formalin, dehydration with increasing alcohol grades (two changes of 70%, 90%, and 100% alcohol), clearing in xylene, embedding in molten paraffin, and cooling to produce tissue blocks were all steps in the tissue processing procedure.
Haematoxylin and Eosin (H&E) staining was applied to blocks of study tissues that had been cut into slices 5 μ thick. In order to stain, the following steps were taken: dewax in xylene, hydrate with decreasing alcohol grades (100%, 90%, and 70% alcohol) and distilled water, stain in hematoxylin, wash under running water, distinguish in 1% acid alcohol (1% HCl in 70% alcohol) for, blue in tap water for, rinse in distilled water, stain in eosin, rinse in distilled water, dehydrate with increasing alcohol grades (70%, 90%, and 100% alcohol), clear in xylene, and mount using Distrene polystyrene xylene (DPX).
According to Johnson scoring system, the spermatogenesis was evaluated with five sections per slide and 10 seminiferous tubules per field with a scale of 1–10 points [43, 44].
The immunohistochemical staining procedures were done as the following: sections were dewaxed and immersed in a solution of 0.05 M citrate buffer, pH 6.8 for antigen retrieval. These sections were then treated with 0.3% (H2O2) and protein block. Then, sections were incubated with polyclonal anti-caspase 3 antibodies (Invitrogen, Cat# PA5-77,887, dilution 1/100), and monoclonal mouse of Ki67 antibody, Dako, USA M7248 and 1/100 dilution. After rinsing with phosphate buffered saline, they were incubated with a goat anti-rabbit secondary antibody (Cat# K4003, EnVision + ™ System Horseradish Peroxidase Labelled Polymer; Dako) for 30 min at room temperature. Slides were visualized with DAB kit and eventually stained with Mayer's hematoxylin as a counter stain. The immunolabelling indices of both caspase 3 and Ki67 were presented as a percentage of positive expression in a total 1000 cells per 8 HPF.
Means ± Standard deviation of means (Mean ± S.D) was used to express the degree of results variability. A one-way ANOVA analysis of variance was used to assess the data statistically (the Prism pad computer software, USA) then the Newman-keuls T-test, and to assess for treatment differences, the least significant difference (L.S.D.) was utilized. When the P-value of the results is < (0.001), they are considered statistically significant.
Initially, all rats had almost the same body weight at the start of the experiment. However, by the end of the experiment, both the LA group and the LA-MSCs group experienced a significant decrease in body weight compared to the control group (P < 0.001, P < 0.01), respectively. Surprisingly, there was a significant increase in the weight of LA-MSCs group when compared to LA group (P < 0.01) (Table 1).
Regarding testicular weight, LA group exhibits a significant reduction in comparison to both control and LA-MSCs groups (P < 0.001). In contrast, there was no significant difference between control and LA-MSCs group (Table 1).
As shown in Table 2, the sperm count, and viability were significantly decreased in LA group (P < 0.001) in comparison to the control and LA-MSCs group (P < 0.05). Interestingly LA- MSCs group shows a significant increase when compared to LA group (P < 0.01). In control group, no pus cells were found in semen, but LA group exhibits a significant increase in comparison to control group (P < 0.001), while LA-MSCs group shows a considerable decrease when compared to LA group (P < 0.001).
Regarding sperm motility: grade A was absent in LA group, shows a significant increase in LA-MSCs group (P < 0.001). For grade B, both LA and LA-MSCs groups show a non-significant differences compared to the control group. For grade C, no significant difference was observed among groups.
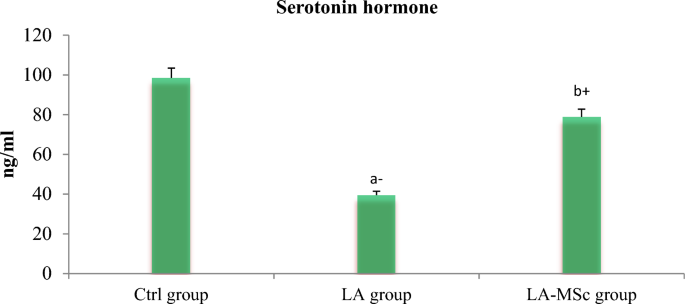
Table 3 shows the serum levels of the reproductive hormones, serotonin and cortisol in the tested group. Compared to the control group, serum levels of GnRH, testosterone, FSH, and serotonin were significantly decreased (P < 0.001) in the LA group (Figs. 1, 2 and 3). In contrast, the group of LA-MSCs showed significant increase in the all previously mentioned hormones compared to the LA group. Serum cortisol shows non-significant difference among groups (Fig. 4).
Serum levels of gonadotropin-releasing hormone (GnRH), and testosterone in the control group, LA group, and LA-MSCs group of adult male albino rats. Ctrl group: control group; LA group: Lead acetate group and LA- MSCs group: lead acetate co-treated with mesenchymal stem cells group. + Significant increase at (p < 0.05). a → significantly different from control group.—Significant decrease at (p < 0.05). b → significantly different from Lead group. All Values are mean ± S.D (n = 7)
Serum levels of follicle-stimulating hormone (FSH) in the control group, LA group, and LA-MSCs group of adult male albino rats. Ctrl group: control group; LA group: Lead acetate group and LA-MSCs group: lead acetate co-treated with mesenchymal stem cells group. + Significant increase at (p < 0.05). a → significantly different from control group.—Significant decrease at (p < 0.05). b → significantly different from Lead group. All Values are mean ± S.D (n = 7)
Serum levels of serotonin in the control group, LA group, and LA-MSCs group of adult male albino rats. Ctrl group: control group; LA group: Lead acetate group and LA-MSCs group: lead acetate co-treated with mesenchymal stem cells group. + Significant increase at (p < 0.05). a → significantly different from control group.—Significant decrease at (p < 0.05). b → significantly different from Lead group. All Values are mean ± S.D (n = 7)
Serum levels of cortisol in the control group, LA group, and LA-MSCs group of adult male albino rats. Ctrl group: control group; LA group: Lead acetate group and LA-MSCs group: lead acetate co-treated with mesenchymal stem cells group. All Values are mean ± S.D (n = 7)
As shown in Table 4, the level of MDA was significantly increased in LA group in comparison to control and LA-MSCs group (Fig. 5). In addition, there was a significant decrease in the levels of SOD, CAT and GPX in LA group in comparison to control group (P < 0.001) (Fig. 6 and 7). LA- MSCs group showed a significant increase in the three parameters in comparison to LA group.
Levels of malondialdehyde (MDA) in the testicular tissue of the control group, LA group, and LA-MSCs group of adult male albino rats. Ctrl group: control group; LA group: Lead acetate group and LA-MSCs group: lead acetate co-treated with mesenchymal stem cells group. + Significant increase at (p < 0.05). a → significantly different from control group.—Significant decrease at (p < 0.05). b → significantly different from Lead group. All Values are mean ± S.D (n = 7)
Levels of superoxide dismutase (SOD), and glutathione peroxidase (GPx) in the testicular tissue of the control group, LA group, and LA-MSCs group of adult male albino rats. Ctrl group: control group; LA group: Lead acetate group and LA-MSCc group: lead acetate co-treated with mesenchymal stem cells group. + Significant increase at (p < 0.05). a → significantly different from control group—Significant decrease at (p < 0.05). b → significantly different from Lead group. All Values are mean ± S.D (n = 7)
Levels of catalase (CAT) in the testicular tissue of the control group, LA group, and LA-MSCs group of adult male albino rats. Ctrl group: control group; LA group: Lead acetate group and LA-MSCc group: lead acetate co-treated with mesenchymal stem cells group. + Significant increase at (p < 0.05). a → significantly different from control group. - Significant decrease at (p < 0.05). b → significantly different from Lead group. All Values are mean ± S.D (n = 7)
In this investigation, Partial sequences of the mt16S rRNA gene in each of the three groups were identified. The fragment sizes ranged from 537 to 540 bp, with minimal size variation between groups. The16S rRNA’s partial nucleotide sequences of were add in the GenBank with accession numbers (OM570616- OM570618). The resulting concatenated alignment comprised 540 base pairs, of which 534 were sites that were conserved. The average A + T attribution was 60.8, surpassing than the C + G. Each group's sequence length, A + T and C + G contents, as well as their average, were shown in (Table 5).
Between the control and treated groups, the P-distances varied from 0.0000 to 0.0031. All groups combined had a distance value of 0.01%. The genetic effects of lead acetate were reflected in the highest P-distance (0.0031) between control group and LA group. However, there was a lower value (0.0019) between control group and LA-MSCs group (Table 6).
For every clade recovered by Neighbour Joining (NJ) clades, the study evaluated the level of support using bootstrap values. The phylogenetic analysis revealed that LA group formed a separate cluster, while the remaining (OM570616.1, MZ782915.1—Control and OM570618.1—Lead acetate + stem cells) found in one clad (Fig. 8).
Phylogenetic tree using the Neighbour Joining method among the three groups and Rattus norvegicus MZ782915.1from GenBank based on partial sequences of (16S rRNA) gene
Figure 9 showed the pathological changes that occurred in the testis in the three tested groups using the same magnification. Testis of ctrl group showing normal seminiferous tubules lined with normal spermatogenic cell layers with presence of free sperm in the lumen. While, the testis of LA group showing marked degenerative and necrotic changes within the seminiferous tubules, desquamation of some necrotic cells and edema within the interstitial tissue. In addition, sever diffuse degeneration and necrosis in the spermatocytes was also observed compared to control one. After the treatment with MSCs, the testes are showing marked improvement of the degenerative changes within the spermatogenic cells with appearance of sperms within the lumen.
a Testis from the control group showing normal seminiferous tubules lined with intact spermatogenic cell layers (S) and abundant free sperm in the lumen (arrows). b Testis from the lead acetate group showing marked degenerative and necrotic changes in the seminiferous tubules (S), desquamation of necrotic cells, and interstitial edema (arrow). c Testis from the lead acetate + mesenchymal stem cells (MSCs) group showing a marked reduction in degenerative changes in spermatogenic cells (arrow), with the reappearance of sperm in the lumen. H&E staining, bar = 50 µm, × 200.
Johnsen score was found 10 in the ctrl group that refers to maximum spermatogenesis activity, while it was 3.3 in LA group that refer to complete absence of spermatogenesis. Whereas the LA-MSCs group showed enhancement in the spermatogenic status as Johnsen score was found 8.6.
The immunohistochemistry investigation of caspase3 and Ki67 were presented as a percentage of positive expression. Results revealed an obvious increase in the average percentage of Caspase3 antibody in the testicular tissue of LA group, while it is significantly decreased in the testis of rats treated with MSCs (LA-MSCs group). In contrast, statistics showed a normal KI67 percentage in the control group (2.2%), while the LA group showed a highly significant decreasing level of KI67 percentage (1.7%). The group of LA-MSCs showed a noticeable enhancement of KI67 percentage (2.9%) (Fig. 10).
a Photomicrographs of testicular tissue from control animals immunostained with Ki67 antibody, showing strong nuclear expression of Ki67 in spermatogenic cells (arrows). b Photomicrographs of testicular tissue from the lead acetate group immunostained with Ki67 antibody, showing a significant decrease in Ki67 expression within spermatogenic cell layers (arrows). c Photomicrographs of testicular tissue from the lead acetate + mesenchymal stem cells (MSCs) group immunostained with Ki67 antibody, showing a marked increase in Ki67 expression in spermatogenic cells (arrows). d Photomicrographs of testicular tissue from control animals immunostained with caspase-3 antibody, showing mild cytoplasmic expression of caspase-3 in spermatogenic cells (arrows). e Photomicrographs of testicular tissue from the lead acetate group immunostained with caspase-3 antibody, showing significant increase in caspase-3 expression in spermatogenic cell layers (arrows indicating nuclear expression). f Photomicrographs of testicular tissue from the lead acetate + MSCs group immunostained with caspase-3 antibody, showing a marked decrease in caspase-3 expression in spermatogenic cells (arrows). Ki67 and caspase-3 IHC, bar = 50 µm, × 200.
Due to its pervasiveness in soil, water, and air, lead is a widely researched environmental pollutant that still poses a major risk to human health. It is commonly known that lead is hazardous to various systems, including the male reproductive system [45, 46].
In our study, significant decrease of the testes and body weights were observed in LA exposed rats compared to controls as well as MSCs treated group. This indicating that the general metabolic condition of LA treated animals suffers from disturbance in contrast to the animal received MSCs treatment which remains normal. Our result agreed with the previous studies that concerned with lead acetate toxicity [17, 19]. According to the latter research, rats given lead had considerably lower index weights for the testis, epididymis, and accessory sex glands than the control group.
In the current trial, the sperm motility investigation revealed that the lead-treated group experienced a disturbance in sperm motility, compared to the Ctrl and LA-MSC groups, which displayed normal sperm motility. This suggests that MSCs treatment had a positive effect on sperm motility compared to the untreated group. According to a recent study, the sperm motility test showed that the model + MSCs treatment group had significantly higher sperm motility than the LA toxic group. This finding supports the idea that MSCs have a reparative effect on the testicular tissue of mice and improve sperm motility [47]. These results also demonstrate that elevated lead levels are significantly negatively correlated with common semen characteristics and sperm function biomarkers [48, 49].
One of the most significant methods that were applied in variant studies as a method of evaluation of the fertility of animal and human is assessment of the reproductive hormones [50,51,52]. Compared with the Ctrl group in the current study, the contents of reproductive hormones and serotonin in the LA group were significantly decreased, indicating that gonadal hormone secretion was disordered. After the intervention of MSCs, reproductive hormones content and serotonin content increase, and gonadal hormone secretion tended to a normal level. Several researches stated that the decrease in T levels after exposure to LA toxicity may result from the indirect effects of LA on the hypothalamic-pituitary–testicular (HPT) axis, and the structural and functional integrity of male reproductive organs [53, 54]. In line with earlier findings, a recent study that examined the proteins involved in the synthesis of steroid hormones in mouse testes discovered that lead significantly lowers serum testosterone levels and disrupts enzyme activity and gene expression levels during the synthesis of male steroid hormones [54,55,56].
Malonaldehyde was used in different studies as an indicator for oxidative stress either by its evaluation in the blood or in the tested tissues [57,58,59]. Since tissue MDA is a byproduct of peroxidized polyunsaturated fatty acids (PUFA), it is an essential diagnostic metric for identifying oxidative stress. An increase in lipid peroxidation is suggested by the elevated MDA level. MDA levels in rat tissues have been shown to rise in response to pesticides like acetamiprid and heavy metals like lead [51, 60]. In the present study, MDA level increased in the LA group compared to the other two groups. These results were consistent with recent reports that lead increased the levels of lipid peroxide (LPO) and MDA, in testicular tissue [56]. A higher level of oxidative stress (OS) can harm the gonads by causing damage to the nuclear and mitochondrial DNA, as well as the sperm plasma membrane. This is associated with shorter telomere length, the production of 8-OHdG, and mitochondrial DNA fragmentation. Leydig cell activity generally declines as a result of this, affecting proteins including the steroidogenic acute regulatory protein (StAR), which controls mitochondrial cholesterol uptake [61, 62].
The current study's findings unequivocally demonstrated that rats given lead acetate had much lower levels of the antioxidants SOD, catalase, and Gpx. These results are consistent with previous study showed that rats exposed to lead had significantly lower levels of the antioxidant enzymes SOD and catalase activity in their testes [63]. In the current investigation, rats given MSCs plus LA had higher levels of SOD, catalase, and Gpx than rats given lead alone. MSCs can eliminate free radicals, lower the oxidation rate, and increase the expression of the heme oxygenase system, particularly SOD [47].
One of the most dangerous hazards of lead is that it influence the genetic through chromosomal abnormalities [13, 64] and micronucleus [65]. These genotoxic may involve direct DNA damage that affects chromatin stabilization [66] or engaging with repair procedures [67]. In this study, the sequence sizes ranged from 537 to 540 base pairs (bp). According to Simon et al. [38], the 16Sar and 16Sbr primers amplify a fragment between 500 and 650 bp, so the observed sizes fell within the expected range. The final alignment obtained was 540 bp in length, with 534 of those sites being conserved. This aligns with previous studies reporting that mitochondrial genes are highly conserved across many animal species. [68, 69].
The high P-distance (0.0031) found between ctrl group and LA group in the current research reflected the genetic effects of lead acetate. Recent study agreed with these findings and illustrated presence of highest P-distance value (0.002) between the lead acetate-treated mice and the control non-treated one [70]. According to multiple studies, mice exposed to LA at doses of 50, 100, 200, and 400 mg/kg body weight exhibited a significant increase in the incidence of chromosomal abnormalities in their bone marrow [71]. Furthermore, it was noted that sucking rats exposed to lead either subchronically (for nine days) or cutely (in a single intraperitoneal injection) experienced genome damage from low doses of lead acetate, which had no effect on the growth and development of the rats [72]. Consequently, other research linked the direct impact of lead on DNA structure and oxidative processes to the observed damaging effect of lead on mice’s DNA [73, 74]. Additionally, lead ions were found to impair DNA repair mechanisms and reduce the fidelity of DNA synthesis [13, 75].
The results of 16S rRNA sequences displayed that the P-distances between the Ctrl group and LA-MSCs group was 0.0019 which was smaller than that in the LA group. Thus, stem cells reduced the genotoxic effect of LA that may be due to the MSCs were described as multi-potent because of their ability for differentiation into a variety of different cells and tissue lineages [76]. Stem cells models considered as new tools for drug discovery and predictive toxicology due to their exclusive characteristics relevant to stemness, self-renewal and differentiation ability to various cell types [77].
The histological investigation in the present study showed that, compared to the Ctrl group, the LA group's seminiferous tubules displayed significant degenerative and necrotic alterations, including edema in the interstitial tissue and desquamation of some necrotic cells. Similar results were recorded in the previous study that stated male wistar rats exposed to LA exhibited a testicular parenchymal atrophy, various deteriorating histological abnormalities, as well as inhibition of spermatogenesis [78]. Furthermore, a recent study investigating the effects of subacute lead exposure in rats [79] revealed that testicular injuries included degeneration and depletion of germinal cells, alterations in Sertoli cells, reduced semen quality, changes in the shape and size of spermatozoa, and inflammatory responses.
Johnsen’s score was used to evaluate lead-induced regressive histological damage. The observed damage, including disrupted seminiferous tubules and a reduction in spermatogenic cells, as indicated by lower Johnsen’s scores, may be correlated with impaired spermatogenesis [80, 81].
In the current study, the significant increase of the average percentage of Caspase-3 antibody in the LA group indicated the harmful effect of the LA toxicity in the reproductive aspects of the toxicated animal. The current findings are similar to that was reported in the recent studies [82, 83]. It was stated that LA activates caspase-3 in spermatogenic cells, which triggers apoptosis, and it also causes DNA damage and apoptosis in spermatozoa [54]. As a result, lead exposure has lately been identified as a significant contributor to male infertility and testicular dysfunction [54, 84]. Male infertility results from excessive exposure to lead compounds, which lowers semen quality and reproductive capacity [84].
In the present study, KI67 antibody revealed an obvious decrease in its average percentage in the testicular tissue of LA group, while its percentage significantly increased in the LA-MSCs group after treatment by MSCs. Similar findings were reported in the previous studies that indicate the deleterious effect of lead toxicity was not limited to damage of the tissue but extend to decrease the Ki-67 expression that is responsible for cell proliferation [85, 86]. According to earlier research, Ki-67 expression is a prognostic indicator for a number of malignancies and is connected with cell proliferation essential for this malignancies [87]. The latter study shows that the cancer stem cell niche is diminished in human epithelial breast and colon cancer cells when Ki-67 is genetically disrupted. This provides evidence that the commencement of Ki-67 expression and cell proliferation are positively correlated with stem cells.
Finally, MSCs therapy has shown potential in treating medical conditions, specifically lead poisoning-induced male infertility. However, the clinical relevance and/or limitations of translating these findings to human therapy should be investigated further. Research indicates that MSCs injections may enhance germ cell differentiation and support the regeneration of gonadal tissue. Previous experimental studies in animal models of azoospermia have demonstrated promising outcomes using MSCs-based approaches. Although these therapies are not yet considered standard practice in human medicine, the encouraging results provide a hopeful outlook for their future application in routine clinical settings [88, 89].
It is suggested that MSCs treatment may reduce the risk of infertility, especially in men with occupational exposure to lead, such as workers in industries like battery manufacturing, construction (especially demolition or renovation), smelting, and radiator repair. Using MSCs to treat male infertility caused by lead poisoning is a promising area of regenerative medicine. However, several limitations exist in these aspects, such as most data are from preclinical animal models, meaning human trials are needed to confirm safety and efficacy. Additionally, there are ethical and regulatory barriers, as stem cell therapies require strict regulation, particularly regarding cell sourcing, purity, and delivery. Further optimization is needed, including determining the best type of MSCs (e.g., bone marrow, adipose tissue, umbilical cord), the optimal dose and administration route, and avoiding long-term effects and potential risks (e.g., tumorigenicity) [90, 91].
The MSCs is a potential therapeutic for the treatment of LA related testicular dysfunction through the reduction of oxidative stress, reduction of the genotoxic effect, reduction of the apoptosis marker caspase-3, increase the proliferation marker Ki-67 in the testicular tissue and restoration of hormonal imbalance.
This article contains all the data that was created or evaluated during the research.
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open-access funding is provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
The experiment described in this thesis was performed in adherence to the zoology department, faculty of science. Guidelines on the care and use of laboratory animals and approval of the ethic committee of south Valley University were obtained before the study with the Code No. 018/11/22. All animal experiments complied with the ARRIVE guidelines and were carried out in accordance with the U.K. Animals (Scientific Procedures) Act, 1986 and associated guidelines, EU Directive 2010/63/EU for animal experiments.
Not applicable.
There are no competing interests declared by the authors.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Allam, M., Amin, Y.A., Fouad, S.S. et al. Mesenchymal stem cells reduce the genotoxic effect of lead acetate in the testis of male rats and induce testicular cellular proliferation indicated by 16S rRNA sequence, increase the proliferation marker Ki-67 and a reduction in the apoptosis marker caspase-3. Biol Res 58, 29 (2025). https://doi.org/10.1186/s40659-025-00614-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40659-025-00614-5