Cardiovascular Diabetology volume 24, Article number: 189 (2025) Cite this article
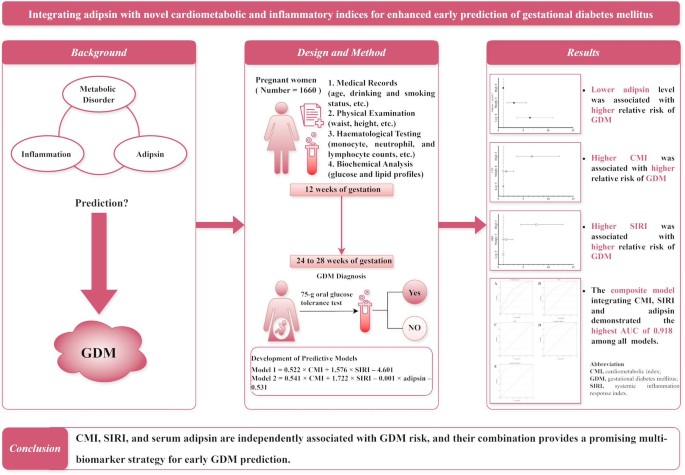
Early identification of individuals at risk for gestational diabetes mellitus (GDM) is essential for mitigating its adverse effects on both maternal and foetal health. This study aimed to evaluate the predictive value of the cardiometabolic index (CMI), systemic inflammation response index (SIRI), and serum adipsin levels for GDM.
A total of 1660 pregnant women were enrolled in this study conducted in Suzhou, China. Baseline clinical data, including blood glucose levels, lipid profiles, and blood cell counts, were collected at 12 weeks of gestation. GDM was diagnosed between 24 and 28 weeks of gestation. Logistic regression and receiver operating characteristic (ROC) curve analyses were performed to assess the associations and predictive performance of CMI, SIRI, and adipsin for GDM.
Compared with non-GDM participants, those with GDM exhibited significantly higher CMI and SIRI values and lower serum adipsin levels at baseline. Increased CMI and SIRI, as well as reduced adipsin levels, were independently associated with a higher risk of GDM in both unadjusted and adjusted models (all P < 0.05). The composite model incorporating all three biomarkers achieved a higher area under the curve (AUC) of 0.918 compared with the individual models for CMI (AUC = 0.825), SIRI (AUC = 0.802), and adipsin (AUC = 0.724).
CMI, SIRI, and serum adipsin are independently associated with GDM risk, and their combination provides a promising multi-biomarker strategy for early GDM prediction. Further studies are needed to validate these findings in diverse populations.
What is currently known about this topic?
What is the key research question?
What is new?
How might this study influence clinical practice?
Gestational diabetes mellitus (GDM) is a common metabolic disorder characterised by glucose intolerance that typically develops during the second or third trimester of gestation. GDM not only increases the risk of maternal preeclampsia and future type 2 diabetes but also contributes to foetal macrosomia and raises the likelihood of long-term metabolic complications in offspring [1, 2]. Identifying reliable and minimally invasive biomarkers for early GDM prediction is crucial for enhancing clinical outcomes and minimising long-term complications, as many existing early predictive indicators have suboptimal sensitivity and specificity [3].
The cardiometabolic index (CMI) is a recently proposed composite marker that reflects both metabolic and cardiovascular risk by incorporating measures of lipid levels and body fat distribution [4]. Similarly, the systemic inflammation response index (SIRI) is a novel biomarker that quantifies systemic inflammation based on neutrophil, monocyte, and lymphocyte counts [5]. Since both metabolic dysregulation and chronic inflammation are implicated in GDM pathogenesis, it is plausible that CMI, SIRI, or both could serve as valuable predictive biomarkers for GDM.
Adipsin, also known as complement factor D, is an adipokine secreted by adipose tissue that plays a vital role in metabolic homeostasis and inflammation modulation [6]. It enhances insulin secretion, preserves β-cell function, and regulates lipid metabolism via the complement system. Although adipsin has been implicated in the pathogenesis of several metabolic diseases, the literature regarding adipsin’s role in metabolic dysfunction remains controversial. Some studies, including meta-analysis, have reported the inverse associations of adipsin with several metabolic and cardiovascular diseases, such as type 2 diabetes [7, 8], insulin resistance [9], non-alcoholic fatty liver disease [10], and atherosclerosis [11]. In contrast, Pan et al. demonstrated that elevated serum adipsin level was associated with metabolic dysfunction-associated fatty liver disease, with both adipsin concentrations and the severity of fatty liver increasing as the number of metabolic risk factors rises [6]. Similarly, Wang et al. found a positive correlation between elevated serum adipsin and cardiovascular risk factors [12]. Although further verification is required, the potential role of adipsin in regulating glucose and lipid metabolism warrants investigation into its association with GDM, given that GDM shares some similar pathophysiological features with type 2 diabetes, including insulin resistance and metabolic dysregulation.
Given the substantial burden of GDM and its associated health complications, the identification of robust biomarkers for early prediction remains clinically pertinent. While previous studies have highlighted the potential individual roles of adipsin, CMI, and the SIRI in metabolic and inflammatory status, their combined predictive potential for GDM has not been thoroughly explored. This study aims to address this gap by assessing the predictive accuracy of these biomarkers, both individually and in combination, providing a novel multi-biomarker approach for early GDM prediction.
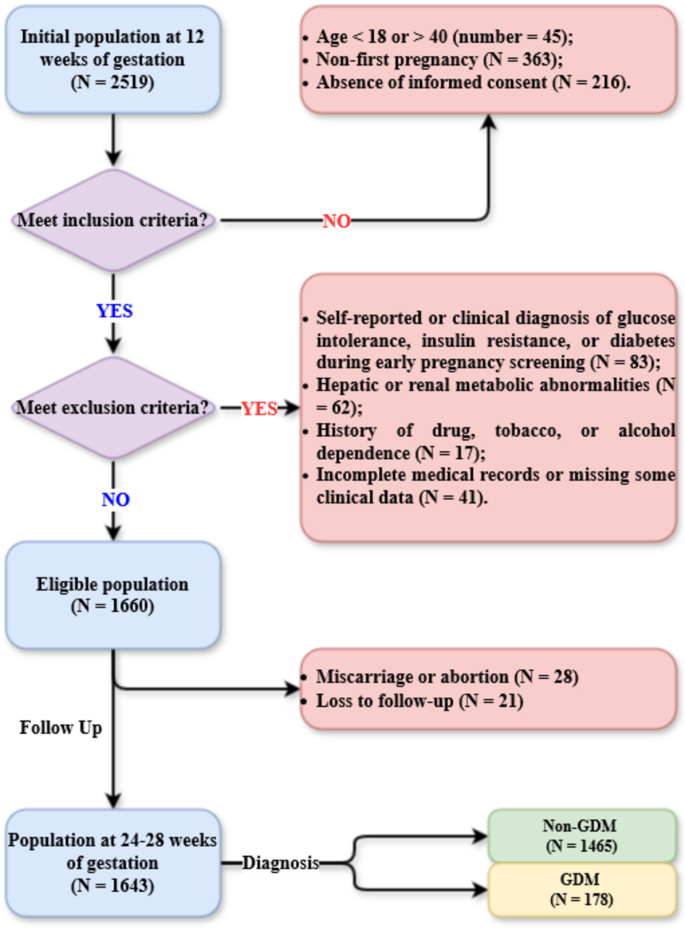
This study initially enrolled all pregnant women (N = 2519) who delivered at the Second Affiliated Hospital of Soochow University between 1 January 2023 and 31 December 2023. The inclusion criteria were as follows: (1) aged between 18 and 40 years; (2) first pregnancy; and (3) provision of signed informed consent. The exclusion criteria were as follows: (1) self-reported or clinically diagnosed glucose intolerance, insulin resistance, or diabetes at the first-trimester (12 weeks of gestation) prenatal check-up; (2) hepatic or renal metabolic disorders, such as metabolic dysfunction-associated fatty liver disease; (3) history of drug, tobacco, or alcohol dependence; and (4) incomplete medical records or missing clinical data. The participant selection and follow-up flowchart is presented in Fig. 1. This study was approved by the Ethics Committee of The Second Affiliated Hospital of Soochow University. The design and all procedures of this study were in accordance with the Declaration of Helsinki.
Basic demographic and clinical information was obtained from hospital medical records. Physical examination was conducted at 12 and 24–28 weeks of gestation. At 12 weeks of gestation, following an overnight fast, venous blood samples were collected for laboratory analysis. A 2 mL whole blood sample was collected into a vacuum tube containing EDTA as an anticoagulant for automated haematological testing. Additionally, a 5 mL whole blood sample was collected into an anticoagulant-free vacuum tube for serum biochemical analysis. For serum preparation, the 5 mL blood sample was left at room temperature for 30 min, after which the clotted blood was centrifuged at 2000 g for 10 min at 4 °C. The resulting serum was immediately aliquoted into separate tubes and stored at − 80 °C to prevent degradation due to freeze-thaw cycles. Monocyte, neutrophil, and lymphocyte counts were measured using an automated haematology analyser (Mindray SAL 8000, Mindray Bio-Medical Electronics Co., Ltd., China). Serum glucose, triglyceride (TG), total cholesterol, and high-density cholesterol (HDL-c) levels were quantified using an automated biochemical analyser (Roche Cobas 501, Roche Diagnostics, USA) via enzymatic methods. Serum adipsin concentrations were determined using a commercial enzyme-linked immunosorbent assay kit (Multiscience Biotech Co., Ltd., China) following the manufacturer’s protocol. The mean minimal detectable concentration of adipsin was 143.37 pg/mL, with intra- and inter-assay coefficients of variation of 5.4% and 6.0%, respectively. The CMI was calculated by multiplying the waist-to-height ratio by the TG-to-HDL-c ratio [4]. The SIRI was calculated as the product of the neutrophil count and monocyte count, divided by the lymphocyte count [5]. Smoking status was defined as lifetime cigarette consumption of ≥ 100 cigarettes, with smokers further categorised as current smokers (smoked within the past 30 days) or former smokers [13]. Alcohol consumption was defined as an average intake of at least 40 g per week over the past 12 months [14].
At 24–28 weeks of gestation, all participants underwent a 75-g oral glucose tolerance test for GDM diagnosis. Following an overnight fast of at least 8 h, a 5 mL fasting venous blood sample was collected into a vacuum tube containing sodium fluoride and potassium oxalate to inhibit glycolysis. Participants were then instructed to consume a 75-g glucose solution within 5 min. Additional 5 mL venous blood samples were collected at 1 h and 2 h post-ingestion using the same type of anticoagulant-containing tubes. All blood samples were immediately mixed to prevent clotting and then centrifuged at 2000 g for 10 min at 4 °C to separate plasma. Plasma glucose levels at each time point were measured using an automated biochemical analyser (Roche Cobas 501, Roche Diagnostics, USA) via enzymatic methods. According to the International Association of Diabetes and Pregnancy Study Groups criteria [15], GDM was diagnosed if any of the following conditions were met: (1) fasting plasma glucose level ≥ 5.1 mmol/L; (2) 1-hour plasma glucose level ≥ 10.0 mmol/L; or (3) 2-hour plasma glucose level ≥ 8.5 mmol/L.
Data are presented as numbers (percentages), means ± standard deviations, or medians (interquartile ranges). Student’s t-test and the Mann-Whitney U-test were used to compare normally and non-normally distributed continuous variables, respectively, between the GDM and non-GDM groups. Categorical variables were compared using the chi-square test. The CMI, SIRI, and adipsin were categorised into tertiles for further analysis, as this approach facilitates clinical and epidemiological interpretation by expressing results as odds ratios for different risk groups, accounts for potential non-linear relationships by avoiding the assumption of linearity, and minimises the influence of extreme values. Multivariable logistic regression analysis was performed to evaluate the associations of CMI, SIRI, and adipsin with GDM, and to develop two predictive composite models for GDM. In the predictive model 1, CMI and SIRI were incorporated as independent variables, with GDM status as the dependent variable. Based on the logistic regression analysis, the equation for predictive model 1 in terms of the log-odds of GDM was generated as follows: logit (p) = 0.522 × CMI + 1.576 × SIRI– 4.601. The probability (p) of GDM was then derived from this equation using the inverse logit transformation: p = exp(logit(p)) / (1 + exp(logit(p))). This generated probability was saved as a new variable (PRE_1) in the statistical software. In the predictive model 2, CMI, SIRI, and serum adipsin levels were entered as independent variables, with GDM as the dependent variable in the logistic regression analysis. Similarly, the equation for predictive model 2 in terms of the log-odds of GDM was: logit (p) = 0.541 × CMI + 1.722 × SIRI– 0.001 × adipsin– 0.531. Following the same inverse logit transformation as in predictive model 1, the probability of predictive model 2 was generated and saved as another new variable (PRE_2) in the statistical software. These two predicted probabilities (PRE_1 and PRE_2) were subsequently used in receiver operating characteristic (ROC) curve analysis to assess and compare the predictive performance of the two composite models for identifying subsequent GDM. All statistical tests were two-tailed, and a P value < 0.05 was considered statistically significant. Statistical analyses were performed using SPSS 20.0 software (IBM Corp., USA).
A total of 1643 participants, with a mean age of 28.0 years, were included in the study. The baseline characteristics of women who underwent clinical examinations at 12 weeks of gestation are presented in Table 1, categorised by those with and without GDM at 28 weeks of gestation. Compared with their non-GDM counterparts, women with GDM were significantly older (31.2 ± 4.2 years versus 27.6 ± 3.9 years, P < 0.001) and had a greater waist circumference (91.1 ± 15.0 cm versus 81.8 ± 11.9 cm, P < 0.001). However, height did not differ significantly between the two groups (P = 0.124). There was also no significant difference in smoking status or alcohol consumption between groups. Although baseline fasting glucose levels were comparable between groups, lipid and inflammatory markers, including TG, HDL-c, and monocyte count, were significantly higher in the GDM group. Additionally, serum adipsin levels were significantly lower in the GDM group compared with the non-GDM group (3925.4 ± 920.8 ng/mL versus 4769.0 ± 1076.7 ng/mL, P < 0.001).
The crude and adjusted relative risks (RRs) with 95% confidence intervals (CIs) are presented in Table 2. For CMI, women in the highest tertile had a significantly higher risk of GDM compared with those in the lowest tertile (crude RR = 12.43, 95% CI: 7.06–21.87; adjusted RR = 6.71, 95% CI: 3.68–12.23; both P < 0.001). The middle tertile was associated with increased risk in the crude model (crude RR = 2.11, 95% CI: 1.10–4.03, P = 0.024), though this was not statistically significant after adjustment (adjusted RR = 1.60, 95% CI: 0.83–3.07, P = 0.169). For the SIRI, the highest tertile was strongly associated with GDM (crude RR = 9.92, 95% CI: 5.89–16.70; adjusted RR = 7.61, 95% CI: 4.47–12.95; both P < 0.001). The middle tertile was not significantly associated with GDM in either model (crude RR = 1.75, 95% CI: 0.95–3.22, P = 0.073; adjusted RR = 1.59, 95% CI: 0.85–2.95, P = 0.145). For adipsin, women in the lowest tertile had a significantly increased risk (crude RR = 8.53, 95% CI: 4.97–14.61; adjusted RR = 6.37, 95% CI: 3.67–11.05; both P < 0.001). Similarly, the middle tertile was also associated with increased risk (crude RR = 3.33, 95% CI: 1.87–5.93; adjusted RR = 3.15, 95% CI: 1.75–5.67; both P < 0.001).
The statistical significance of variables in the predictive composite models was evaluated using the Wald test, and model fit was assessed with the Hosmer-Lemeshow test. In the model 1, the Wald values for CMI, SIRI, and the constant term were 127.61, 113.76, and 453.73, respectively (P < 0.001 for all); in the model 2, the Wald values for CMI, SIRI, adipsin, and the constant term were 115.64, 109.24, 67.76, and 1.21, respectively (P < 0.001 for all except for the constant term, P = 0.271). The Hosmer-Lemeshow test results demonstrated an adequate fit for both composite model 1 (P = 0.517) and composite model 2 (P = 0.459), indicating good agreement between the predicted and observed outcomes.
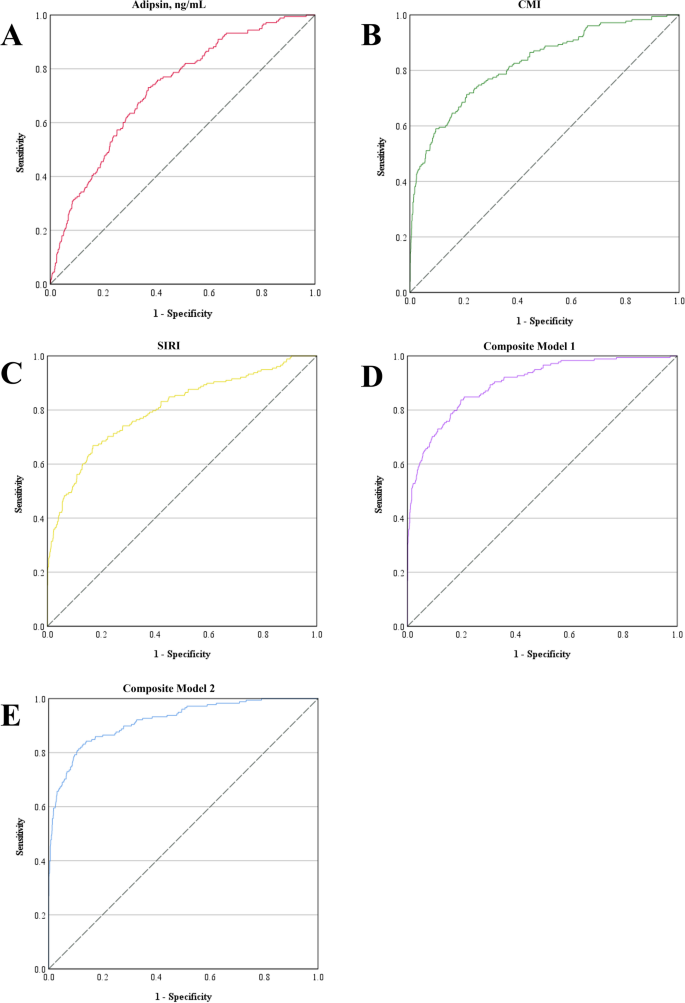
As presented in Fig. 2; Table 3, the area under the curve (AUC) values for adipsin, the CMI, and the SIRI were 0.724, 0.825, and 0.802, respectively. The composite model 1 integrating CMI and SIRI has achieved an AUC of 0.898, while the composite model 2 additionally incorporated adipsin further improving the AUC to 0.918. The Youden index method was used to determine the optimal predicted probability cut-off points. For adipsin, the optimal cut-off was 4385.84 ng/mL, with a sensitivity of 0.730 and a specificity of 0.630. The optimal cut-off for CMI was 1.71, yielding a sensitivity of 0.713 and a specificity of 0.789. For SIRI, the optimal cut-off was 0.83, with corresponding sensitivity and specificity values of 0.669 and 0.831, respectively. The composite model 1 had an optimal cut-off of 0.075, with a sensitivity of 0.837 and a specificity of 0.802. For composite model 2, the optimal cut-off was 0.098, achieving a sensitivity of 0.843 and a specificity of 0.861.
ROC curves for predicting gestational diabetes mellitus. ROC curves were generated to assess the diagnostic performance of A adipsin, B CMI, C SIRI, D the predicted probability of composite model 1, and E the predicted probability of composite model 2. The composite model 1 integrates the CMI and SIRI, whereas the composite model 2 integrates the CMI, SIRI and adipsin. CMI, cardiometabolic index; ROC, receiver operating characteristic; SIRI, systemic inflammation response index
In this study, we investigated the associations of CMI, SIRI, and serum adipsin levels with the risk of GDM in a cohort of pregnant women. Our results indicate that reduced adipsin and elevated CMI and SIRI are significantly associated with an increased risk of GDM. These results suggest that these biomarkers could serve as potential predictors for GDM, with their combination improving predictive accuracy.
According to previous studies, the global prevalence of GDM varies considerably, with rates in the Western Pacific region ranging from 4.5 to 20.3% and a median prevalence of 10.3% [16]. In our study, the incidence of GDM was approximately 10.8% in 2023, which aligns with data from this region but is slightly lower than the 14.8% average reported for mainland China [17]. Possible explanations for this discrepancy include: (1) differences in inclusion and exclusion criteria—for instance, our study excluded women with early insulin resistance and abnormal glucose tolerance, which may have led to a lower reported incidence of GDM; (2) variations in confounding factors such as diet, socioeconomic status, and lifestyle across studies; and (3) our hospital’s long-standing comprehensive health education and pregnancy guidance programme.
The association between CMI and GDM aligns with the role of metabolic dysregulation in the development of type 2 diabetes. A previous large population-based study reported that high CMI was strongly associated with diabetes [4], and this association has also been demonstrated in several recent studies [18,19,20]. However, few studies have examined the relationship between CMI and GDM. In our study, the positive association between CMI and GDM remained statistically significant after adjusting for age, alcohol consumption status, and smoking status, although the strength of association was slightly attenuated. This observation aligns with several previous studies linking lipid disorders to glucose intolerance and insulin resistance during pregnancy [21, 22]. For instance, in a study of 67 pregnant women, Daniel et al. demonstrated that elevated TG levels in early pregnancy were positively associated with insulin resistance and β-cell dysfunction during pregnancy [23]. Given the rising prevalence of metabolic disorders, CMI may serve as a non-invasive, cost-effective tool for the early identification of women at high risk of GDM.
Our findings also highlight the role of systemic inflammation, as measured by SIRI, in the development of GDM. Inflammation is widely recognised as a key factor in the pathogenesis of insulin resistance [24], which is central to the pathophysiology of GDM. We found that women in the highest SIRI tertile had a more than sevenfold increased risk of GDM. These findings support previous studies that link chronic low-grade inflammation to insulin resistance and glucose metabolism abnormalities. Prolonged inflammatory responses, characterised by elevated levels of TNF-α and IL-6, disrupt insulin signalling pathways, reduce insulin sensitivity, and impair pancreatic β-cell function. TNF-α and other cytokines interfere with insulin receptor function by promoting serine phosphorylation of insulin receptor substrate-1, impairing glucose uptake in peripheral tissues such as muscle and adipose tissue. The inflammatory markers that constitute SIRI—neutrophils, lymphocytes, and monocytes—may play distinct roles in GDM-related immune dysregulation. Studies have shown that elevated neutrophil counts in pregnant women with GDM reflect a heightened state of systemic inflammation, as neutrophils are major contributors to reactive oxygen species (ROS) and proinflammatory cytokine production, which exacerbate insulin resistance [25, 26]. In contrast, a reduced lymphocyte count in GDM may indicate immune suppression, consistent with findings that pregnant women with hyperglycaemia exhibit altered adaptive immune responses [27]. Regarding monocytes, increased monocyte levels in GDM have been linked to heightened inflammatory activity, as monocytes can differentiate into macrophages and promote chronic low-grade inflammation by secreting IL-1β, IL-6, and TNF-α. Nevertheless, findings on the association between monocytes and GDM remain inconsistent. Two population-based studies reported significantly elevated monocyte counts in GDM patients [28, 29], while another study found that a decreased gestational monocyte count was associated with GDM development [30]. These discrepancies highlight the limitations of using a single biomarker to predict GDM. The significant association between SIRI and GDM in our study underscores the critical role of inflammation in GDM development and management, reinforcing the potential of composite inflammatory markers like SIRI in GDM risk assessment.
To date, research on adipsin level changes and the mechanisms through which adipsin influences GDM remains limited. Two population-based studies reported that women with GDM have higher circulating adipsin levels than those with normal glucose tolerance. This suggests a possible compensatory mechanism to support insulin secretion and maintain glycaemic control during pregnancy, especially considering that both these studies used blood samples collected on the day of caesarean section at the end of pregnancy [31, 32]. Conversely, another study of 426 pregnant women found that GDM women aged over 35 years had lower adipsin levels, which partially aligns with our findings [33]. The inconsistent results regarding adipsin levels in GDM highlight the complexity of adipokine regulation during pregnancy. This complexity is influenced by factors such as maternal age, gestational stage, and study design, reinforcing the need for further research to clarify the role of adipsin and other adipokines in GDM and their potential as biomarkers or therapeutic targets.
Both SIRI and CMI demonstrated good predictive accuracy for GDM (0.8 ≤ AUC < 0.9), whereas the combination of SIRI, CMI, and serum adipsin markedly improved predictive accuracy to an excellent level (0.9 ≤ AUC < 1.0), although adipsin alone exhibited only fair predictive accuracy (0.7 ≤ AUC < 0.8) [34]. These findings suggest that a multibiomarker approach could enhance the early detection of GDM risk, allowing for timely intervention to mitigate adverse pregnancy outcomes. The clinical implications of CMI and SIRI are substantial. Unlike adipsin, all variables required to calculate CMI and SIRI are routinely measured during pregnancy, eliminating the need for additional invasive or costly tests. Given that the composite model incorporating only CMI and SIRI without adipsin still demonstrated near-excellent predictive accuracy (AUC = 0.898), these two indices alone may provide a practical alternative in clinical settings where adipsin measurement is not feasible.
Our study has several strengths, including a large real-world sample size and cost-effective multibiomarker prediction models. However, certain limitations should be considered. All participants were recruited from a single institution in China, limiting the generalizability of our findings to populations with different genetic and environmental backgrounds. In addition, the underlying mechanistic pathways linking these biomarkers to GDM development were not fully elucidated in this study. Future studies should aim to validate these biomarkers in diverse populations and further explore their underlying mechanisms.
In conclusion, our findings suggest that low adipsin levels, high CMI and SIRI in early pregnancy are significant risk factors for GDM. The combination of these biomarkers provides a promising approach for the early identification of women at risk for GDM, potentially facilitating timely interventions and improving maternal and foetal outcomes. Further research is warranted to confirm these findings and explore their clinical applications in GDM screening.
The datasets used and/or analysed during the current study are available from the corresponding author upon reasonable request and with the permission of the Second Affiliated Hospital of Soochow University.
- CMI:
-
cardiometabolic index
- GDM:
-
gestational diabetes mellitus
- HDL-c:
-
high-density lipoprotein cholesterol
- RR:
-
relative risk
- ROC:
-
receiver operating characteristic
- SIRI:
-
systemic inflammation response index
- TG:
-
triglyceride
We would like to thank all the participants for their participation and cooperation.
This study was supported by grants from the Suzhou Science and Education Strengthening Health Youth Project (KJXW2021011), the Suzhou Medical Innovation and Application Research Project (SKY2023119), the Extracurricular Academic Research Fund for Undergraduates (KY2024266B), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
This study was approved by the Ethics Committee of The Second Affiliated Hospital of Soochow University, and signed informed consent forms were obtained from all participants.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Cai, M., Jiang, X., Xu, X. et al. Integrating adipsin with novel cardiometabolic and inflammatory indices for enhanced early prediction of gestational diabetes mellitus: a prospective cohort study. Cardiovasc Diabetol 24, 189 (2025). https://doi.org/10.1186/s12933-025-02744-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12933-025-02744-2







_1751880097.jpeg)