BMC Medical Imaging volume 25, Article number: 272 (2025) Cite this article
Renal ectopic fat deposition and hypoxia are linked to renal function damage, but regional differences within the kidney and the relationship between renal fat deposition and abnormal oxygen metabolism remain unclear.
To investigate the regional characteristics of the kidney and the interaction between abnormal renal fat deposition and oxygen metabolism in type 2 diabetes mellitus (T2DM).
A total of 26 healthy participants and 105 patients with T2DM underwent Dixon scan to quantify renal cortex and medulla proton-density fat fraction (PDFF) and relaxation rate (R2*). Patients with T2DM were classified into three groups by estimated glomerular filtration rate (eGFR). Differences in PDFF and R2* between groups and across renal compartments were analyzed using One-way ANOVA. Pearson’s correlation analysis assessed variable relationships, and receiver-operating-characteristic curve evaluated diagnostic performance.
R2* values in the medulla were significantly higher than those for the cortex. Both PDFF and R2* values in the renal cortex and medulla differed significantly across the four groups, showing an increasing trend with disease severity: control group < T2DM < diabetic kidney disease (DKD) stage I-II < DKD stage III-IV. PDFF and R2* values exhibited a significant positive correlation. Renal medulla PDFF value demonstrated the best diagnostic performance in distinguishing: (1) T2DM patients without DKD from healthy controls (2), T2DM without DKD from those with DKD stage I-II, and (3) DKD stage I-II from DKD stage III-IV.
Generally, the renal medulla is more vulnerable to hypoxic injury, and disruptions in renal oxygen metabolism closely linked ectopic fat deposition. Quantitative MRI offers a promising approach for early detection and monitoring of diabetes-related renal damage.
Diabetes mellitus is a chronic metabolic disorder posing a major global public health challenge [1]. Diabetic kidney disease (DKD), the most prevalent microvascular complication of diabetes, is the leading cause of end-stage renal disease (ESRD) and is strongly associated with increased mortality in diabetic patients [2]. Approximately 50% of individuals with type 2 diabetes mellitus (T2DM) are susceptible to developing DKD [3]. In China, the large population base has contributed to an estimated 20 million cases of ESRD attributable to diabetes, imposing a significant societal and familial burden [4, 5]. Early diagnosis and intervention in DKD can delay and prevent the further deterioration of renal function in diabetic patients. However, the pathophysiology of DKD is complex, involving multiple pathways and mediators [6].
Although the precise mechanisms underlying kidney damage in T2DM remain under investigation, key contributors include dysregulated glucose and lipid metabolism, altered renal hemodynamics, and chronic inflammation [7,8,9,10]. Recent evidence highlights a strong association between hypoxia and renal ectopic fat depositions in kidney damage in patients with T2DM. The accumulation of fat in the kidneys leads to damage of renal mesangial cells, proximal tubular epithelial cells, and podocytes. These detrimental processes may play an important role in the initiation and advancement of DKD [11]. Particularly, an increase in renal fat content disrupts microvascular function by mechanical compression, inflammatory responses, and oxidative stress to reduce renal blood flow, which compromises renal perfusion and leads to tissue hypoxia [10]. Simultaneously, the surplus fat stored in the kidneys contributes to insulin resistance and harms mitochondrial function within cells, disrupting normal oxygen metabolic processes [12]. This interference negatively affects the cells’ ability to utilize oxygen and produce energy. Furthermore, renal lipid accumulation in renal tissues exacerbates fibrosis, further aggravating hypoxia [13]. Ultimately, these factors collectively lead to diminished kidney function and heightened hypoxia. At the same time, renal hypoxia leads to metabolic dysfunction, inflammation, and oxidative stress, which may collectively promote abnormal fat accumulation in the kidneys [9]. This creates a harmful feedback loop, further amplifying the pathological mechanisms driving the progression of DKD. However, renal biopsy, combined with immunohistochemical staining, is currently the gold standard for evaluating renal fat deposition and hypoxia in patients with T2DM [7, 14]. It is inherently invasive and carries risks of complications such as bleeding and pain. Additionally, sampling limitations may result in diagnostic inaccuracies, as the obtained tissue may not be representative of the overall renal pathology. Therefore, the regional differences in renal fat deposition and abnormal oxygen metabolism in the kidney are unclear. This highlights the necessity for developing dependable and noninvasive methods to facilitate early diagnosis and ongoing monitoring of renal fat deposition and hypoxia in patients with T2DM.
Quantitative magnetic resonance imaging (MRI) offers a comprehensive array of noninvasive techniques for evaluating renal function, encompassing parameters such as diffusion, perfusion, oxygenation, and hemodynamics. Among these, blood oxygen level-dependent (BOLD) MRI has gained widespread application in the assessment of renal oxygenation changes across various kidney pathologies by exploiting the paramagnetic properties of deoxyhemoglobin. Specifically, the relaxation rate (R2*) is inversely correlated with oxyhemoglobin concentration, such that a rise in R2* values is associated with a reduction in local tissue oxygenation [15]. While BOLD MRI provides valuable insights into renal oxygen metabolism, its scope is limited to the quantification of R2*, and it lacks the capacity to measure renal fat deposition quantitatively.
The Dixon technology is a widely recognized quantitative MRI technique that produces separate water-only and fat-only images by utilizing the chemical shift phenomenon between fat and water protons [16]. Iterative decomposition of water and fat with echo asymmetry and least square estimation quantification (IDEAL-IQ) is a three-point Dixon technique. It produces water, fat, R2*, and proton density fat fraction (PDFF) images by in a single breath-hold [17, 18]. PDFF maps enable precise quantification of fat content, while R2* maps can be employed for quantitative analysis of paramagnetic substances, such as iron. Given that hemoglobin contains iron, the R2* value is directly proportional to deoxyhemoglobin content, providing an indirect reflection of local tissue oxygenation [19, 20]. IDEAL-IQ has been widely utilized in fat quantification and iron overload research in the liver, pancreas, and skeletal system [18, 21,22,23,24]. However, studies investigating the use of IDEAL-IQ for evaluating renal hypoxia and the extent of renal function impairment remain notably limited.
Consequently, the aim is to reveal regional differences and interactions between renal ectopic fat deposition and hypoxia in patients with T2DM. Additionally, this study aims to assess the effectiveness of IDEAL-IQ imaging in identifying kidney damage.
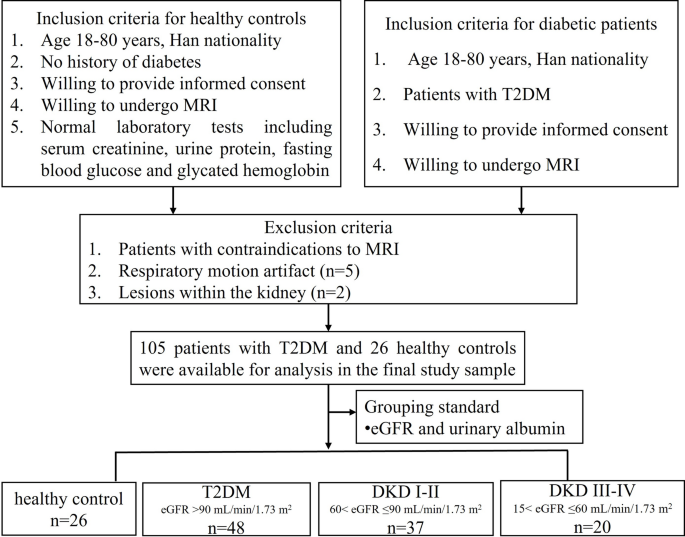
This cross-sectional study, conducted between January 2022 and February 2024 at Guizhou Provincial People’s Hospital. A total of 112 patients with T2DM were consecutively enrolled from the Departments of Endocrinology and Nephrology for this study. Of these, seven individuals were excluded for specific reasons: five due to omitted due to respiratory motion artifacts resulting from inadequate breath-holding, which significantly compromised image quality, and two due to excluded due to the presence of either large simple kidney cysts (> 2 cm) or more than three cysts or other renal lesions. The diagnosis of DKD was determined by the treating physician, based on pathological biopsy findings, estimated glomerular filtration rate (eGFR), urine albumin-to-creatinine ratio (UACR), and the duration of type 2 diabetes mellitus (T2DM), among other factors including diabetic retinopathy status, and longitudinal changes in serum creatinine (Scr) [25]. The remaining 105 patients were categorized into three groups based on the diagnostic criteria for DKD, using eGFR and albuminuria levels. Patients with an eGFR ≥ 90 mL/min/1.73 m² were classified as having T2DM without DKD. Those with 90 > eGFR ≥ 60 mL/min/1.73 m2 were diagnosed with T2DM and DKD stages I-II, while patients with 60 > eGFR ≥ 15 mL/min/1.73 m2 were categorized as having T2DM and DKD stages III-IV. At the initial visit, all patients underwent a comprehensive physical examination, including anthropometric assessments and blood and urine sample collection. Demographic and laboratory data, including age, gender, height, weight, and T2DM history, were collected. Laboratory assessments included Scr, eGFR, UACR, serum glucose (GLU) and microalbuminuria. Additionally, 26 healthy volunteers were recruited as controls, and demographic information such as age, gender, weight, and height were collected. The control group comprised individuals without a history of hypertension, diabetes, vascular disease, or primary chronic kidney conditions. Participants were excluded if they were pregnant or lactating, or had liver disorders, infections, cancer, urinary tract obstructions, renal artery blockages, heart failure, claustrophobia, or any implanted pacemakers or ferromagnetic objects. The detailed inclusion and exclusion criteria are outlined in Fig. 1.
All MRI examinations were conducted using a 3.0-T MRI scanner (Discovery MR 750 W, GE Healthcare, Milwaukee, WI, USA). Standard eight-channel abdominal surface receiver coils were utilized, with participants positioned supine. A respiratory belt was positioned around the abdomen to track and record respiratory signals. Prior to the examination, all participants underwent breath-hold training at the end of expiration. Each examination comprised coronal and axial T2-weighted single-shot fast spin-echo sequences. Furthermore, breath-hold axial IDEAL-IQ scans were obtained during end-expiration. The IDEAL-IQ imaging parameters were as follows: repetition time, 6.2 ms; echo time, 2.5 ms; slice thickness, 5 mm; matrix size, 160 × 160; field of view, 420 × 336 mm; and acquisition time, 17 s. Water-phase, fat-phase, fat fraction, and R2* maps were automatically reconstructed.
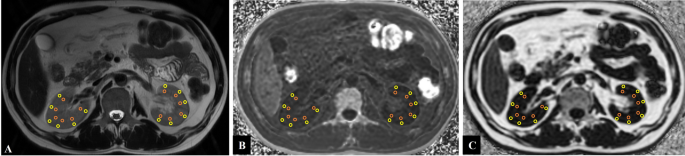
Image analyses were carried out on anonymized data utilizing a picture archiving and communication system (AW.4.6), conducted by two experienced abdominal radiologists (TC, 9 years of experience; RL, 3 years of experience), both of whom were blinded to the demographic and clinical information, except for the identification of benign renal lesions like cysts. T2-weighted imaging (T2WI) served as an anatomical reference to differentiate between the renal cortex and medulla. Six regions of interest (ROIs), each with an area of approximately 10–25 mm², were carefully placed on both the renal cortex and medulla, ensuring avoidance of artifacts or contamination from renal sinus fat, perirenal fat, or benign renal lesions. For each kidney, the PDFF and R2* values of the cortex and medulla were calculated as the average of the six ROIs. To reflect the overall function of bilateral renal function, the PDFF and R2* values for the cortex and medulla from each kidney were averaged. Figure 2 presents representative examples of ROIs overlaid on the PDFF map, R2* map, and T2WI images for both kidneys.
Examples of ROIs placements in the bilateral kidneys: PDFF and R2* values of cortex and medulla were measured in the central slice of renal hilum. (A) Six ROIs were selected per kidney in the renal cortex (yellow circle) and medulla (orange circle) on the T2WI map; (B) The same ROIs were copied from the T2WI map and manually placed on the R2* map to ensure consistency. (C) Likewise, the same ROIs were copied from the T2WI map and applied to the fat fraction map. To ensure consistency and comparability of the data, we ensured that the selected lesions in the T2WI, R2* and fat fractions maps were located in the same plane. (ROIs, regions of interest; PDFF, proton-density fat fraction; R2*: relaxation rate)
Statistical analyses were conducted using SPSS version 26.0 software (IBM, Chicago, USA). Continuous variables that adhered to a normal distribution were reported as mean ± standard deviation (SD), whereas non-normally distributed variables were expressed as medians along with interquartile ranges. Comparisons between groups for non-normally distributed data were conducted using the Kruskal-Wallis H test. Categorical variables were reported as frequencies and compared using the chi-square tests. The intra-class correlation coefficient (ICC) assessed inter-rater reliability for renal PDFF and R2* measurements. Paired t-tests were used to compare PDFF and R2* values between the left and right kidneys to evaluate the uniformity of renal fat deposition and hypoxia. Differences in clinical data and renal PDFF and R2* values among the four groups were analyzed using one-way ANOVA. Post hoc pairwise comparisons were performed using Fisher’s Least Significant Difference tests. Pearson’s correlation analysis was used to assess the relationships between variables. The diagnostic performance of IDEAL-IQ in distinguishing healthy controls, T2DM, DKD stage I-II, and DKD stage III-IV was assessed using receiver operating characteristic (ROC) curves. The areas under the curve (AUCs) were compared to determine statistical significance. All statistical analyses were two-tailed, and a significance threshold of p < 0.05 was established.
Table 1 provides a summary of the baseline characteristics for all participants. The gender distribution among the four groups did not show any significant differences (P = 0.276). The ICC for PDFF values was 0.845 for the cortex and 0.853 for the medulla. For the R2* values, the ICC was 0.867 for the cortex and 0.912 for the medulla.
PDFF and R2*values did not significantly differ between the left and right kidneys (PDFF, cortex, P = 0.659; medulla, P = 0.198; R2*, cortex, P = 0.093; medulla, P = 0.508) (Table 2). Table 3 displays the mean values and SD of PDFF and R2* values for each group. There were no significant differences in PDFF values of the cortex and medulla among the four groups (control, P = 0.868; T2DM, P = 0.114; DKD stage I-II, P = 0.567; DKD stage III-IV, P = 0.075). In contrast, the R2* values for the medulla were significantly higher than those for the cortex across all groups (control, P < 0.001; T2DM, P < 0.001; DKD stage I-II, P = 0.035; DKD stage III-IV, P < 0.001). Additionally, both PDFF and R2* values significantly differed among the groups, following the trend: control < T2DM < DKD stage I-II < DKD stage III-IV (PDFF: control, 0.98 ± 0.09, 0.99 ± 0.14; T2DM, 1.18 ± 0.07, 1.20 ± 0.03; DKD stage I-II, 1.26 ± 0.04, 1.27 ± 0.04; DKD stage III-IV, 1.35 ± 0.04, 1.33 ± 0.03. R2*: control, 14.71 ± 2.25, 16.48 ± 2.33; T2DM, 17.01 ± 1.47, 18.00 ± 1.62; DKD stage I-II, 20.23 ± 1.39, 20.82 ± 1.50; DKD stage III-IV, 23.03 ± 1.36, 24.58 ± 1.31).
The correlations between PDFF, R2*, and biochemical parameters are summarized in Table 4. Among the combined group of healthy controls, T2DM, and DKD patients, a significant positive relationship was observed between PDFF and R2* values (cortex PDFF: cortex R2*, r = 0.717; medulla R2*, r = 0.672; medulla PDFF: cortex R2*, r = 0.692; medulla R2*, r = 0.628). For both patients with T2DM and DKD, a notable positive correlation was identified between PDFF values and Scr (cortex, r = 0.522; medulla, r = 0.622), ACR (cortex, r = 0.283; medulla, r = 0.381) and microalbuminuria (cortex, r = 0.284; medulla, r = 0.351). Conversely, there was a significant negative correlation between PDFF values and eGFR (cortex, r = − 0.568; medulla, r = − 0.694). Similarly, R2* values demonstrated positive correlations with Scr (cortex, r = 0.622; medulla, r = 0.689), ACR (cortex, r = 0.366; medulla, r = 0.427) and microalbuminuria (cortex, r = 0.357; medulla, r = 0.381), while showing a negative correlation with eGFR (cortex, r = − 0.672, medulla, r = − 0.718). No significant correlations were found between PDFF or R2* values and BMI (PDFF, cortex, r = 0.159, P = 0.121, medulla, r = 0.147, P = 0.051; R2*, cortex, r = 0.164, P = 0.111, medulla, r = 0.256, P = 0.012) and GLU (PDFF, cortex, r = − 0.043, P = 0.679, medulla, r = − 0.034, P = 0.744; R2*, cortex, r = − 0.108, P = 0.296, medulla, r = − 0.082, P = 0.427).
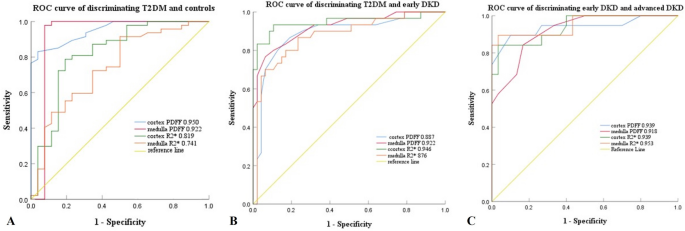
The ROC curves for the cortex and medulla PDFF and R2* values used to differentiate between the four groups (healthy controls, T2DM, DKD stage I-II, and DKD stage III-IV) are depicted in Fig. 3, with the corresponding diagnostic performance metrics listed in Table 5. Of all the PDFF and R2* values, the PDFF values of the renal medulla demonstrated the highest diagnostic accuracy in distinguishing T2DM from healthy controls, T2DM from DKD stage I-II, and DKD stage I-II from DKD stage III-IV.
Receiver operating characteristic curves of the diagnostic performance of PDFF (%) and R2* (seconds− 1) values of the renal cortex and medulla. (A) discriminating T2DM from control group (B) discriminating early DKD from T2DM (C) discriminating advanced DKD from early DKD. Numbers are areas under the curves for each parameter (PDFF, proton-density fat fraction; R2*, relaxation rate; HC, healthy control; T2DM, type 2 diabetes mellitus; DKD, diabetic kidney disease)
We investigated renal R2* and PDFF values derived from IDEAL-IQ imaging to evaluate renal function in T2DM patients with varying degrees of kidney damage. Our findings indicate that as diabetic renal damage progresses, PDFF and R2* values in both the renal cortex and medulla progressively increase. Specifically, T2DM patients exhibited significantly elevated PDFF and R2* values in both regions compared to healthy controls. Additionally, patients with DKD had higher PDFF and R2* values than both healthy controls and T2DM patients without DKD, with the highest values observed in patients with moderate to severe renal damage compared to those with mild damage. In addition, R2* values in the medulla were greater than those cortex, highlighting that the renal medulla is more vulnerable to hypoxic injury. Moreover, we identified a positive correlation between renal hypoxia and increased renal fat deposition. These findings underscore the regional characteristics and interaction between abnormal renal fat deposition and oxygen metabolism in T2DM. IDEAL-IQ may thus serve as a promising quantitative imaging modality for tracking changes in renal fat content and oxygenation, providing valuable insights into the progression of renal dysfunction in diabetes.
The absence of a significant BMI-renal fat deposition correlation in our study implies that renal lipid accumulation is not strictly dependent on systemic adiposity. Ectopic renal fat deposition likely arises from organ-specific mechanisms, including insulin resistance, localized metabolic dysregulation, and aberrant lipid handling, rather than general obesity [13]. Therefore, BMI inability to differentiate fat distribution patterns (subcutaneous vs. visceral vs. ectopic) further limits its utility in assessing lipid-driven renal pathology. This dissociation underscores the necessity for direct renal fat quantification, via imaging or histopathology, to evaluate its role as a discrete biomarker of metabolic kidney disease.
Although the accuracy of IDEAL-IQ technology in quantifying renal fat fraction has yet to be validated against kidney biopsies, it has been successfully validated in other tissues [26, 27]. In previous studies, we demonstrated the feasibility of IDEAL-IQ for assessing renal parenchymal fat deposition [28]. Furthermore, renal parenchymal and perirenal fat deposition have been demonstrated in T2DM patients by quantitative MRI, highlighting the potential roles in DKD pathophysiology [14, 29, 30]. However, distinctions between cortical and medullary fat deposition have not been fully explored. In this study, we separately evaluated fat deposition in the cortex and medulla and found no significant differences between these regions, consistent with previous findings [20].
Susceptibility artifacts, motion-related distortions, and limited spatial resolution affect BOLD MRI accuracy, particularly in the medulla. Additionally, factors such as hydration status, medications, and systemic hemodynamics can impact results [31,32,33]. Although there is limited literature on the application of Dixon sequences for renal oxygenation, our results indicate that multi-echo Dixon-based R2* quantification holds promise as a valuable tool for evaluating renal oxygenation in patients with T2DM. Furthermore, our study explored differences in cortical and medullary renal hypoxia, revealing that R2* values in the medulla were markedly greater than in the cortex. This finding is consistent with previous studies and can be attributed to the natural oxygen partial pressure gradient between the renal cortex and medulla [20, 34]. The renal cortex, with its higher blood perfusion, is more resilient to hypoxia, whereas the medulla, which has lower oxygen levels but higher oxygen consumption due to tubular reabsorption, is more susceptible to ischemia and hypoxia [35]. These findings corroborate earlier research, highlighting that the renal medulla is more vulnerable to hypoxic injury, with greater structural and functional damage observed in this region. In contrast, a study examining patients with stage 5 DKD found no notable differences in R2* values between the medulla and cortex in individuals with moderate-to-severe renal dysfunction [36]. This discrepancy may be due to our exclusion of patients undergoing renal dialysis, which could have influenced the results. The characteristic severe tissue alterations in advanced renal failure may confound water-fat separation in IDEAL-IQ and compromise fat fraction measurement accuracy.
We confirmed that IDEAL-IQ is capable of detecting subtle changes in renal fat deposition and hypoxia across different stages of the T2DM population, which has significant clinical implications. Even in diabetic patients without complications, there is a noticeable increase in lipid content and hypoxia in both the renal cortex and medulla. This increase becomes more pronounced in patients with accompanying DKD. These findings are consistent with the histological and biochemical results reported in previous human and animal studies [32, 37, 38]. Although renal PDFF values remain relatively low in patients with T2DM and DKD, even slight increases in renal fat content have considerable pathophysiological significance [39]. Multiple studies have established that the accumulation of fat within the kidneys is an independent risk factor for renal injury. Ectopic renal fat deposition is associated with lipotoxicity, inflammation, and fibrosis [40, 41]. This study demonstrates that ectopic lipid deposition in the kidneys can occur in the early stages of T2DM. Thus, early intervention to reverse or reduce ectopic lipid deposition in the kidneys may offer a viable strategy for protecting renal function in T2DM patients.
Renal fat deposition and hypoxia are significantly correlated with the severity of renal injury in T2DM patients. Renal fat accumulation and chronic hypoxia synergistically drive DKD through interconnected metabolic and oxidative stress pathways [33]. Fat accumulation in the kidneys induces lipotoxicity, disrupting mitochondrial energy metabolism and activating oxidative stress cascades [35]. This process damages glomerular filtration barriers by promoting podocyte loss and extracellular matrix remodeling [42]. Concurrently, persistent hypoxia-inducible factors (HIF-1α and HIF-2α) signaling pathways exacerbate renal fibrosis and inflammation [33]. Notably, lipid overload and hypoxia reciprocally amplify reactive oxygen species production, overwhelming endogenous antioxidant defenses and establishing a self-perpetuating cycle of cellular injury [40]. These pathophysiological processes disrupt interorgan communication and may play a pivotal role in the development and advancement of DKD [13, 43]. These mechanisms highlight therapeutic opportunities to concurrently target lipid metabolism, hypoxia adaptation, and oxidative stress in DKD management.
In light of these findings, we further analyzed the correlation between renal fat deposition and hypoxia. Results shows that increased fat deposition has been correlated with lower oxygen levels within the kidney. However, the mechanisms connecting renal fat deposition and hypoxia remain incompletely understood, but current evidence suggests that fat accumulation disrupts normal metabolic processes, leading to reduced oxygen utilization. Firstly, excessive fat triggers insulin resistance, impairing glucose metabolism and reducing energy availability to kidney cells [7]. Additionally, free fatty acids and inflammatory mediators induce oxidative stress and mitochondrial damage, further compromising cellular oxygen use and energy production [12]. Fat deposition also alters renal hemodynamics, resulting in impaired oxygen delivery and reduced metabolic efficiency [13, 40]. These processes ultimately contribute to diminished kidney function and heightened hypoxia. Simultaneously, renal hypoxia promotes ectopic fat deposition through various mechanisms. Hypoxia-induced oxidative stress impairs mitochondrial function, reduces fatty acid oxidation, and facilitates lipid accumulation in renal cells [31, 44]. Moreover, local fat deposits release inflammatory cytokines that aggravate insulin resistance, impede lipid metabolism, and perpetuate a cycle of fat accumulation [45, 46]. This establishes a harmful cycle between renal hypoxia and ectopic fat deposition, ultimately accelerating the progression of DKD. Previous preclinical animal studies using MRI have demonstrated the impact of renal fat deposition on kidney oxygenation [32]. Our study extends these findings to human subjects, providing further evidence supporting the relationship between renal fat deposition and hypoxia, thereby reinforcing its clinical significance.
Specially, therapeutic strategies aimed at reducing renal fat accumulation, such as sodium-glucose cotransporter 2 (SGLT2) inhibitors like canagliflozin - have shown promise in improving renal oxygenation and mitigating the damaging effects of hypoxia in DKD [35, 47]. These drugs exhibit several renal-protective mechanisms, including the restoration of tubuloglomerular feedback, reduced intrarenal renin-angiotensin-aldosterone system (RAAS) activation, and increased production of ketone bodies, which collectively help mitigate lipid accumulation and hypoxia in the kidneys [48]. In summary, the interplay between renal fat deposition and hypoxia is a significant contributor to the pathogenesis of DKD. This dual interaction raises critical therapeutic implications, suggesting that interventions targeting both renal fat deposition and hypoxia may improve patient outcomes.
Our study demonstrates that quantitative MRI exhibits high diagnostic efficacy, sensitivity, and specificity in monitoring changes in renal fat deposition and hypoxia across various stages of T2DM. Evidence suggests that early-stage DKD may be reversible [49]. Therefore, early diagnosis and an objective assessment of the severity of kidney damage are crucial for devising appropriate treatment strategies, improving prognosis, and reducing complications. Traditionally, DKD is diagnosed through continuous monitoring of UACR and eGFR. However, the accuracy, sensitivity, and specificity of these markers are often debated, especially in patients with early-stage DKD or uncomplicated diabetes, where ACR or eGFR may remain normal [49]. Consequently, invasive renal biopsy is still considered the gold standard for diagnosing DKD. This highlights the challenge of detecting and preventing diabetic nephropathy at an early stage, a key limitation in predicting disease progression. To address this issue, novel biomarkers are necessary. In our study, we observed that diabetic patients with normal urinary albumin levels exhibited significantly higher PDFF and R2* values compared to healthy controls, suggesting that these MRI-derived metrics may detect abnormal renal function earlier than UACR. Additionally, even when conventional laboratory tests return normal results, subtle damage to renal microstructures, reflected in decreased oxygen levels and increased lipid deposition, can already be present. Thus, non-invasive MRI imaging may offer an additional tool to assess renal function in T2DM patients, enabling the early detection of renal pathophysiological changes driven by hyperglycemia. Early clinical intervention based on these findings could prove effective in preventing or delaying the onset of renal parenchymal damage in T2DM patients.
Our study has some limitations that warrant consideration. Firstly, the correlation between pathological findings and quantitative MRI was not explored, as not all patients had progressed to a stage of DKD where renal biopsy was required. Additionally, the cross-sectional design restricts our ability to infer causal relationships between renal parenchymal fat deposition and hypoxia. Longitudinal studies are needed to clarify these associations over time. Future research should also examine the effects of medical interventions.
In conclusion, the renal medulla is more vulnerable to hypoxic injury than the cortex, and disruptions in renal oxygen metabolism are closely linked to ectopic fat deposition. Quantitative MRI enables the indirect measurement of renal tissue blood oxygen levels and fat accumulation, offering the potential to detect kidney function alterations before morphological changes occur. Therefore, IDEAL-IQ may emerge as a promising imaging tool for the early identification and accurate staging of renal damage in T2DM.
The raw datasets used during the current study are available from the corresponding author on reasonable request.
- AUC:
-
Area under the curve
- BMI:
-
Body mass index
- BOLD:
-
Blood oxygen level-dependent
- DKD:
-
Diabetic kidney disease
- DM:
-
Diabetes mellitus
- eGFR:
-
Estimated glomerular filtration rate
- HC:
-
Healthy control
- ICC:
-
Intra-class correlation coefficient
- IDEAL-IQ:
-
Iterative decomposition of water and fat with echo asymmetry and least squares estimation quantification sequence
- MRI:
-
Magnetic resonance imaging
- MRS:
-
Magnetic resonance spectroscopy
- PDFF:
-
Proton density fat fraction
- ROC:
-
Receiver-operating-characteristic
- ROIs:
-
Region of interest
- R2*:
-
Relaxation rate
- SD:
-
Standard deviation
- Scr:
-
Serum creatinine
- T2DM:
-
Type 2 diabetes mellitus
- WHR:
-
Waist-hip ratio
This study was supported by National Natural Science Foundation of China (82060314, 82460344), Guizhou hundred levels of personnel training program project QKHPTRC-GCC [2023] 083 and Guizhou Science and Technology Project QKHZC [2021] YB037.
The study protocol and design were approved by the Institutional Review Board of Guizhou Provincial People’s Hospital (approval number: KY 2022-02). Informed written consent was taken from all participants.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Liu, J., Yang, D., Tian, C. et al. Magnetic resonance imaging for noninvasive assessment of renal fat deposition and hypoxia in diabetic kidney disease. BMC Med Imaging 25, 272 (2025). https://doi.org/10.1186/s12880-025-01816-9







_1751880097.jpeg)




