BMC Psychology volume 13, Article number: 496 (2025) Cite this article
The aim of this study was to utilize network analysis to explore the interconnections among anxiety, depressive, and insomnia symptoms in depressed patients in China.
The study included two surveys, the baseline survey was conducted from May 18, 2020 to June 18, 2020, and the follow-up survey was conducted 5 months later. A total of 4476 patients completed the baseline survey, and 1877 of them completed the follow-up survey. Depression symptoms were evaluated using the 9-item Patient Health Questionnaire-9 (PHQ-9), anxiety symptoms were evaluated using the 7-item Generalized Anxiety Disorder (GAD-7), and insomnia symptoms were evaluated using the 7-item Insomnia Severity Index (ISI). The centrality indices are utilized in the network analysis, and using Network Comparison Test (NCT) to evaluate the differences between the network structures at two different time points.
Network analysis revealed that the central symptom value was ISI5 (“Interfere with your daily functioning”) in the baseline networks and ISI4 (“Worried/distressed”) in the follow-up networks, the symptom with the bridge symptom value in both networks was PHQ9-3 (“Sleep”). The NCT results revealed no significant differences in edge weights and global strength among participants who completed both baseline and follow-up surveys.
Our results suggest that central symptom (e.g., “Interfere with your daily functioning”,“Worried/distressed”) and bridge symptom PHQ9-3 (“Sleep”) can be prioritized as a target for intervention and treatment in patients with depression.
Depression is a complex mental health disorder characterized by symptoms that extend beyond persistent low mood and loss of interest [1]. In fact, many individuals with depression experience a constellation of interconnected symptoms, with anxiety and sleep disturbances being among the most prevalent [2]. Anxiety symptoms may manifest as excessive worry, restlessness, or physical tension, exacerbating the distress of depression and complicating daily life for patients [3]. Insomnia and other sleep problems are also common, with patients struggling to fall asleep, experiencing frequent nighttime awakenings, or waking up too early and being unable to return to sleep [4]. These sleep disturbances not only diminish patients’ quality of life but can also amplify the severity of depressive symptoms. The interplay between a depressed mood and anxiety can lead to sleep difficulties, and insufficient sleep, in turn, can worsen both depressive and anxiety symptoms [5]. This complex interweaving of symptoms underscores the need for a comprehensive understanding and treatment approach to address these interrelated conditions effectively.
Most studies on the concurrent presence of anxiety, depression, and insomnia have relied primarily on syndromic assessments using standard scale total scores, although these scores are composed of diverse individual symptoms that may have different psychoneurological mechanisms [6, 7]. To address these concerns, network analysis has been used as a novel methodology for examining the intricate and ever-changing connections among individual mental symptoms [8]. This approach is rooted in the idea that a psychiatric disorder consists of a complex network of interconnected symptoms [9], with a focus on understanding the nature and intensity of the relationships among these symptoms [6, 10]. Network analysis has been proved to be a valuable tool in comprehending the interrelationships among particular symptoms and various psychiatric diseases [11].
Several research studies have employed network analysis to examine the relationships among symptoms of anxiety, depression, and insomnia. For instance, a study conducted network analysis of comorbid insomnia and depressive symptoms among psychiatric practitioners found that “Distress caused by sleep difficulties” (ISI7) and “Sleep maintenance” (ISI2) had the highest strength centrality, followed by “Motor dysfunction” (PHQ8) and “Sad mood” (PHQ2). Furthermore, the nodes “Sleep dissatisfaction” (ISI4), “Fatigue” (PHQ4), and “Motor dysfunction” (PHQ8) had the highest bridge strengths in linking depression and insomnia communities [12]. Another study investigated the network structure of comorbid anxiety and insomnia symptoms among clinicians with depressive symptoms. The study found that ISI7 (“Interference with daytime functioning”), ISI4 (“Sleep dissatisfaction”) and ISI5 (“Noticeability of sleep problem by others”) were the most central symptoms in the network model, while PHQ3 (“Sleep”) and PHQ4 (“Fatigue”) were the main bridge symptoms [13]. A network analysis of depressive and anxiety symptoms in Chinese college students identified “Fatigue” (PHQ4), “Restlessness” (GAD5), “Uncontrollable worrying” (GAD2), “Worry too much” (GAD3) as key central symptoms. Additionally, “Fatigue” (PHQ4), “Restlessness” (GAD5) and “Irritability” (GAD6) consistently acted as bridge symptoms [14]. An investigation of depressive and anxiety symptoms in adolescents revealed that central symptoms included the nodes PHQ2 (“Sad mood”), GAD6 (“Irritability”), GAD3 (“Worry too much”), while nodes PHQ6 (“Guilty”), PHQ2 (“Sad mood”), and PHQ9 (“Suicide ideation”) were bridge symptoms [15].
Although previous studies have explored the network structure among symptoms of anxiety, depression, and insomnia, these investigations have primarily focused on diverse populations such as college students [14], adolescents [15], and the elderly patients with mental illness [16], nurses [17], the general population [18], OSA patient [19], and individuals with insomnia [20]. However, research specifically examining the network relationships among these three symptom categories in patients diagnosed with clinical depression remains notably insufficient. This significant research gap needs to be addressed, particularly given that depression is characterized by high prevalence and complex symptomatology. In previous studies, data collection has predominantly been cross-sectional, involving a single time point measurement [6, 7, 14]. This approach limits the assessment of the stability of symptom network structures over time. Collecting data across multiple time points enables effective evaluation of symptom network stability and its evolutionary process over time [21]. This study employs a five-month follow-up design aimed at precisely capturing the potential impact of seasonal changes on insomnia, anxiety, and depressive symptoms in patients with depression. This time span extends from late spring/early summer to late autumn, encompassing significant seasonal transition phases, thereby providing a unique perspective for examining how symptom network structures are influenced by seasonal factors. In-depth analysis of the dynamic reorganization process of symptom networks during seasonal changes will provide important insights into the stability and vulnerability of psychological networks, further enriching our understanding of the dynamic characteristics of depressive symptom networks.
The aim of this study was to utilize network analysis to investigate the relationships and differences among anxiety, depression and insomnia symptoms in patients with depression at baseline and 5 months later (follow-up).
The study was conducted in outpatient clinics of 56 hospitals in 31 provincial administrative regions in mainland China. The study utilized convenience sampling technique. Self-assessment questionnaires were released and collected through a platform specifically designed for this study. Patients presenting to the outpatient clinics were invited to participate in the study after being diagnosed to meet the inclusion criteria by an attending psychiatrist. Before starting the study, all participants were informed about the purpose and significance of the survey. The participants needed to sign an informed consent form on the platform. Participants needed to answer all the questions in order to submit the questionnaire successfully. The data were automatically saved in the system. Participants were invited to engage in a subsequent survey conducted 5 months later.
Eligibility for inclusion was based on the following criteria: (1) a diagnosis of major depressive disorder (MDD) in accordance with the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5); (2) the ability to proficiently read and understand a survey questionnaire; (3) the patient voluntarily participated in the survey; and (4) the patient was 18 years or older. Exclusion was determined by the following conditions: (1) being under 18 years of age; or (2) the patient had a significant cognitive impairment. This study was conducted in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University (approval number: NFEC-2019-008).
The survey consisted of demographic information, and psychological indicators, including those for depression, anxiety, and insomnia. The depressive symptoms were evaluated using the 9-item Patient Health Questionnaire-9 (PHQ-9) [22]. Each item of the PHQ-9 pertains to a depressive symptom, scored on a scale from “0” (not at all) to “3” (nearly every day). The cumulative scores on the PHQ-9 scale range from 0 to 27, where higher values suggest increased severity of depressive symptoms. The PHQ-9 has been validated for its reliability and validity in evaluating depressive symptoms among Chinese individuals [23]. To assess anxiety symptoms, we employed the 7-item Generalized Anxiety Disorder (GAD-7) in this study. The GAD-7 evaluates typical anxiety symptoms, with each item scored from 0 (not at all) to 3 (nearly every day), and the overall scores range from 0 to 21, reflecting the gravity of anxiety symptoms [24]. The GAD-7 has been established as a reliable and valid instrument for assessing anxiety in China’s general outpatient population [25]. The assessment of insomnia symptoms was conducted using the 7-item Insomnia Severity Index (ISI) [26]. The ISI is a brief self-report instrument that measures perceived insomnia severity and impact. It evaluates 7 dimensions of insomnia. Each item is rated on a 5-point Likert scale (0–4), with total scores ranging from 0 to 28. Higher scores indicate more severe insomnia symptoms. The ISI scores are categorized as follows: no insomnia symptoms (0 to 7 points), mild insomnia symptoms (8 to 14 points), moderate insomnia symptoms (15 to 21 points), and severe insomnia symptoms (21 to 28 points) [27]. Validation studies have confirmed the reliability of the Chinese version of the ISI among Chinese populations [28].
Network estimation. For conducting the network analysis, we employed the bootnet and qgraph packages [29] within the R program (version 4.3.0). Symptoms were symbolized by nodes, and the connections between them were illustrated as edges [6, 30]. The network included 23 nodes/symptoms, including 9 depressive nodes, 7 anxiety nodes, and 7 insomnia nodes. In order to improve the comprehensibility of the outcomes and reduce the occurrence of false connections, we implemented a graphical Least Absolute Shrinkage and Selection Operator (LASSO) [29, 31, 32] to regulate the network models. The edge weights in our network represent partial correlation coefficients, which measure the direct relationship between two symptoms while controlling for all other symptoms in the network. A tuning parameter for the approach of model selection known as Extended Bayesian Information Criterion (EBIC) is commonly assigned a value within the range of 0 to 0.5 [33, 34].
In order to evaluate the importance of each node within the network, the expected influence (EI) was calculated for each node, defined as the sum of the absolute values of its connection weights [29]. These calculations were executed through the ‘centralityPlot’ function inside the qgraph package [35]. The resulting centrality values were then normalized (z-scored) and visualized in a plot for each node. The ‘bridge’ function in networktools was used to identify specific symptoms that serve as pathways in connections among symptoms [32], with a particular focus on bridge expected influence as the centrality metric. Bridge symptoms with higher expected influence values reflect greater risk of transmission from one community to others, compared to bridges with lower expected influence values [9, 30]. The network visualization was carried out employing the Fruchterman-Reingold algorithm [36]. The predictability of the nodes within the network model was estimated using the “mgm” R package [37].
Estimation of network accuracy and stability. Employing the “bootnet” R package (version 1.4.3), we evaluated the network solution’s robustness, calculated the edge weight accuracy and assessed the reliability of centrality measures. The accuracy of edge weights was appraised through the computation of confidence intervals (CIs), using non-parametric bootstrapping method [38, 39]. The dataset was randomly resampled to generate new datasets, a narrower CI indicates a more reliable network construct [29]. We executed 1000 permutations and implemented a bootstrap differential test to compare variations in network characteristics. These discrepancies were utilized to compare differences in edge weights and node centrality metrics [40]. A subset bootstrap approach was engaged to appraise the consistency of the centrality indices (e.g., strengths), calculating correlation stability coefficients (CS) [41]. The CS should not be less than 0.25, and preferably above 0.50 [29].
Comparison of networks between the baseline and follow-up surveys. A Network Comparison Test (NCT) was performed to assess changes between the baseline and follow-up surveys [42]. Given the high attrition rate in this study, and the significant differences in some demographic variables between the dropout group and the follow-up group, directly comparing these two datasets could introduce confounding effects. Therefore, we specifically compared the network structures of participants who completed both baseline and follow-up assessments (n = 1877), which minimized the influence of confounding variables and allowed for a more valid comparison of temporal changes. The NCT employs a two-tailed permutation approach that compares two networks using multiple invariance measures [42, 43]. The NCT was run 1000 times, focusing on the comparative analysis of global strength, which denotes the aggregate of the absolute edge weights between the two networks [44]. Significance was considered at a level of p < 0.05 (two-tailed).
We recruited 5237 adult participants from mainland China into this study. Among them, 4476 individuals satisfied the study’s inclusion criteria and completed the initial baseline assessment. The percentage of females was 63.4% and the age was 36.36 ± 11.88 years (mean ± SD). The scores for PHQ-9, GAD-7 and ISI were 8.19 ± 6.75, 5.97 ± 5.65, and 9.2 ± 7.19, mean ± SD, respectively. A total of 1877 individuals participated in the follow-up survey, comprising 64% females. The age was 38.12 ± 12.21 years (mean ± SD). The mean scores for PHQ-9, GAD-7, and ISI were 5.31 ± 5.56, 3.79 ± 4.59 and 5.91 ± 5.98, mean ± SD, respectively. Table 1 presents an overview of the demographic attributes of the participants in the baseline and follow-up surveys.
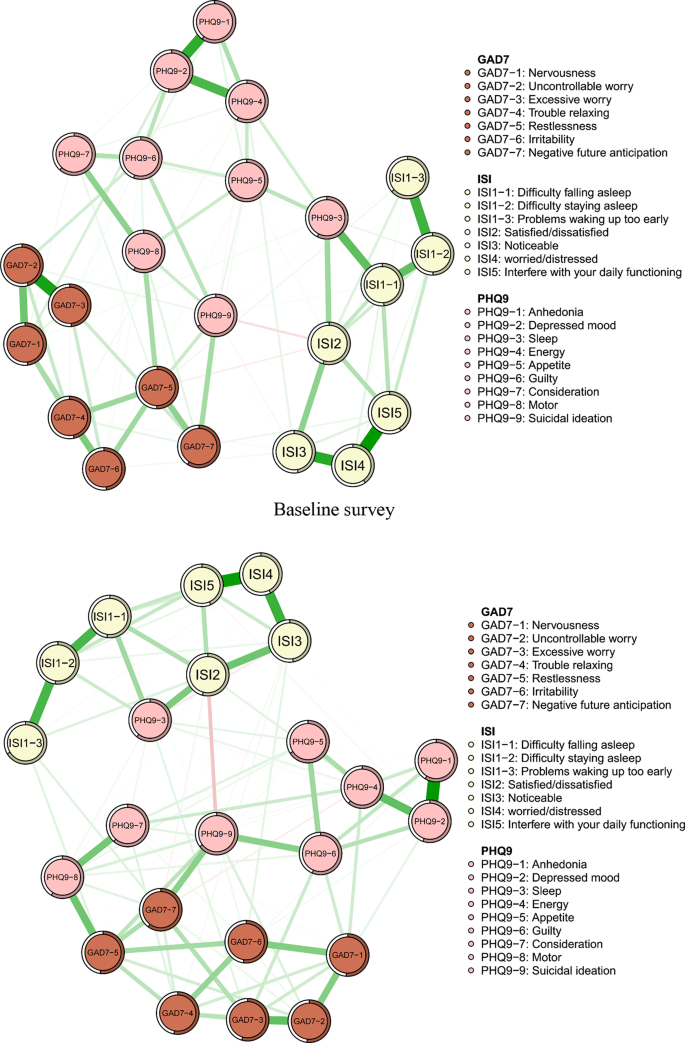
As seen in Fig. 1, the 23 nodes of baseline survey network showed that 169 of 253 edges were estimated to be non-zero edges (66.8%), reflecting considerable interconnectedness between two symptoms. Similarly, the 23 nodes of the follow-up survey network indicated that 163 of 253 edges were estimated to be non-zero edges (64.4%).
In the baseline survey network, the top three strongest edges were the ISI4 and ISI5 (“Worried/distressed” - “Interfere with your daily functioning”), GAD7-2 and GAD7-3 (“Uncontrollable worry” - “Excessive worry”), and ISI3 and ISI4 (“Noticeable” - “Worried/distressed”). In the follow-up survey, the edge between the ISI4 (“Worried/distressed”) and ISI5 (“Interfere with your daily functioning”) was identified as the most robust, followed by the edge between the ISI3 and ISI4 (“Noticeable” - “Worried/distressed”), and the edges between the ISI3 and ISI5 (“Noticeable” - “Interfere with your daily functioning”). For both surveys, the strongest edges were distributed within an individual community for insomnia, anxiety or depression, see Fig. 1, Supplementary Table S1 (baseline) and Supplementary Table S2 (follow-up).
The predictability values of the nodes in the baseline survey exhibited a range of 56–83.9%, averaging at 71.1%, indicating that approximately 71% of the variability among nodes in the network was accounted for by their neighboring nodes (Table S3). The predictability values of nodes in the follow-up survey ranged from 56.5 to 82.2%, with an average of 68.9%. For both surveys, the ISI4 (“Worried/distressed”), symptoms of insomnia, exhibited the highest level of predictability. The PHQ9-9 (“Suicidal ideation”) and ISI1-3 (“Problems waking up too early”), had the lowest level of predictability in the baseline survey and follow-up survey, respectively (Table S3).
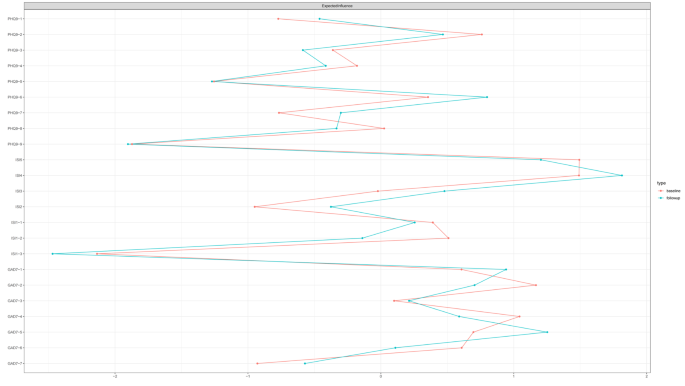
Figure 2 illustrates the centrality measures for all nodes across both surveys. For the baseline survey, the expected influence centrality was highest for ISI5 (“Interfere with your daily functioning”), followed by the ISI4 (“Worried/distressed”). In the follow-up survey network, the expected influence centrality was highest for ISI4 (“worried/distressed”), followed by GAD7-5 (“Restlessness”).
For bridge symptoms, the baseline survey indicated that the PHQ9-3 (“Sleep”) exhibited the highest bridge expected influence values, followed by PHQ9-8 (“Motor”), and GAD7-5 (“Restlessness”). The PHQ9-3 (“Sleep”) exhibited the highest bridge expected influence values, followed by GAD7-5 (“Restlessness”), and GAD7-1 (“Nervousness”) presented the highest expected influence values in the follow-up survey (Fig. S2).
To evaluate the accuracy and stability of the network estimation, we used a bootstrapping process consisting of 2500 iterations, as shown in Fig. S1 and Fig. S3. Figure 3 demonstrates the stability of centrality indices by using a case dropping subset bootstrap analysis. The CS coefficients for expected influence was 0.75 in the baseline survey. Similarly, in the follow-up survey as well, the correlation stability coefficients for expected influence was 0.75 (as shown in Fig. 3 & S3).
Figure S4 shows the outcomes of the bootstrap difference tests conducted during the survey. In the baseline survey, edge weights included ISI4 - ISI5 (“Worried/distressed” - “Interfere with your daily functioning”) and ISI3 - ISI4 (“Noticeable” - “Worried/distressed”) were significantly stronger than other edges. In the follow-up survey, the edge weights included PHQ9-1 - PHQ9-2 (“Anhedonia”– “Depressed mood”) and ISI4-ISI5 (“Worried/distressed” - “Interfere with your daily functioning”) were significantly stronger than other edges.
The baseline survey the non-parametric bootstrapped difference test for strength demonstrated that the nodes ISI5 (“Interfere with your daily functioning”) and GAD7-5 (“Restlessness”) had the highest strength compared to other symptoms. In the follow-up survey, the node of ISI4 (“Worried/distressed”) and GAD7-5 (“Restlessness”) had the highest strength (Fig. S5).
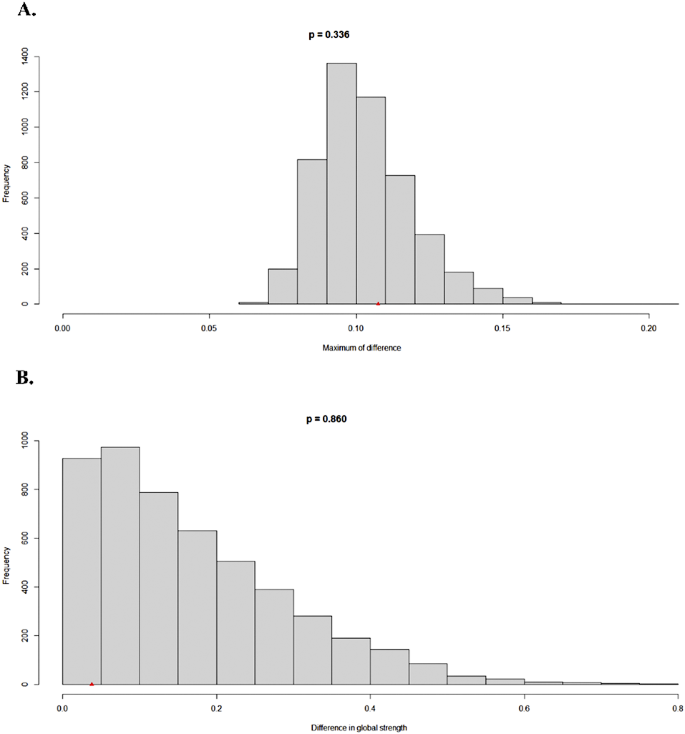
Based on the same 1877 participants who completed the follow-up, we conducted a Network Comparison Test (NCT) analysis to compare the baseline and follow-up networks. The results indicated no significant differences between the two time points in terms of network structure (M = 0.107, p = 0.337) or global strength (S = 0.038, p = 0.860).
As shown in Fig. 4, panel A displays the network edge invariance test results with frequency distribution of maximum differences, illustrating that the observed difference (M = 0.107) falls within the expected range (p = 0.337). Panel B shows the network global invariance test with frequency distribution of differences in global strength, confirming that the observed difference in global strength (S = 0.038) was not statistically significant (p = 0.860). These findings suggest that the overall network structure remained stable between baseline and follow-up assessments.
As far as we know, this is the first network analysis study to examine symptoms of anxiety, depression, and insomnia in a nationwide sample of patients with depression at two time points and to compare the network structures between these time points.
In both networks, our findings indicated that the strongest edges were confined within individual mental disorder community, rather than spanning across distinct disorder communities, aligning with the outcomes of prior network analysis research [45,46,47]. In our study the most robust edge in both networks was between the insomnia symptoms ISI4 (“Worried/Distressed”) and ISI5 (“Interfere with your daily functioning”). The second strongest edge in the baseline survey was between the GAD7-2 (“Uncontrollable worry”) and GAD7-3 (“Excessive worry”); the second strongest in follow-up survey edge was between the ISI3 (“Noticeable”) and ISI4 (“Worried/distressed”). A network analysis conducted on the interplay of depression, anxiety, insomnia, and life quality among the residents of Macau revealed that the strongest edge in the whole network was between the ISI1(“Difficulty falling asleep”) and ISI2 (“Difficulty staying asleep”), followed by the edge between the PHQ9-1 (“Little interest or pleasure in doing things”) and PHQ9-2 (“Feeling down, depressed, or hopeless”) [48]. The inconsistency of the findings may be due to differences in the population. Other strong edges in both networks in our study were observed between two depression symptoms, indicating that feeling nervous and anxious was prominent in depressed patient in China.
The expected influence centrality of nodes effectively pinpoints certain symptoms that have the greatest impact on the overall psychopathological symptom network within this population. The insomnia symptoms ISI5 (“Interfere with your daily functioning”) and ISI4 (“Worried/Distressed”) exhibited the highest centrality value in the baseline network and follow-up network, respectively. This indicated that the ISI5 and ISI4 had the most significant influence on the entire symptom network in the baseline network and follow-up network, respectively. It is therefore plausible to hypothesize that interventions aiming at improving daytime functioning and reduce worry about sleep may alleviate other symptoms in the network. Worry about sleep and interfere with daytime functioning are triggered by misperceptions of insomnia. Cognitive Behavioral Therapy for Insomnia (CBTI) or e-aid CBTI can be used to help patients identify and change their misperceptions and irrational beliefs towards sleep, and to reshape rational concepts and positive attitudes towards sleep and achieve the goal of alleviating symptoms of insomnia [49,50,51,52].
The bridge symptoms among different communities of mental disorders have a notable impact on psychiatric comorbidity. It is important to prioritize these bridge symptoms as treatment targets, with a focus on identifying specific symptoms that are key in lessening the likelihood of spread among psychiatric disorders within network models [9]. In both baseline and follow-up surveys, the PHQ9-3 (“sleep”) was found to be the bridge symptom in the anxiety-depressive-insomnia symptom network. Our results align with those of prior studies. Tao et al. conducted a network analysis among college students and found that the PHQ9-3 (“sleep”) emerged as a centrality and bridge symptoms for anxiety, depression, and sleep symptoms [53]. An examination of the co-occurrence of anxiety and insomnia among clinicians exhibiting depressive symptoms also revealed that PHQ9-3 (“Sleep”) was the bridge symptom [13]. The PHQ9-3 refers to having trouble falling or staying asleep, or sleeping too much. Pharmacological treatments such as benzodiazepines [54,55,56] and non-benzodiazepines [57] have been shown to be effective in improving trouble falling asleep and difficulties maintaining sleep. Pitolisant, a novel antagonist/inverse agonist of the histamine-3 receptor, is utilized for alleviating excessive daytime sleepiness in narcoleptic or obstructive sleep apnea adult patients. Solriamfetol, a novel inhibitor of norepinephrine and dopamine reuptake, has gained approval for the treatment of hypersomnolence due to its demonstrated effectiveness [58]. Furthermore, meta-analyses have indicated that psychological therapies, including CBTI, are employed to enhance sleep onset latency and increase overall sleep duration [39, 59].
Other bridge symptoms identified in our models included PHQ9-8 “Motor problems” (baseline survey) and GAD7-5 “Restlessness” (follow-up survey). A network analysis of anxiety-depression-insomnia symptoms among the population of Macau also showed that GAD5 and PHQ8 were the bridge symptoms [60]. The PHQ8 and GAD5 were identified as bridge symptoms in a study analyzing relationships of night shifts, anxiety, insomnia and depression among nurses in China [17]. These results suggest that the PHQ8 and GAD5 are stable bridge symptoms in network analyses across different groups of participants.
While our study was designed with a five-month follow-up period specifically to capture potential seasonal effects on symptom networks, our analysis revealed no significant differences in network structure between baseline and follow-up measurements among participants who completed both assessments. This stability suggests that the symptom interrelationships in patients with depression may be more resilient to seasonal influences than initially hypothesized. One possible explanation is that the core symptom structure of depression, anxiety, and insomnia might represent relatively stable trait-like patterns that persist despite environmental changes. Nevertheless, the consistency of bridge symptoms across time points, particularly PHQ9-3 (“Sleep”), provides valuable clinical information by identifying stable therapeutic targets regardless of seasonal context.
This study has several strengths, such as utilizing a large sample size and a multi-center study design, and applying network analysis methods. And the longitudinal approach enables researchers to examine the temporal stability of network structures, providing insights into which symptom relationships remain consistent and which may fluctuate over time. Nevertheless, several limitations need to be addressed. Firstly, this study employed convenience sampling technique, which may introduce selection bias. Future research should consider adopting more rigorous random sampling methods to enhance the generalizability and representativeness of the results. Secondly, the number of participants in the follow-up surveys was only 42% of that in the baseline survey. Future research should implement more effective retention strategies to minimize participant loss and ensure more representative longitudinal data. Thirdly, while the ISI is a well-validated measure of insomnia severity in the Chinese population, future studies would benefit from including both subjective and objective sleep measures to provide a more comprehensive evaluation of insomnia symptoms and their relationships with depression and anxiety.
To summarize, this study identified that the PHQ9-3 (“sleep”) was the key bridge symptom in the anxiety-depression-insomnia network in patients with depression in China. The findings from this research demonstrate that implementing treatments that target on difficulties with initiating or maintaining sleep, may have a positive impact on alleviating concurrent symptoms of depression, anxiety, and insomnia in patients with depression.
Symptom network of depressive, anxiety and insomnia symptoms among depressed patients. In the diagram, symptom nodes with stronger connections are positioned closer to each other. Positive correlations are depicted by green lines
Centrality measures of all symptoms within the network at different time periods.The figure shows expected influence of all symptoms within the network (z-scores)
Stability of centrality indices by case dropping subset bootstrap. The horizontal axis of the graph depicts the percentage of cases from the original sample utilized at each increment. Meanwhile, the vertical axis represents the mean correlations between the centrality indices derived from the original network and those obtained from the networks that were re-estimated after progressively removing cases
Data and materials are available from the corresponding author upon reasonable request.
- PHQ-9:
-
The9-item Patient Health Questionnaire-9
- GAD-7:
-
GAD-7 The7-item Generalized Anxiety Disorder
- ISI:
-
The7-item Insomnia Severity Index
- NCT:
-
Network Comparison Test
- MDD:
-
major depressive disorder
- DSM-5:
-
the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- LASSO:
-
Least Absolute Shrinkage and Selection Operator
- EBIC:
-
Extended Bayesian Information Criterion
- CIs:
-
confidence intervals
- CS:
-
correlation stability coefficients
- CBTI:
-
Cognitive Behavioral Therapy for Insomnia
We would like to thank all participants in this study.
This work was supported by the National Key R&D Program of China [grant number 2021YFC2501500], National Natural Science Foundation of China [grant number 82271525], Nanfang Hospital Clinical Research Project of Southern Medical University [grant number 2021CR009], and National Natural Science Foundation of China [grant number 82071488].
This study was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University. All participants provided informed consent after the study procedures were described to them.
Not applicable.
The authors declare no competing interests.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
Luo, X., Fang, L., Du, S. et al. Anxiety, depressive and insomnia symptoms among patients with depression: a network perspective. BMC Psychol 13, 496 (2025). https://doi.org/10.1186/s40359-025-02826-6