BMC Nutrition volume 11, Article number: 67 (2025) Cite this article
We investigated the potential association between dietary mineral patterns and longitudinal change of glycemic status among individuals with prediabetes (Pre-DM).
This study included 1456 individuals with Pre-DM (mean age of 47.2 ± 12.8, and 52.5% men) who participated in the third (2006–2008) and fourth (2009–2011) examinations of the Tehran Lipid and Glucose Study (TLGS) that followed up until 2015–2017. The participants’ habitual dietary intakes of minerals were assessed using a semi-quantitative food frequency questionnaire (FFQ) at baseline. Principle factor analysis (PCA) identified three major mineral patterns (with a total variance of 92.3%), including multi-mineral (MM) (characterized by higher loads of phosphorous, zinc, calcium, magnesium, and copper), chromium-selenium (Cr-Se), and iron-manganese (Fe-Mn) patterns. Cox proportional hazard models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of developing type 2 diabetes (T2D) and regression to normal glucose regulation (NGR) across tertile categories of mineral patterns score.
After a median follow-up of 5.8 years, the incidence rates of T2D and NGR was 23.8% and 46.8%, respectively. After adjustment of T2D risk score (i.e., composed of age, sex, family history of diabetes, history of gestational diabetes, body mass index, and physical activity level) and dietary confounders, Cr-Se and Fe-Mn patterns were associated with an increased chance of returning to NGR by 26% (HR = 1.26, 95% CI = 1.02–1.55) and 42% (HR = 1.42, 95% CI = 1.14–1.76), respectively. Fe-Mn pattern was also associated with a reduced risk of developing T2D (HR = 0.67, 95% CI = 0.49–0.92).
Our findings emphasize the potential benefits of dietary Fe-Mn and Cr-Se intakes in pre-diabetic individuals.
Pre-diabetes (Pre-DM) is a condition characterized by intermediate hyperglycemia, diagnosed through isolated impaired fasting glucose (iIFG), isolated impaired glucose tolerance (iIGT), or a combination of both (IFG-IGT) [1, 2]. In 2021, the International Diabetes Federation (IDF) reported a global prevalence of 9.1% for iIGT, which is projected to increase to 10.0% by 2045 [1, 2]. Individuals with Pre-DM have a 25% risk of progressing to type 2 diabetes (T2D) within 3–5 years, with a lifetime risk exceeding 70% [3]. Our recent studies among individuals with Pre-DM highlight the beneficial impact of intensive lifestyle interventions, including physical activity [4], weight management [5], and dietary modifications [5, 6], on reducing the risk of developing T2D and restoration of normal glycemic regulation (NGR).
Dietary minerals are essential regulators of glucose metabolism and insulin signaling, with deficiencies in specific minerals being linked to an increased risk of T2D [7]. Key minerals such as magnesium, zinc, chromium, selenium, and iron play critical roles in insulin function and glucose homeostasis [7, 8, 9]. Chromium has been shown to enhance glucose homeostasis and improve insulin sensitivity by increasing the basal and insulin-stimulated levels of p38MAPK in insulin-resistant cells [10]. Additionally, glucose and lipid metabolism are linked to selenium levels in both healthy adults and individuals with T2D [11]. Notably, subjects with T2D often experience manganese deficiency, and supplementation with manganese has proven effective in promoting insulin secretion [12]. Conversely, iron may disrupt insulin secretion and signaling; however, its regular dietary intake has been found to be inversely associated with fasting plasma glucose levels in patients with acute pancreatitis [13].
Several studies have reported conflicting associations between a single dietary mineral intake and the risk of T2D [8, 14–17]. However, there is a significant gap in understanding how the mineral patterns influence the progression or regression of Pre-DM; little is known about the role of minerals in the intermediate stage of Pre-DM, particularly regarding the long-term changes in glycemic status. Additionally, previous studies did not assess the mineral pattern intake, but focused only on single supplementation. This gap highlights the importance of our study, which aims to investigate the potential association between dietary mineral patterns and the longitudinal changes in glycemic status among Iranian adults with Pre-DM over a 5.8-year follow-up period. Our study findings may provide some insights into whether specific mineral intakes could contribute to T2D prevention and restoration of NGR, offering new avenues for early dietary interventions.
This prospective cohort study was conducted within the Tehran Lipid and Glucose Study (TLGS) framework, a population-based investigation initiated in 1999 to examine non-communicable disease (NCD) prevalence and prevention in Tehran’s 13th district (Iran) [18]. The TLGS employed a standardized protocol to assess NCD risk factors every three years. This research included adults aged 21 and older with identified Pre-DM at the third (2006–2008) and fourth (2009–2011) TLGS examinations. A rigorous exclusion process based on missing data for 2-hour serum glucose (2 h-SG), glucose-lowering medications, having normal glycemia [i.e., normal fasting glucose (NFG) and normal glucose tolerance (NGT)], prevalent T2D, and unassessed usual dietary intake yielded a baseline study population of 1640 participants. These individuals were followed for reversion to NGR or progression to T2D until the sixth examination (2015–2017), representing a median follow-up of 5.8 years. After accounting for loss to follow-up (n = 54), the final analytic sample comprised 1586 participants.
Detailed methodologies for data collection and variable assessment within the TLGS have been previously reported [18]. Trained personnel conducted triennial follow-up visits to obtain and update information on family history of NCDs, tobacco use, medication regimen, physical activity level (PAL), and anthropometric indices [weight, height, waist circumference, and body mass index (BMI)].
Details of PAL assessment using the validated Iranian version of the Modifiable Activity Questionnaire (MAQ) have been described elsewhere [4]. This instrument captured both leisure time and occupational PAL. Participants were categorized into two PAL groups based on their estimated weekly metabolic equivalent of task (MET) minutes: a low PAL group (< 600 MET-min/week) and a moderate-to-high PAL group (≥ 600 MET-min/week) [19].
Blood pressure (BP) measurements were implemented between 8:00 and 12:00 AM by the trained TLGS staff, manually using an appropriate-sized cuff using Omron M7 sphygmomanometer (HEM-780-E), i.e., calibrated by the Institute of Standards and Industrial Research of Iran [20]. Participants rested seated for 15 min before two BP readings, separated by a 30-second interval. The average of these readings constituted the final BP value.
Detailed biochemical measurements in the TLGS have been described elsewhere [21]. Briefly, fasting blood samples were collected from participants after a 12–14 h fast to determine baseline and follow-up levels of fasting serum glucose (FSG), triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C). Additionally, a standard oral glucose tolerance test (OGTT) was administered to all consenting adults aged 21 years and older who were not on anti-hyperglycemic medications. The OGTT involved a 75-gram oral glucose load, followed by a two-hour measurement of blood glucose (2 h-SG) levels.
The participants' habitual dietary intakes were assessed using a semi-quantitative food frequency questionnaire (FFQ) at the third (2006–2008) and fourth (2009–2011) TLGS examinations [22, 23]. In brief, participants reported their consumption frequency of various foods over the past year, using household measures to estimate portion sizes, which were then converted to grams. The FFQ has demonstrated validity and reliability in assessing nutrients compared to more intensive dietary assessment method including 24-hour recalls [18]. The validity and reliability of the FFQ were previously evaluated in a random sample by comparing data from two FFQs administered one year apart, as well as by comparing the FFQ data with twelve 24-hour dietary recalls. The FFQ demonstrated acceptable validity and reliability for total dietary fat, with correlation coefficients of 0.59 and 0.38 between the FFQ and multiple 24-hour dietary recalls, and 0.43 and 0.42 between the two FFQs for males and females, respectively [18]. Additionally, a study examining the reliability, validity, and stability of dietary patterns derived from the FFQ found that the dietary patterns were reasonably reliable and valid over time within the population [24]. Residual energy-adjusted intake of minerals were included in the principle component analysis (PCA).
Glycemic status was categorized according to the American Diabetes Association (ADA) criteria [25]. Participants with NGR exhibited FSG levels below 100 mg/dL (NFG) and 2-hour serum glucose (2 h-SG) levels below 140 mg/dL (NGT). Prediabetes was defined by IFG (FSG between 100 and 126 mg/dL), IGT (2 h-SG between 140 and 200 mg/dL), or a combination of IFG and IGT. Type 2 diabetes was indicated by an FSG ≥ 126 mg/dL, 2 h-SG ≥ 200 mg/dL, or glucose-lowering medications. A positive family history (FHD) of T2D was defined as having at least one first-degree relative with the condition.
Individualized T2D risk assessment was conducted using the ADA risk prediction model. This model incorporates several risk factors [26], including age (age < 40 y, 40–49 y, 50–59 y, and ≥ 60 y, is scored as 0, 1, 2, and 3, respectively), sex (men = 1, women = 0), history of gestational diabetes mellitus (GDM, yes = 1, no = 0), FHD (yes = 1, no = 0), history of hypertension (HTN, yes = 1, no = 0), PA (yes = 0, no = 1), and body weight status (BMI < 25, 25–30, 30–35, and ≥ 35 kg/m2, is scored as 0, 1, 2, and 3, respectively). PAL was categorized as either low (< 600 MET-min/week) or moderate-to-high (≥ 600 MET-min/week), with corresponding scores of 1 and 0, respectively. A total risk score was calculated, with a score of 5 or higher indicating a heightened risk of developing T2D.
Statistical analyses were conducted using the SPSS for Windows version 20 (SPSS Inc., Chicago, IL, USA) and STATA version 14 SE (StataCorp, TX, USA). Baseline characteristics of the study participants were compared across glycemic outcomes overtime, using analysis of variance (ANOVA) for continuous variables or chi-square test for the categorical variables. Data are reported as mean (SD) or percent.
To determine dietary mineral patterns, the PCA was used. PCA is a data-driven method unrestricted by the investigator’s à priori hypothesis and is commonly used to obtain dietary patterns in population-based studies. In brief, this method reduced a set of predictors (including dietary minerals, including phosphorous, zinc, calcium, magnesium, copper, chromium, selenium, iron, and manganese) to a smaller number of components (mineral patterns) based on linear combinations of the predictors. Due to a high correlation between mineral intakes, the promax rotation (an oblique rotation) was used. Considering eigenvalues > 0.4, the scree plot, and the interpretability of the factors, 3 patterns were obtained. Minerals with an absolute component loading ≥ 0.55 were selected to describe the pattern, although all minerals contributed to the calculation of the pattern score. The Kaiser-Mayer-Olkin statistic, considered a measure of sampling adequacy, was 0.86, indicating a good appropriateness of factor analysis. To evaluate whether the correlation matrix was suitable for factor analysis, Bartlett’s test of sphericity was used; P value for Bartlett’s test of sphericity was < 0.001.
Cox proportional hazard models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for Pre-DM regression to NGR or progression to T2D across tertile categories of mineral patterns. Potential confounding variables were identified based on the literature and validated by statistical evidence. A univariate analysis was conducted to assess these potential confounders, and variables with a p-value (PE) less than 0.2 were included in the final multivariable model. The PE (p-value for entry) was used to determine which variables should be retained in the multivariable model [27]. Two Cox models were conducted: Model 1 was adjusted for ADA-risk score; and Model 2 was additionally adjusted for total energy intake (kcal/d), total fat (g/d) and fiber (g/d) intakes. The study defined the event date (diagnosis of NGR or T2D) for cases as the midpoint between the follow-up visit where the outcome was first detected and the preceding visit. Follow-up time was then calculated as the difference between this midpoint and the date the participant entered the study. For censored subjects, their survival time was simply the interval between their first and last observation dates. The proportional hazards assumption was evaluated using Kaplan-Meier estimation and the scaled Schoenfeld residuals, although no clear violations were observed. Correlations (and P values) between PCA-derived mineral patterns score and food groups were performed using Pearson correlation coefficients, and correlation plots were visualized using the STATA heatmap function.
A cohort of 1586 individuals with prediabetes (mean age 47.2 ± 12.9 y) was followed for a median of 5.8 years. After this follow-up period, the incidence rates of T2D and NGR was 23.8% and 46.8%, respectively. Baseline characteristics of the study participants across glycemic outcomes overtime are presented in Table 1. Participants who regressed to normoglycemia during the study follow-up were more likely to be younger, had lower values of BMI, WC, SBP, DBP and ADA-T2D risk score, and lower FSG, and 2 h-SG levels compared to pre-diabetics and diabetics. TG-to-HDL ratio was significantly lower than diabetics, but not pre-diabetics. A significant between-groups difference was also found for BP-lowering medications and High-risk of T2D score.
Table 2 component loading of major dietary mineral patterns. Principle factor analysis identified three major mineral patterns (with a total variance of 92.3%), including (1) multi-mineral (MM) pattern with a maximum variance of 74.5%, i.e., characterized by higher loads of phosphorous, zinc, calcium, magnesium, and copper; (2) chromium-selenium (Cr-Se) pattern (i.e., characterized by positive loadings for chromium and selenium), and iron-manganese (Fe-Mn) pattern (i.e., characterized by higher loads of iron and manganese).
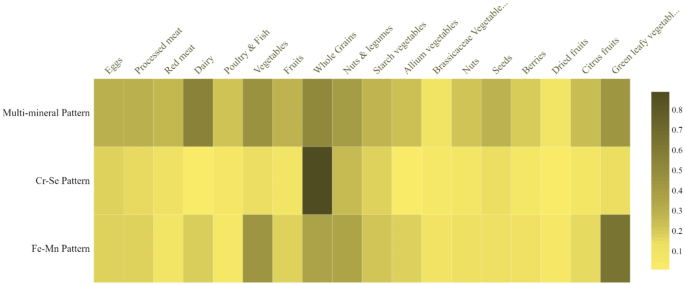
Heatmap of the correlations between PCA-derived mineral patterns scores and food groups are illustrated as Fig. 1. The second mineral pattern (Cr-Se) was highly correlated with whole grains, while Fe-Mn pattern was strongly correlated with green leafy vegetables, total vegetables, whole grains, nuts and legumes.
Heatmap of the correlations between PCA-derived mineral patterns scores and food groups. Cr, Chromium; Se, selenium; Fe, iron; Mn, manganese
The associations (HR and 95% CIs) of dietary mineral patterns and Pre-DM regression and progression are presented in Table 3. After adjustment of T2D risk score (i.e., composed of age, sex, FHD, history of GDM, BMI, and PAL) as well as dietary confounders (including energy intake, total fat and fiber intakes), Cr-Se and Fe-Mn patterns were associated with an increased chance of returning to NGR by 26% (HR = 1.26, 95% CI = 1.02–1.55) and 42% (HR = 1.42, 95% CI = 1.14–1.76), respectively. Fe-Mn pattern was also associated with a reduced risk of developing T2D (HR = 0.67, 95% CI = 0.49–0.92). MM pattern was not associated with either Pre-DM regression or progression.
Upon a median follow-up of 5.8 years among adults with Pre-DM, our findings demonstrate that specific dietary mineral patterns, particularly Cr-Se and Fe-Mn, are significantly associated with favorable changes in glycemic status. The Cr-Se and Fe-Mn patterns were more likely associated with a 26% and 42% higher likelihood, respectively, of returning to NGR. Moreover, the Fe-Mn pattern was associated with a 33% reduced risk of developing T2D. These findings, which were independent of established T2D risk factors and dietary confounders, underscore the potential role of specific mineral intakes in modulating glycemic outcomes and highlight the importance of targeted dietary interventions for the prevention and management of T2D.
Consistent with our findings, Norbitt et al. reported an inverse relationship between Mn intake and the severity of key glycemic markers in new cases of T2D following acute pancreatitis [28]. Specifically, each 1 mg decrease in Mn intake was associated with an increase in HbA1c by 0.17 mmol/mol and fasting serum glucose by 0.02 mmol/L [28]. Similarly, a cross-sectional study of 2,402 Chinese adults found that higher serum Mn levels were inversely associated with the prevalence of Pre-DM, particularly among elderly women [29]. Further supporting these findings, Du et al. analyzed data from two large Chinese cohort studies and consistently demonstrated a significant inverse association between Mn intake and T2D risk [30]. The Women’s Health Initiative (WHI) study, which included 84,285 postmenopausal women, also identified lower T2D incidence with higher manganese intake independent of known risk factors [31].
While Mn has been shown to improve insulin action and glucose tolerance in diabetic mouse models [33], the mechanistic link in human remains unclear. Recent studies have proposed that Mn may play a critical role through its involvement in the superoxide dismutase (SOD) enzymes, particularly manganese-dependent SOD (Mn-SOD), which is essential for scavenging reactive oxygen species (ROS) in mitochondrial oxidative stress [34]. Mitochondrial oxidative stress is a well-known contributor to the pathogenesis of dysglycemia through impaired β-cell function and insulin resistance [35, 36]. The protective role of Mn-SOD in mitigating oxidative stress may help preserve β-cell function, thus preventing the onset of insulin resistance and, ultimately, T2D [37]. Clinicians are now increasingly recommending the mitigation of oxidative stress during the prediabetic phase as a strategy to prevent the progression to T2D, and a Mn-rich diet may be a beneficial dietary modification.
The association between dietary iron intake and the risk of T2D remains complex and multifaceted. In our study, the dietary Fe-Mn pattern was predominantly linked to non-heme iron sources, such as green leafy vegetables, whole grains, nuts, and legumes; while meats, known for their heme iron content, had a low correlation with this pattern. This distinction is important given that non-heme iron has a significantly lower absorption rate compared to heme iron, being absorbed at only one-fifth to one-tenth the rate of heme iron [38]. Despite this lower absorption, there is no strong evidence suggesting that non-heme and heme iron differ in their roles once absorbed.
Interestingly, while iron is widely recognized as a strong pro-oxidant that can exacerbate metabolic disorders and increase the risk of T2D [39, 40], our findings suggest that the Fe-Mn pattern, which is higher in non-heme iron sources, is protective against diabetes risk. This observation aligns with emerging research indicating that it is primarily heme iron, rather than non-heme iron that is associated with an increased risk of T2D [39]. A large meta-analysis involving 323,788 participants and 28,837 T2D cases demonstrated a 20% increased risk of diabetes with higher heme iron intake, while non-heme iron intake showed no such association [41]. Moreover, cohort studies such as the Cardiovascular Disease Association Study (CAVAS), which examined 16,666 participants, identified a progressive association between higher total iron intake and increased diabetes risk, particularly among Korean men [42, 43]. In contrast, prospective studies focusing on non-heme iron have highlighted its protective role [38]. For example, an 11-year follow-up study of 35,698 postmenopausal women found a positive association between heme iron and T2D risk, but also revealed a protective effect of non-heme iron intake [38]. These observations underscore the importance of distinguishing between heme and non-heme iron when assessing dietary risk factors for T2D. While excessive heme iron intake may contribute to T2D development through its pro-oxidant properties, non-heme iron, often derived from plant-based sources, appears to offer a protective effect, potentially through its role in maintaining optimal oxidative balance and promoting insulin sensitivity [44].
Previous research indicates that Fe and Mn compete for absorption in the gastrointestinal tract [32]. However, our results reveal that these minerals can coexist in the same dietary pattern, contributing to a reduced risk of diabetes. This paradox may be attributed to several factors: first, the antioxidant properties of manganese, particularly via Mn-SOD, may counterbalance the oxidative effects associated with Fe. Additionally, a higher intake of Mn might modulate Fe absorption, leading to a beneficial equilibrium. Notably, the majority of Fe intake in our population comes from non-heme sources, which are absorbed at a significantly lower rate than heme-Fe. This lower absorption not only mitigates T2D risk but could also offer protective effects [42, 43, 45]. Furthermore, non-heme Fe sources are typically rich in nutrients [46], have lower glycemic indices, and possess higher antioxidant properties, all of which have been linked to reduced risk of Pre-DM and T2D [47]. Our earlier findings from the TLGS population further support this by demonstrating an inverse relationship between T2D risk and adherence to a healthy dietary pattern rich in plant-based foods, reinforcing the importance of dietary sources in metabolic health [48].
Chromium is recognized as an essential trace element that significantly enhances glucose metabolism [49]. Notably, Rafiei et al. highlighted the prevalence of Cr deficiency among Iranian individuals with Pre-DM and emphasized the necessity of screening for this deficiency according to the ADA guidelines to address its impact on subjects with Pre-DM [50]. Consistent with these findings, lower Cr levels have also been reported in both Pre-DM and T2D subjects in Jordan [51] and China [52]. Collectively, these studies underscore the importance of Cr intake in mitigating potential deficiencies, which may be crucial for controlling the prevalence of dysglycemia.
Chromium functions as a physiological enhancer of insulin activity [16]. It has been proposed that the interaction between kinase and phosphatase enzyme activities is essential for facilitating the rapid uptake of glucose by cells [49]. Specifically, Cr inhibits the activity of phosphotyrosine phosphatase, the enzyme that removes phosphate groups from the insulin receptor, thereby contributing to reduced insulin sensitivity [49]. Conversely, Cr may enhance the kinase activity of the insulin receptor (IR-β), which in turn boosts the activity of Akt and phosphoinositide 3-kinase (PI3K) [52, 53]. This activation promotes AMP-activated protein kinase (AMPK) activity, facilitates the translocation of GLUT4 vesicles from the cytoplasm to the cell membrane for glucose uptake, and mitigates oxidative stress within the cells [52, 53]. These mechanisms collectively underline the role of Cr in improving insulin sensitivity and glucose homeostasis.
Whole grains have been identified as the predominant component in the second mineral pattern (Cr-Se) among our population. While our results suggest a protective effect of dietary Se intake on reversion from Pre-DM to NGR, other studies have reported higher serum Se levels in individuals with T2D. A key distinction lies in the measurement of Se exposure. Our study relied on dietary intake, which reflects long-term consumption of Se-rich foods, while many other studies assessed Se through biomarkers such as serum or plasma levels. These biomarkers may be influenced by acute changes in Se status, illness, or metabolic disturbances, which could explain the observed association between elevated serum Se levels and increased T2D risk. Additionally, baseline Se status and the presence of other interacting minerals may modulate the effects of Se on glucose metabolism. For instance, low Se intake, combined with deficiencies in other essential minerals, could result in a higher risk of insulin resistance, while adequate Se intake from food sources may confer protective benefits. A higher serum Se levels was reported in individuals with T2D [54, 55, 56], despite in patients with gestational diabetes (GDM) [57]. Notably, elevated Se levels in T2D seems to be more likely a consequence rather than a cause of T2D [58]. A cross-sectional analysis from the CODING (Complex Diseases in the Newfoundland Population: Environment and Genetics) study, involving 2,420 non-diabetic adults, found an inverse relationship between Se intake and IR when total dietary Se intake was below 1.6 µg/kg/day [59]. Furthermore, an analysis of two large-scale US cohorts, consisting of 3,630 women and 3,535 men, concluded that individuals with higher intake of Se had a lower risk of developing T2D [60]. Despite established role of Se as an antioxidant mineral and its link to certain selenoproteins involved in glucose metabolism [61], the association between Se intake and risk of T2D remains highly complex.
In this regard, a meta-analysis pooling data from both observational studies found a direct association between selenium (Se) exposure and diabetes risk [62]. It is important to note that, in contrast to our study, the exposure assessment of Se in the included studies of this meta-analysis differed, with measurements taken from nails, urine, serum, blood, and plasma, which could influence the consistency and accuracy of the results. On the other hand, a separate meta-analysis of five randomized controlled trials (RCTs) involving participants with metabolic syndrome demonstrated a beneficial effect of Se supplementation on serum insulin levels and the Quantitative Insulin Sensitivity Check Index (QUICKI), although no significant improvements were observed in lipid profiles or FSG [63]. These discrepancies may be attributed to differences in study design, baseline Se levels, and the sources of Se measurement used across the studies, which likely contributed to varying outcomes.
The interaction between Se levels and glucose regulation affects both hyperglycemic states and the risk of hypoglycemia in individuals with Se deficiency [64]. Se deficient diets tend to dysregulate glucose homeostasis in consequence of decreased insulin secretion and expression of several selenoprotein genes [11]. Evidence indicates that Seconcentration in Iranian diabetic patients is significantly lower compared to non-diabetic individuals [66]. Consequently, to facilitate the reversion of Pre-DM states to NGR or to maintain glycemic control in populations at risk, particularly in regions where Se deficiency is common, it may be scientifically justified to promote the consumption of selenium-rich dietary sources.
Based on our findings, dietary recommendations for pre-diabetics should emphasize on an increased intake of leafy greens, whole grains, legumes and nuts, which are known as a good dietary sources of non-heme iron, manganese, selenium and chromium, which may enhance insulin sensitivity and reduce diabetes risk.
The strengths of this study include the use of a validated FFQ, which significantly enhances the reliability of dietary data. Additionally, the repeated, precise measurements of glycemic parameters and other relevant covariates strengthen the accuracy of tracking both disease progression and regression. By controlling for known risk factors of T2D, the study ensures a robust analysis, though the potential impact of unmeasured confounders remains a limitation. However, several limitations should be acknowledged when interpreting the findings. First, the reliance on baseline dietary data to identify major dietary mineral patterns may underestimate changes in diet over time. Future studies should consider using repeated dietary assessments or more robust dietary tracking methods to better capture dynamic dietary patterns. Moreover, the study did not include serum or urinary mineral levels, which are clinically important for assessing potential deficiencies or toxicities in the population, as well as for evaluating associations between mineral intake and the glycemic outcomes. Future studies should incorporate measurements of serum and urinary minerals to validate dietary intake data and better understand the relationship between mineral status and T2D risk. While we have adjusted for several potential confounders, other factors such as physical activity, genetic predispositions, and environmental exposures could further influence the outcomes. Future research should consider including a broader range of confounders to strengthen the findings. The geographic specificity of the study population limits the generalizability of the results to other populations with different genetic backgrounds, lifestyle factors, and dietary behaviors. Genetic variations in mineral metabolism pathways [65] can significantly influence individual responses to dietary mineral intake, potentially altering the effectiveness of dietary interventions in managing or preventing diabetes. Future studies that incorporate genomic and epigenetic analyses could offer more nuanced insights into the role of gene-diet interactions in diabetes prevention, helping to identify personalized approaches for improving glycemic control. Additionally, the classification of glycemic status based on a single measurement of FSG and 2 h-SG may introduce some degree of classification error, as these measurements may not fully capture variations in glycemic control over time. Longitudinal studies that assess glycemic status through repeated measures or by incorporating additional biomarkers (such as HbA1c) would help improve the accuracy of glycemic classification and better reflect the dynamics of T2D risk.
This prospective cohort study contributes valuable insights into the relationship between mineral intake and the reversion or progression of Pre-DM. Our findings suggest that individuals with Pre-DM may benefit from mineral patterns rich in Cr-Se and Fe-Mn, supporting the biological plausibility of these minerals’ involvement in glucose regulation and T2D prevention. Future research should explore the relationship between dietary mineral intake and Pre-DM outcomes, with an emphasis on baseline serum and urinary mineral levels, as well as food sources of these minerals, over extended follow-up periods. Such studies would provide more definitive evidence and help clarify the role of dietary minerals in Pre-DM management.
Data will be available upon forwarding the request to the corresponding author ([email protected]) and confirmation of the director of RIES ([email protected]).
We thank the Tehran Lipid and Glucose Study participants and the field investigators of the Tehran Lipid and Glucose Study for their cooperation and assistance in physical examinations, biochemical evaluation and database management. This study, was supported by Shahid Beheshti University of Medical Sciences.
This work was not supported by any funding agency.
Written informed consent was obtained from all participants. The ethics research committee of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran, approved the study protocol (IR.SBMU.ENDOCRINE.REC.1401.080). The study protocol was carried out according to the relevant guidelines expressed in the Declaration of Helsinki.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Jalali, M., Bahadoran, Z., Mirmiran, P. et al. Dietary mineral patterns are associated with the pre-diabetes regression and progression: the Tehran lipid and glucose study (TLGS). BMC Nutr 11, 67 (2025). https://doi.org/10.1186/s40795-025-01047-9